ABSTRACT
Background:
No reports about factors that predict prognosis after second-line chemotherapy for metastatic colorectal cancer have been published.
Methods:
We retrospectively analyzed 124 patients with metastatic colorectal cancer who received irinotecan-based second-line chemotherapy after first-line folinic acid/5-fluorouracil (5-FU)/oxaliplatin (FOLFOX) with or without bevacizumab.
Results:
A multivariate Cox model revealed 5 prognostic factors for worse survival: ECOG performance status 2, pathologically poorly differentiated adenocarcinoma, peritoneal metastasis, progression-free survival of first-line FOLFOX < 6 months, and lactate dehydrogenase ≥ 400 IU/L. When patients were categorized into 3 risk groups—patients without any prognostic factors (low-risk, n = 55), patients with one prognostic factor (intermediate-risk, n = 32), and patients with 2 or more prognostic factors (high-risk, n = 37)—overall survival from initiation of second-line chemotherapy was 23.5, 14.6, and 5.5 months, respectively. The proportion of patients who were eligible to receive further chemotherapy after disease progression was significantly lower in the high-risk group (41%) than in the intermediate- (67%) and low-risk (95%) groups.
Conclusion:
Several prognostic factors for survival after second-line therapy and probability of receiving third-line chemotherapy were identified. This risk classification system might be useful for determining which patients should receive cetuximab in the second-line setting rather than the third-line setting.
Folinic acid/5-fluorouracil (5-FU)/oxaliplatin (FOLFOX) plus bevacizumab is the most widely used first-line chemotherapy regimen for metastatic colorectal cancer (MCRC).1,2 After failure of FOLFOX, FOLFIRI [folinic acid/ (5-FU)/irinotecan] or irinotecan monotherapy is usually administered in the second-line setting.3,4 The results of a large observational study have also suggested that continued use of bevacizumab during second-line therapy may provide additional benefit.1
Cetuximab, a recombinant, human-mouse chimeric monoclonal IgG1 antibody that specifically targets epidermal growth factor receptor (EGFR) has been shown to improve the prognosis of MCRC significantly compared to best supportive care alone in the third-line setting.5 Furthermore, combining cetuximab with irinotecan results in a higher response rate than cetuximab alone, even in patients with irinotecan-refractory disease, suggesting that cetuximab may restore chemosensitivity in these patients.6
The EPIC trial was a large phase III study that compared irinotecan plus cetuximab to irinotecan monotherapy as second-line treatment in patients with MCRC following failure of oxaliplatin-based therapy.7 Although the primary end point of improved survival was not achieved (10.7 vs 10.0 months, p = .71), patients in the combination arm experienced a superior response rate and progression-free survival (PFS). Approximately half of the patients in the irinotecan monotherapy arm received cetuximab after irinotecan failure, which may have contributed to the similar overall survival rates in the 2 arms. However, 35% of patients in the irinotecan group were unable to receive any third-line chemotherapy, most likely due to rapid tumor progression.7 Thus, it was suggested that cetuximab with irinotecan may be better than irinotecan as second-line therapy for patients with rapidly progressing disease. So far, no reports about factors that predict the prognosis after second-line irinotecan or probability of receiving third-line therapy have been published. To address this issue, we conducted the following retrospective analysis of MCRC patients who received irinotecan-based chemotherapy as second-line treatment after first-line FOLFOX.
PATIENTS AND METHODS
This was a retrospective cohort study of MCRC patients who received irinotecan-based chemotherapy as second-line treatment after first-line FOLFOX. Irinotecan-based chemotherapy consisted of FOLFIRI (2-hr infusion of leucovorin isomers at 200 mg/m2 followed by bolus 5-FU 400 mg/m2 plus a 46-hr infusion of 5-FU 2,400 mg/m2 every 2 weeks, with irinotecan 150 mg/m2 as a 1.5-hr infusion on day 1) with or without bevacizumab (5 mg/m2 every 2 weeks), irinotecan monotherapy (irinotecan 150 mg/m2 every 2 weeks), or S-1 plus irinotecan (S-1 40 mg/m2 twice daily for 14 consecutive days followed by a 2-week rest, with irinotecan 100 mg/m2 every 2 weeks). Individual regimens were selected at the discretion the physicians or as called for in clinical trials.
