Abstract
Conversion of fatty acid hydroperoxides to epoxyalcohols is a well known secondary reaction of lipoxygenases, described for S-specific lipoxygenases forming epoxyalcohols with a trans-epoxide configuration. Here we report on R-specific lipoxygenase synthesis of a cis-epoxyalcohol. Although arachidonic and dihomo-γ-linolenic acids are metabolized by extracts of the Caribbean coral Plexaura homomalla via 8R-lipoxygenase and allene oxide synthase activities, 20:3ω6 forms an additional prominent product, identified using UV, GC-MS, and NMR in comparison to synthetic standards as 8R,9S-cis-epoxy-10S-erythro-hydroxy-eicosa-11Z,14Z-dienoic acid. Both oxygens of 18O-labeled 8R-hydroperoxide are retained in the product, indicating a hydroperoxide isomerase activity. Recombinant allene oxide synthase formed only allene epoxide from 8R-hydroperoxy-20:3ω6, whereas two different 8R-lipoxygenases selectively produced the epoxyalcohol.A biosynthetic scheme is proposed in which a partial rotation of the reacting intermediate is required to give the observed erythro epoxyalcohol product. This characteristic and the synthesis of cis-epoxy epoxyalcohol may be a feature of R-specific lipoxygenases.
Keywords: arachidonic acid, total synthesis, Sharpless epoxidation, trans epoxide, hepoxilin, 18O2 incorporation, gas liquid chromatography-mass spectrometry, Plexaura homomalla
In the late 1960s and early 1970s, high concentrations of prostaglandin esters were identified in the Caribbean sea whip coral Plexaura homomalla (1, 2), and for a few years this abundant octacoral served as a source of prostaglandins for research (3). Due to differences in the prostaglandin profile from that typically seen in mammalian systems, for some time it was suspected that there existed a different prostaglandin biosynthetic pathway in coral (4, 5). Although this putative noncyclooxygenase pathway of prostaglandin synthesis turned out to be a red herring and cyclooxygenase accounts for the biosynthesis (6, 7), research into polyunsaturated fatty acid metabolism in coral extracts uncovered other interesting biochemistry. Bundy and colleagues studied the related coral Pseudoplexaura porosa and uncovered 8R-lipoxygenase activity, the first known existence of an R-specific lipoxygenase (8). 8R-LOX was subsequently found to be widespread in corals including in P. homomalla (5, 9) as well as in many marine invertebrates (10), and a 12R-LOX is highly conserved and functionally essential in mammals (11, 12). A second novel activity detected in coral extracts was allene oxide synthase (5, 13), which transforms the 8R-LOX product, 8R-hydroperoxy-eicosatetraenoic acid, to an allene epoxide (5), a proposed intermediate in biosynthesis of cyclopentenones such as the clavulones (14–16), and which hydrolyzes in vitro to an α-ketol derivative (5).
The work described in the present paper was initiated in the early 1990s, before the cloning of P. homomalla cyclooxygenases and lipoxygenases. It concerns an unexpected difference we observed in the metabolism of arachidonic acid (20:4ω6) and dihomo-γ-linolenic acid (20:3ω6) in extracts of P. homomalla; a prominent, relatively polar, product is formed specifically from 20:3ω6. This difference had been noted before in work from the E. J. Corey laboratory (17). Although the naturally occurring prostaglandin products in P. homomalla are all 2-series derived from arachidonic acid, we included a study of the metabolic fate of 20:3ω6 because it was originally reported as a substrate for the enzymatic activity in the coral (18) and because study of 20:3ω6 metabolism in P. homomalla is not complicated by the presence of large amounts of endogenous products. With the availability of cloned recombinant enzymes from P. homomalla, we recently returned to the issue of the origin of this extra product from 20:3ω6. The novel product we characterize herein is formed specifically by 8R-lipoxygenase metabolism, and its unusual stereochemistry may represent a feature of the secondary reactions of R- as opposed to S-lipoxygenases.
MATERIALS AND METHODS
Arachidonic (C20:4ω6) and dihomo-γ-linolenic acids (C20:3ω6) were purchased from NuChek Prep Inc. (Elysian, MN). [1-14C]20:4ω6 and [1-14C]20:3ω6 were purchased from Perkin Elmer Life Sciences (Waltham, MA). Plexaura homomalla was collected in the Florida Keys and placed on dry ice until long-term storage in the laboratory at −70°C.