Principal inclusion criteria were presence of histologically proven, inoperable colorectal cancer, age < 80 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2, sufficient bone marrow function, and normal liver and renal function. Treatment failure (defined as disease progression/discontinuation due to toxicity) within 6 months of the last dose of first-line fluoropyrimidine and oxaliplatin treatment for metastatic disease was required. Prior bevacizumab was allowed. These criteria were very similar to those of the EPIC study. Written informed consent was obtained from all patients prior to chemotherapy.
Among patients with MCRC treated at our institution between October 2005 and December 2008, 124 patients who fulfilled the inclusion criteria were identified. Detailed patient characteristics prior to initiation of second-line chemotherapy were acquired from hospital patient records. Objective tumor response of first-line FOLFOX was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST).8 PFS associated with first-line FOLFOX was measured from the beginning of treatment to the date of progression.
Statistical Methods
The primary end point of this study was evaluation of the association between several prognostic factors and overall survival, which was defined as the interval between the date of initiation of second-line treatment and the date of death or last follow-up using the Kaplan-Meier method. Progression-free survival was also measured from the beginning of second-line treatment to the date of disease progression.
To evaluate the prognostic factors associated with overall survival, univariate and multivariate Cox proportional hazards modeling was applied. The hazard ratio (HR) along with the 95% confidence interval (95% CI) was used as a measure of association in this study. Forward and backward stepwise methods were used for model building. Threshold p values for inclusion or exclusion in the model were defined as .10 and .20, respectively.
Factors included in the uni- and multivariate analyses were age (< 65 vs ≥ 65 years), gender (male vs female), ECOG PS (0–1 vs 2), peritoneal metastasis (yes vs no), liver metastasis (yes vs no), number of metastatic sites (1–2 vs ≥ 3), pathologic type (moderately or well-differentiated adenocarcinoma vs poorly differentiated adenocarcinoma), serum alkaline phosphatase (ALP) level (< 400 vs ≥ 400 IU/L), serum lactate dehydrogenase (LDH) level (< 400 vs ≥ 400 IU/L), serum carcinoembryonic antigen (CEA) level (< 500 vs ≥ 500 ng/mL), leukocyte count (< 8.0 × 109/L vs ≥ 8.0 × 109/L), response to first-line FOLFOX (responder vs nonresponder), and PFS associated with first-line FOLFOX (< 6 months vs ≥ 6 months). “Responders” were defined as patients who achieved a complete response or partial response, while “nonresponders” were patients with stable disease or progressive disease.
Distribution of subject characteristics was assessed by the chi-square test or the Fisher exact test, as appropriate. Statistical analyses were performed using STATA ver. 10 (StataCorp LP, College Station, TX). All tests were 2-sided, and p values < .05 were considered to be statistically significant.
RESULTS
Detailed patient characteristics are shown in Table 1. All 124 patients experienced disease progression prior to second-line chemotherapy. Oxaliplatin was discontinued due to neuropathy or allergy prior to disease progression in 59 patients; most of these patients continued 5-FU/leucovorin with or without bevacizumab until disease progression. First-line FOLFOX resulted in a partial response in 54 patients (43.5%), stable disease in 47 patients (37.9%), and progressive disease in 23 patients (18.5%). Median PFS associated with first-line FOLFOX was 7.3 months (95% CI, 6.2–8.0).
Table 1.
Patient characteristics
| Characteristic | Number of patients (N = 124) | |
|---|---|---|
| Median age, years (range) | 63 (23–79) | |
| Gender | Male/female | 74/50 |
| Performance status | 0–1/2 | 111/13 |
| Pathology | Wel or mod/por | 113/11 |
| Peritoneal metastasis | Yes/no | 26/98 |
| Liver metastasis | Yes/no | 69/55 |
| Metastatic sites | 1–2/> 3 | 99/25 |
| First-line treatment | FOLFOX/FOLFOX+BV | 107/17 |
| Response to first-line FOLFOX | CR/PR/SD/PD | 0/54/47/23 |
| PFS of first-line FOLFOX | < 6 months/> 6 months | 49/75 |
| Cause of oxaliplatin discontinuation | Disease progression/other | 65/59 |
| Leukocyte count (/L) | < 8×109/≥ 8×109 | 110/14 |
| ALP (IU/L) | < 400/≥ 400 | 70/54 |
| LDH (IU/L) | < 400/≥ 400 | 98/26 |
| CEA (ng/mL) | < 500/> 500 | 110/14 |
Abbreviations: ALP = alkaline phosphatase; CEA = carcinoembryonic antigen; CR = complete response; LDH = lactate dehydrogenase; mod = moderately differentiated adenocarcinoma; PD = progressive disease; PFS = progression-free survival; por = poorly differentiated adenocarcinoma; PR = partial response; SD = stable disease; wel = well-differentiated adenocarcinoma.