Incubation with coral extracts
Frozen P. homomalla was cut into small pieces with scissors and placed in 10 vols of 50 mM Tris, pH 8, containing 1 M NaCl on ice and homogenized using a Polytron blender (Brinkmann) in 10-s bursts. The homogenate was allowed to settle under gravity for up to 30 min; aliquots of the supernatant were diluted 10-fold into fresh buffer for incubations with fatty acid substrates (100 μM), typically for 5 min at room temperature. Products were extracted by the addition of 1 M KH2PO4 plus sufficient 1 N HCl to give pH 4, followed by extraction with 2 vols of ethyl acetate. The organic phase was collected, washed with water to remove traces of acid, and taken to dryness under nitrogen. The extracts were redissolved in a small volume of MeOH before HPLC analysis.
Acetone powders of P. homomalla were prepared as described (5) and stored at −70°C until use. Typically, a 3 mg/ml suspension/solution in 50 mM Tris (pH 8) containing 1 M NaCl was prepared for incubations with substrates (5 min at room temperature). For recovery of 8-hydroperoxides from these incubations, the 3 mg/ml suspension was diluted 10-fold, and the incubation time was extended to 20 min; a few milligrams of 8R-HPETE or 8R-HPETrE could be prepared and purified from 0.5 l of the dilute acetone powder incubations. Products were extracted as described above. If required, before HPLC, hydroperoxides were reduced using a molar excess of triphenylphosphine in MeOH (5 min at room temperature).
HPLC analyses
Typically, aliquots of the extracts were analyzed initially by RP-HPLC using an ODS Ultrasphere 5μ column (Beckman) (25 × 0.46 cm) or Waters Symmetry column (25 × 0.46 cm) using a solvent of MeOH/H2O/HAc (80/20/0.01 or 75/25/0.01 by volume) at a flow rate of 1 ml/min with on-line UV detection (1100 series diode array detector; Agilent, Santa Clara, CA) and radioactive monitoring (Radiomatic Flo-One). Larger amounts (0.5–1 mg of total fatty acids) were injected for collection of products, or a semi-preparative column (Ultrasphere ODS, 25 × 1 cm; Beckmann) was used for larger quantities. Further analysis and purification was achieved by SP-HPLC using a 5-μ silica column (Alltech) or a Beckmann Ultrasphere 5 μ silica column using a solvent of hexane/isopropanol/glacial acetic acid (100/2/0.1 for H(P)ETE free acids and 100/1 for methyl esters; 100/5/0.1 and 100:3 for more polar derivatives and their methyl esters) run at 1 or 2 ml/min.
Synthesis of the threo-epoxy (8R, 9S, 10R)- and erythro-epoxy (8R, 9S, 10S)-eicosanoates
The synthetic approach is outlined in Supporting Data and supplementary Scheme 1.
Expression and purification of 8R-lipoxygenase
cDNA of the 8R-LOX domain of the P. homomalla peroxidase-lipoxygenase fusion protein (19) was subcloned into the pET3a vector (with an N-terminal His4 tag), and the protein was expressed in Escherichia coli BL21 (DE3) cells and purified by nickel affinity chromatography according to a previously published protocol (20). For clarity, this 8R-lipoxygenase is referred to herein as the recombinant 8R-LOX.
The second P. homomalla 8R-lipoxygenase tested here was the soluble enzyme purified in 1996 (21); aliquots from the original purification were stored at −70°C and these retained sufficient activity for use 15 years later. This enzyme is referred to here as the soluble 8R-LOX.
Incubation with enzymes
Side-by-side incubations were performed at room temperature in 1 ml of 50 mM Tris pH 8.0 containing 500 mM NaCl, 2 mM CaCl2 and 0.01% Emulphogene detergent using [14C]20:3ω6 or [14C]20:4ω6 fatty acids (each 25 μg/ml and 300,000 CPM) and recombinant 8R-LOX (10 μg in 1 ml) or soluble 8R-LOX. Under these conditions the recombinant enzyme completely metabolized 50 μM 20:4ω6 or 20:3ω6 substrate within 1 min, while 50 μl (∼50 μg) of the soluble 8R-LOX converted 50 μM 20:4ω6 or 20:3ω6 to the corresponding 8R-hydroperoxide in 5 min; an additional 20 μl enzyme was added to promote further metabolism of the 8R-hydroperoxide. Incubations were conducted in a 1 ml quartz cuvette and the rate of reaction monitored by repetitive scanning from 350-200 nm using a Lambda-35 spectrophotometer (Perkin-Elmer). Reactions were stopped by addition of 500 μl MeOH and the solution placed on ice. After addition of 3 ml of water, 100 μl 1M KH2PO4, and 40 μl 1N HCl to give pH ∼4.5, the samples were extracted using C18 Oasis cartridge and eluted with MeOH and analyzed by HPLC as described above.