Second-line chemotherapy was administered as follows: FOLFIRI, 71 patients; irinotecan, 39 patients; and S-1 plus irinotecan, 14 patients. Bevacizumab was also used in 21 patients. The median treatment duration of second-line chemotherapy was 3.8 months (95% CI, 3–4.8).
At the time of analysis, 74 (59.6%) patients had died, with a median follow-up of 24.1 months since initiation of second-line chemotherapy. Median overall survival for all patients was 14.6 months (95% CI, 10.8–18.8). Median PFS was 3.8 months (95% CI, 2.9–5.2).
Salvage Chemotherapy
Among the 124 patients, 115 patients experienced disease progression despite second-line irinotecan-based chemotherapy; 82 of these patients (71%) received salvage chemotherapy as follows: anti-EGFR antibody (including cetuximab and panitumumab; n = 33), mitomycin-C plus irinotecan (n = 11), FOLFOX reintroduction with bevacizumab (n = 15), hepatic arterial infusion chemotherapy mainly using 5-FU (n = 10), and other regimens (n = 13). KRAS status was evaluated in 40 patients; 25 of these patients were determined to have cancers with a wild-type KRAS genotype.
Survival Analyses and Probability of Receiving Salvage Chemotherapy
Tables 2 and 3 show the results of univariate and multivariate analyses of baseline and clinical characteristics as prognostic factors for survival, including objective response and PFS associated with first-line FOLFOX. According to a multivariate Cox model, 5 prognostic factors for worse survival were identified: PS 2, pathologically poorly differentiated adenocarcinoma, peritoneal metastasis, PFS associated with first-line FOLFOX < 6 months, and LDH ≥ 400 IU/L.
Table 2.
Univariate survival analysis
| Characteristic | Cut-off | n | HR | 95% CI | p value |
|---|---|---|---|---|---|
| Age (years) | < 65 | 50 | 0.85 | 0.53–1.36 | .51 |
| ≥ 65 | 74 | ref | |||
| Gender | Male | 74 | 0.61 | 0.38–0.98 | .04 |
| Female | 50 | ref | |||
| Performance status | 0–1 | 111 | ref | ||
| 2 | 13 | 4.2 | 2.3–7.6 | < .001 | |
| Pathology | Well to mod | 115 | ref | ||
| Por | 9 | 3.4 | 1.7–6.9 | .001 | |
| Peritoneal metastasis | Yes | 26 | 3.1 | 1.87–5.1 | < .001 |
| No | 98 | ref | |||
| Liver metastasis | Yes | 69 | 1.37 | 0.86–2.1 | .18 |
| No | 55 | ref | |||
| Metastatic site | 1 or 2 | 99 | ref | ||
| ≥ 3 | 25 | 1.94 | 1.12–3.36 | .017 | |
| Response to FOLFOX | Responder | 54 | ref | ||
| Nonresponder | 70 | 1.92 | 1.18–3.2 | .008 | |
| Cause of oxaliplatin discontinuation | Progression | 65 | 2.18 | 1.36–3.49 | .001 |
| Other | 59 | ref | |||
| PFS of first-line FOLFOX (months) | < 6 months | 49 | 2.95 | 1.81–4.81 | < .001 |
| ≥ 6 months | 75 | ref | |||
| Leukocyte count (/L) | < 8×109 | 110 | ref | ||
| > 8×109 | 14 | 3.7 | 1.97–6.9 | < .001 | |
| ALP (IU/L) | < 400 | 70 | ref | ||
| ≥ 400 | 54 | 1.81 | 1.13–2.9 | .013 | |
| LDH (IU/L) | < 400 | 98 | ref | ||
| ≥ 400 | 26 | 2.78 | 1.61–4.8 | < .001 | |
| CEA (ng/mL) | < 500 | 110 | ref | ||
| ≥ 500 | 14 | 2.36 | 1.26–4.1 | .007 | |
Abbreviations: ALP = alkaline phosphatase; CEA = carcinoembryonic antigen; CI = confidence interval; CR = complete response; HR = hazard ratio; LDH = lactate dehydrogenase; mod = moderately differentiated adenocarcinoma; PD = progressive disease; PFS = progression-free survival; por = poorly differentiated adenocarcinoma; PR = partial response; ref = reference value; SD = stable disease; wel = well-differentiated adenocarcinoma.