GC-MS analysis of 18O2 incorporation in product from coral
Incubation of 20:3ω6 (100 μM) with an extract of P. homomalla acetone powder (3 mg powder/ml pH 8 buffer) was conducted under an atmosphere of 18O2. The products were purified initially by RP-HPLC (MeOH/H2O/HAc, 80/20/0.01 by volume), and then further purified by SP-HPLC (Hex/IPA/HAc, 100/5/0.1 by volume for the epoxyalcohol). Aliquots of HETrE (prepared by TPP reduction) and epoxyalcohol from the 18O2 incubation, together with unlabeled samples, were hydrogenated (H2, palladium on carbon in ethanol for 2 min) and after addition of water and extraction with ethyl acetate, they were converted to the pentafluorobenzyl (PFB) ester TMS ether derivative. The 18O content was determined by GC-MS analysis in the negative ion/chemical ionization mode using a Nermag R10-10B instrument with a 5 m SPB-1 capillary column programmed from 150° to 300° at 20°/min. The samples were subjected to rapid repetitive scanning over a 10 a.m.u. mass range (0.2 s per scan) covering the prominent M-181 ion (loss of PFB, resulting in the RCOO- ion of product); approximately 30 scans were collected during elution of the GC peak, and these were averaged for calculation of the relative ion abundances. For analysis of hydrogenated HETrE as the PFB-TMS derivative, the range scanned was m/z 397-406 (M-181 ion for unlabeled molecules at m/z 399, 18O1-labeled at m/z 401, and 18O2-labeled at m/z 403). For the hydrogenated epoxyalcohol product as the PFB-TMS derivative, the scanning range was m/z 411-420 with M-181 for the unlabeled molecules at m/z 413, and the 18O-labeled molecules at m/z 415 or 417.
LC-MS analysis of 18O incorporation in product from 8R-LOX
[18O2]8R-HPETrE was prepared using recombinant 8R-LOX (from the P. homomalla fusion protein) reacted with C20.3ω6 (20 μg/ml) in pH 8.0 Tris buffer (5 ml) under an atmosphere of 18O2; (the 100 ml bulb of oxygen gas contained about ∼35% of normal air (16O2), because it had been used three times previously for 18O syntheses). The [18O2]8R-HPETrE labeled in the hydroperoxy group was purified by SP-HPLC and reacted with recombinant 8R-LOX under a normal atmosphere to produce the corresponding epoxyalcohol. The 18O contents of the 8R-HPETrE and its corresponding epoxyalcohol product (which share the same molecular weight, 338 for the unlabeled species) were measured by negative ion electrospray LC-MS using a ThermoFinnigan TSQ Quantum instrument by rapid repetitive scanning over the mass range encompassing the M-H anions (m/z 330-350, 5 scans/sec). A total of 20–30 scans over the HPLC peaks were averaged to obtain the partial mass spectra of labeled and unlabeled epoxyalcohol and 8R-HPETrE.
NMR analysis
1H NMR and 1H,1H COSY NMR spectra were recorded on a Bruker 400 MHz or Bruker DRX 500 MHz spectrometer at 298 K. The parts/million values are reported relative to residual nondeuterated solvent (δ = 7.16 ppm for C6H6, 7.26 ppm for CDCl3).
RESULTS
Metabolism in extracts of P. homomalla
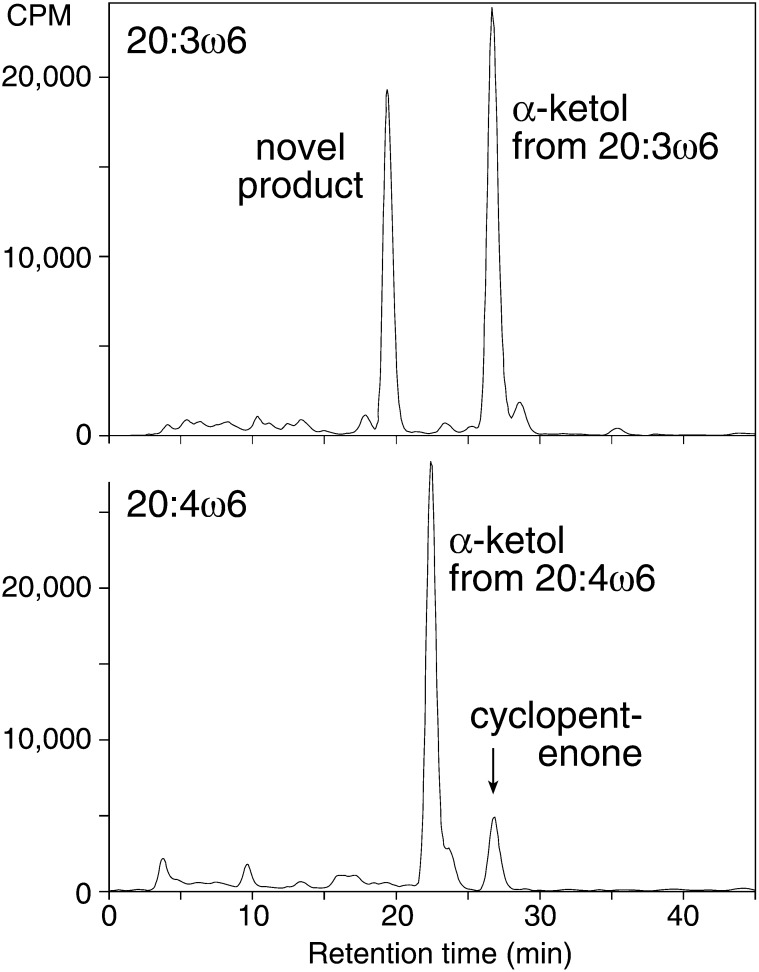
As originally reported (5), when arachidonic acid (20:4ω6) is incubated with extracts of P. homomalla, the fatty acid is rapidly metabolized by 8R-LOX, and the resulting 8R-HPETE is further transformed by allene oxide synthase, leading to the appearance of α-ketol and cyclopentenone end products (Fig. 1, lower panel). Metabolism of dihomo-γ-linolenic acid (20:3ω6) is similar, except for the appearance of a prominent, more polar product that is absent (or present in insignificant amounts) in the arachidonic acid incubations (Fig. 1).
Fig.1.
RP-HPLC analysis of products formed from 20:3ω6 and 20:4ω6 in homogenates of Plexaura homomalla. The fatty acids [14C]20:3ω6 or [14C]20:4ω6 (100 μM, 500,000 CPM) were incubated side-by-side with a homogenate of P. homomalla for 5 min at room temperature in 50 mM Tris buffer (pH 8) containing 500 mM NaCl. The samples were extracted using a C18 cartridge and analyzed by RP-HPLC using a Beckman ODS Ultrasphere column (5μ, 25 × 0.46 cm) with a solvent of MeOH/H2O/HAc (75/25/0.01 by volume) and a flow rate of 1 ml/min with on-l ine UV detection (Hewlett-Packard 1040A diode array detector) and radioactive monitoring (Radiomatic Flo-One detector).
Identification of the novel 20:3ω6 product
The structure was established based on UV, NMR, and GC-MS data. The purified polar product displayed only end absorbance in the UV data, indicating no conjugated double bonds. A quantity of ∼100 μg was prepared, and the proton NMR and COSY spectra were recorded in CDCl3. These results (see supplementary Table I) indicated the presence of an 8,9-cis epoxide [H8, dd, 3.03 ppm; H9, dt, 2.92 ppm; J8,9 = 4 Hz; cf. cis epoxides 4-5 Hz, trans epoxides ∼2 Hz (22)], with α-hydroxyl at C-10 and two cis double bonds at 11,12 and 14,15. So far this established the covalent structure as 8,9-cis-epoxy-10-hydroxy-eicosa-11Z,14Z-dienoic acid, an epoxyalcohol of the hepoxilin B-type that is distinctive in being a cis-epoxide (23).
Determination of the C-10 hydroxyl configuration
To establish the relative stereochemistry of the epoxide to the C-10 hydroxyl, two saturated analogs of the natural product were prepared by total chemical synthesis as outlined in the supplementary data and in supplementary Scheme I.