Table 3.
Multivariate survival analysis1
| Factors | HR | p value | 95% CI |
|---|---|---|---|
| Performance status 2 | 4.8 | < .001 | 2.55–10.2 |
| Pathologic por | 3.50 | .002 | 1.60–7.96 |
| Peritoneal met | 2.10 | .009 | 1.20–3.68 |
| LDH ≥ 400 (IU/L) | 2.05 | .019 | 1.13–3.74 |
| PFS < 6 months | 1.80 | .040 | 1.08–3.01 |
Adjusted by gender, liver metastasis, metastatic sites, response to FOLFOX, cause of oxaliplatin discontinuation, leukocyte count, ALP, and CEA.
Abbreviations: CI = confidence interval; HR = hazard ratio; LDH = lactate dehydrogenase; met = metastasis; PFS = progression-free survival; por = poorly differentiated adenocarcinoma.
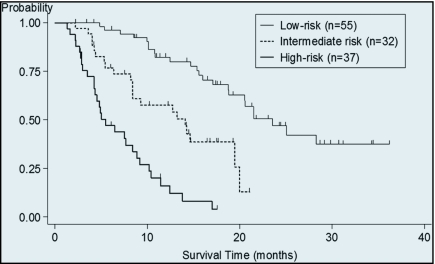
A multivariate prognostic model was constructed by incorporating all 5 prognostic factors, and patients were categorized into 3 risk groups: patients without any prognostic factors (low-risk, n = 55), patients with 1 prognostic factor (intermediate-risk, n = 32), and patients with 2 or more prognostic factors (high-risk, n = 37). Overall survival from initiation of second-line chemotherapy was 23.5 months (95% CI, 18.7–not reached), 14.6 months (95% CI, 8.4–19.9), and 5.5 months (95% CI, 4.2–8.9), respectively (Figure 1).
Figure 1.
Overall survival according to risk group. Median overall survival from initiation of second-line chemotherapy was 23.5 months (95% CI, 18.7–not reached) in the low-risk group, 14.6 months (95% CI, 8.4–19.9) in the intermediate-risk group, and 5.5 months (95% CI, 4.2–8.9) in the high-risk group.
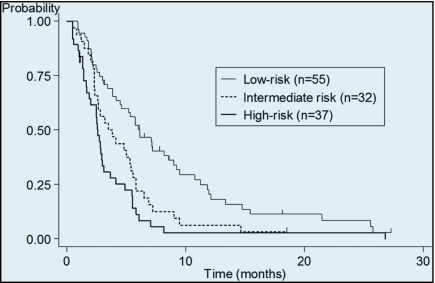
Significant survival differences among the 3 risk groups were observed (p < .001). PFS of second-line chemotherapy of each risk groups was 6.1 months (95% CI, 4.1–8.5), 3.4 months (95% CI, 2.3–5.4), and 2.6 months (95% CI, 1.6–2.9), respectively (Figure 2), and significant differences were observed between each groups (p < .001). If we limited the patients who did not receive anti-EGFR antibody (n = 91), a similar difference in overall survival was observed in these 3 risk groups (median 18.8 months vs 14.1months vs 5.0 months, p < .001).
Figure 2.
Progression free survival according to risk group. Median progression free survival from initiation of second-line chemotherapy was 6.1 months (95% CI, 4.1–8.5) in the low-risk group, 3.4 months (95% CI, 2.3–5.4) in the intermediate-risk group, and 2.6 months (95% CI, 1.6–2.9) in the high-risk group.
Salvage chemotherapy after disease progression was performed in 95% (46 of 48 progressed patients) of good-risk patients, 67% (21 of 31 progressed patients) of intermediate-risk patients, and 41% (15 of 36 progressed patients) of high-risk patients; all between-group differences were statistically significant (p < .001).
DISCUSSION
In this study, we identified 5 independent prognostic factors in patients with MCRC undergoing irinotecan-based second-line chemotherapy after first-line FOLFOX. Additionally, we defined 3 risk groups using these 5 prognostic factors that significantly differed in survival rate and probability of receiving further salvage chemotherapy. To the best of our knowledge, this is the first report to evaluate pretreatment clinical prognostic factors in MCRC patients undergoing second-line therapy. These results may be useful when selecting the appropriate treatment line for cetuximab.