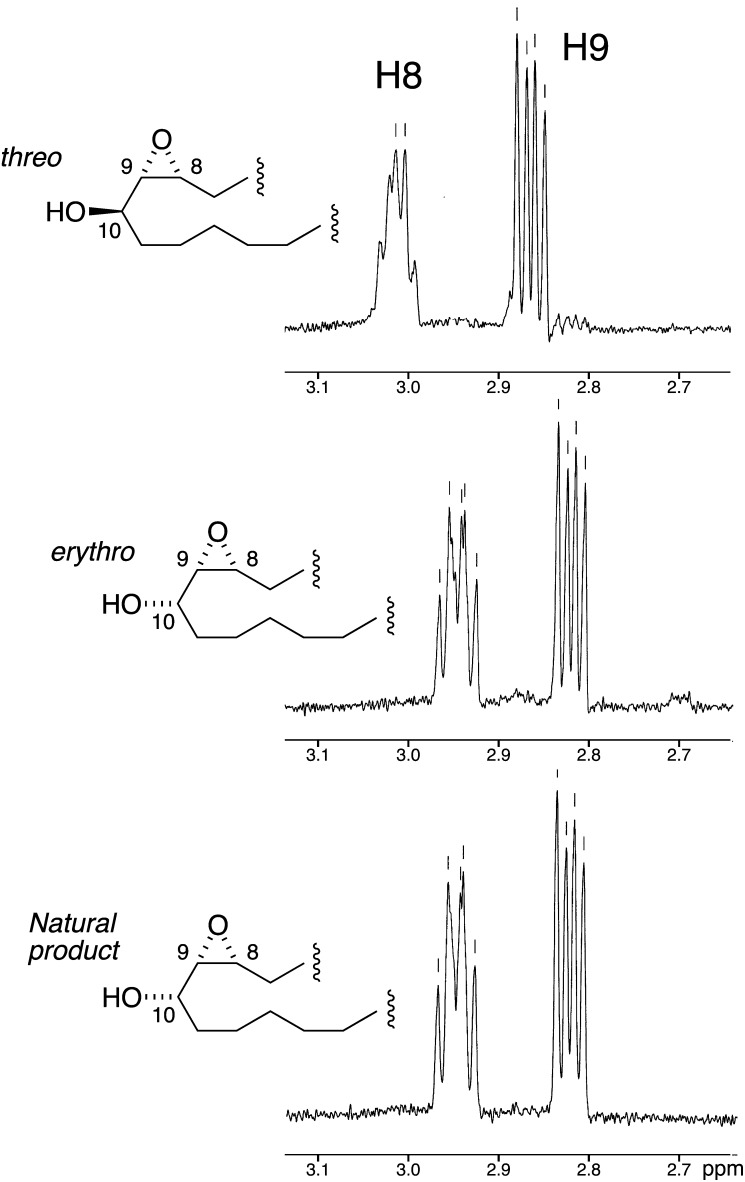
These synthetic standards, 8R,9S-cis-epoxy-10-hydroxy-eicosanoates with the 9,10 erythro and threo relative configurations, were first analyzed by GC-MS (EI mode) in comparison to the hydrogenated natural product as the methyl ester TMS ether derivatives. The threo 8,9-cis-epoxy-10-hydroxy-eicosanoate standard eluted before the erythro diastereomer (5 m SPB-1 capillary column, 150° to 300° at 20°/min) each as well resolved peaks with retention times of 4 min 52 s and 5 min 1 s, respectively. Their mass spectra had a noticeably different pattern of ion fragments, especially at the lower m/z values (see supplementary Fig. I). Significant ions in the threo methyl ester TMS derivative were observed at m/z values of 413 (6%), 397 (1%), 321 (3%), 257 (35%), 243 (base peak), and 211, 183, and 165 (all ∼15–20%), 143 (29%), and m/z 129 (60%). The later eluting erythro standard had structurally diagnostic ions at m/z values of 413 (M-15, 2% relative abundance), 397 (M-31, 1%), 287 (C1-C10, 18%), 271 (287-16, 4%), 257 (8%), 243 (C10-C20, base peak), with other prominent ions at m/z values of 211 (8%), 197 (16%), 165 (90%), and 129 (41%). The ion assignments were confirmed by analysis of the mass spectra of the corresponding TMS ester TMS ether derivatives (data not shown). The erythro standard had an indistinguishable mass spectrum and retention time to the hydrogenated epoxyalcohol product of P. homomalla. Their structural identity was confirmed by comparison of the NMR spectra of the saturated natural product with the synthetic standards (Fig. 2). These data confirmed the erythro relative configuration at 9,10 in the natural product. Because P. homomalla exhibits only 8R-LOX activity, the cis epoxide moiety can be assigned as the 8R,9S enantiomer. Thus, the complete structure of the novel product from 20:3ω6 is established as 8R,9S-cis-epoxy-10S-hydroxy-eicosa-11Z,14Z-dienoic acid. Metabolism in the coral extracts is summarized in Scheme 1.
Fig.2.
Partial 1H-NMR spectra of the hydrogenated P. homomalla product from 20:3ω6 with erythro and threo epoxyalcohol fatty acid standards. The natural product was converted to the methyl ester, hydrogenated, and repurified for comparison with the corresponding synthetic 8R,9S-cis-epoxy-10-hydroxy-eicosanoates. The region encompassing the epoxide protons is illustrated. In the threo isomer the epoxide protons are located at δ 3.02 (1H, H8) and 2.87 (1H, dd, J8,9 = 4.35 Hz, J9,10 = 7.94 Hz), and in the erythro diastereomer at δ 2.95 (1H, ddd, H8) and 2.82 (1H, dd, J8,9 = 4.2 Hz, J9,10 = 7.69 Hz). Other signals in each diastereomer were observed at δ 3.65 (3H, s, -OC H3 ), 3.5 (1H, m, H10), 2.3 (2H, t, H2), 1.7–1.45 (m), 1.45–1.2 (m), and 0.88 (3H, t, H20). The spectra were recorded in CDCl3 using a Bruker 400 MHz instrument.
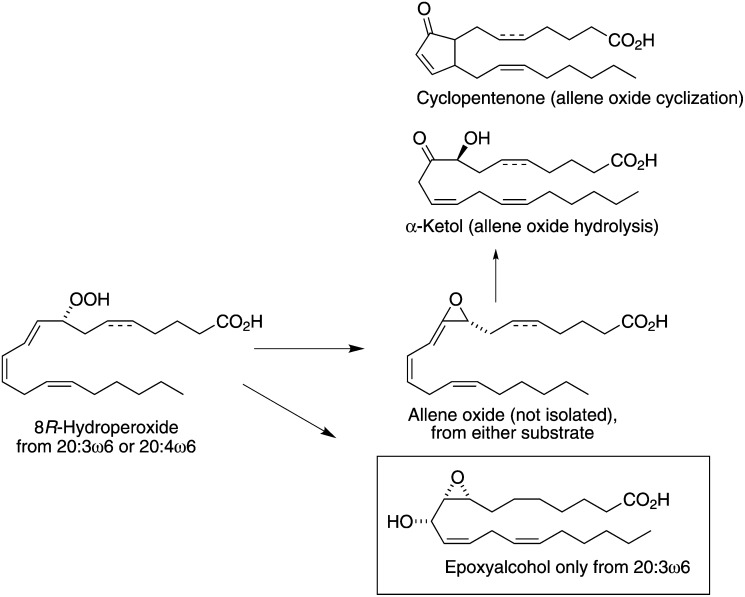
Scheme 1.
Biosynthesis from 20:4 ω 6 and 20:3 ω 6 in P. homomalla.
Origin of the epoxyalcohol oxygens in the novel 20:3ω6 product
To investigate the mechanism of formation of the epoxyalcohol, 20:3ω6 substrate (100 μM) was incubated with extracts of P. homomalla acetone powder (10 ml) under an atmosphere of 18O2 at room temperature for 10 min. The products were extracted and purified by reverse-phase (RP)-HPLC and straight-phase (SP)-HPLC; aliquots were hydrogenated and then analyzed for 18O content by GC-MS of the PFB ester TMS ether derivatives. The ion profile in the hydrogenated epoxyalcohol (see supplementary Fig. I) gave a ratio of 216O:18O-16O:218O of ∼6:4:90, indicating that most of the molecules of epoxyalcohol contain two oxygen atoms from 18O2 (see supplementary Fig. II). Because the precursor of the epoxyalcohol is 8R-HPETrE (an assumption proved formally using purified enzymes, vide infra), these results are compatible with essentially complete retention of the hydroperoxy oxygens from the precursor 8R-HPETrE.