Cetuximab appears to improve the prognosis of MCRC patients when used in the third-line setting compared to best supportive care alone, and irinotecan plus cetuximab has been shown to result in a higher response rate in patients with irinotecan-refractory MCRC (over half of whom also had oxaliplatin-refractory disease) compared to cetuximab alone.5,6 In contrast, the combination of irinotecan plus cetuximab did not improve overall survival in the second-line setting following first-line oxaliplatin-based chemotherapy.7
Based on these results, it may be optimal to use cetuximab in the third-line setting due to its toxicity profile and ability to restore irinotecan responsiveness even after irinotecan failure. However, considering the efficacy of cetuximab in MCRC, opportunities to administer cetuximab to MCRC patients, particularly those with wild-type KRAS disease, should not be missed.9–12
Our risk classification results suggest that cetuximab is not required during second-line treatment in low-risk patients due to their favorable prognosis (almost as long as first-line treatment [> 20 months]) and higher probability of receiving salvage chemotherapy (> 90%). In contrast, it might be optimal to use cetuximab in the second-line setting for high-risk patients with wild-type KRAS disease, to ensure that the opportunity to use cetuximab is not lost.
Determination of the optimal treatment for patients with intermediate-risk disease is more challenging and should therefore be conducted on an individual basis. For example, as PS2 had a significantly higher HR compared to other prognostic factors, cetuximab may be appropriate in second-line treatment of PS2 patients without prognostic factors. Risk classification may also be important for designing future clinical trials evaluating second-line treatment of MCRC and should be included as a stratifying factor considering the significantly different prognosis of each risk group.
This analysis had several methodologic limitations. First, it was a retrospective cohort design that evaluated the association between various prognostic factors and overall survival in patients who received several irinotecan-containing regimens (FOLFIRI, irinotecan, and S-1 plus irinotecan). However, the classification system used in this study has also proven to be similarly useful when patients are stratified by treatment regimen or bevacizumab use.
Second, the utility of salvage chemotherapy other than cetuximab or panitumumab is unknown, as no other treatment has been demonstrated to prolong the survival of patients with MCRC. However, the probability of receiving salvage chemotherapy in our study suggests the possibility that patients may have a chance to receive benefit from third-line chemotherapy, including anti-EGFR antibody therapy (in wild-type KRAS cases).
Third, KRAS status was not evaluated in all patients, since most of the patients initiated treatment before the introduction of cetuximab. As cetuximab should only be used in patients with wild-type KRAS disease, KRAS status should be evaluated in all patients prior to selection of third-line chemotherapy. Finally, the moderate sample size of this study necessitates confirmation of these results in a large cohort study, similar to the EPIC study.
In summary, several prognostic factors for survival after second-line therapy for MCRC and probability of receiving salvage chemotherapy were identified in this study. This risk classification system might be useful for determining which patients should receive cetuximab in the second-line setting rather than the third-line setting.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Grothey A, Sugrue MM, Purdie DM, et al. : Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Berry SR, Van Cutsem E, Kretzschmar A, et al. : Final efficacy results for bevacizumab plus standard first-line chemotherapies in patients with metastatic colorectal cancer: first BEAT. 2008 ASCO Annual Meeting Proceedings 25(15S), Abstract No. 4025, 2008 [Google Scholar]
- 3. Tournigand C, André T, Achille E, et al. : FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237, 2004 [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Colon Cancer V, 22009 [DOI] [PubMed] [Google Scholar]
- 5. Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. : Cetuximab for the treatment of colorectal cancer. New Engl J Med 357:2040229–2048, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham D, Humblet Y, Siena S, et al. : Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. New Engl J Med 351:337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Sobrero AF, Maurel J, Fehrenbacher L, et al. : EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Di Fiore F, Blanchard F, Charbonnier F, et al. : Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer 96:1166–1169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lièvre A, Bachet JB, Boige V, et al. : KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379, 2008 [DOI] [PubMed] [Google Scholar]
- 11. De Roock W, Piessevaux H, De Schutter J, et al. : KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol 19:508–515, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Karapetis CS, Khambata-Ford S, Jonker DJ, et al. : K-ras mutations and benefit from cetuximab in advanced colorectal cancer. New Engl J Med 359:1757–1765, 2008 [DOI] [PubMed] [Google Scholar]