Lack of product using allene oxide synthase
There are several precedents for the transformation of fatty acid hydroperoxides to epoxyalcohols catalyzed by allene oxide synthase (AOS) and related enzymes (24–26), and it seemed possible that this might account for the formation of the 20:3ω6-derived epoxyalcohol. However, experiments with the expressed AOS domain of the P. homomalla AOS-LOX fusion protein (19) produced only allene oxide as product [detected as the major α-ketol hydrolysis product and cyclopentenone (5)] from 8R-HPETE or 8R-HPETrE (data not shown).
Formation of epoxyalcohol by 8R-LOX enzymes
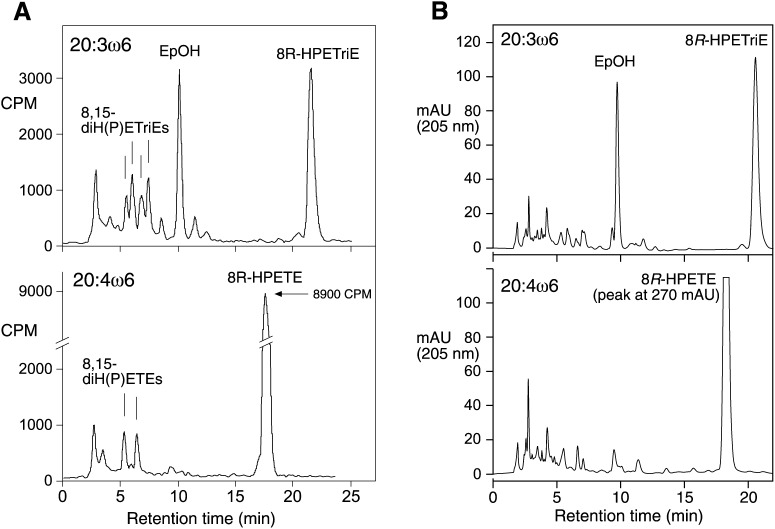
By contrast, use of the recombinant LOX domain of the AOS-LOX fusion protein gave positive results. When sufficient enzyme was used to quickly transform (<1 min) all the fatty acid to the corresponding 8R-hydroperoxide, further reaction generated secondary products. When observed by repetitive scanning in the UV, the rapid appearance of the conjugated diene at 237 nm was followed by the gradual decrease in intensity at this wavelength, with the appearance of a new chromophore characteristic of a conjugated triene(s) centered on ∼270 nm and a weaker broad absorbance in the area of 300–350 nm. The main product of the 20:3ω6 reaction absorbs relatively weakly, at 205 nm, and is not detected by UV scanning (see below). In side-by-side incubations monitored in the UV, it was apparent that the 20:3ω6-derived 8R-HPETrE disappeared more quickly than the corresponding arachidonic acid-derived 8R-HPETE. These side-by-side reactions were also conducted using 14C-labeled fatty acid substrate, and, after extraction of these samples using C18 cartridges, RP-HPLC analysis showed distinctly different profiles of products (Fig. 3A). The results confirmed the more extensive metabolism of the 20:3ω6-derived 8R-HPETrE (less remaining compared with 8R-HPETE) and, more significantly, the prominent appearance of a polar product unique to 20:3ω6 metabolism. This distinctive peak at ∼10 min is the most abundant secondary product from 20:3ω6, detected at 205 nm in the UV. In larger-scale incubations, this polar product from 20:3ω6 was prepared in sufficient amounts for structural analysis by 1H-NMR (see supplemental Tables I and II). On the basis of these data, the 8R-LOX product was shown to be identical to the coral epoxyalcohol 8R,9S-cis-epoxy-10S-hydroxy-eicosa-11Z,14Z-dienoic acid.
Fig.3.
RP-HPLC analysis of products formed from 20:3ω6 and 20:4ω6 by two purified 8R-lipoxygenases. A: Recombinant 8R-LOX (20) (10 μg in 1 ml) was reacted with [14C]20:3ω6 or [14C]20:4ω6 fatty acids (each 25 μg/ml and 300,000 CPM) in 50 mM Tris pH 8 containing 500 mM NaCl, 2 mM CaCl2, and 0.01% Emulphogene detergent for 10 min at room temperature. An aliquot of the extract was analyzed by RP-HPLC using a Waters Symmetry column (25 × 0.46 cm), a solvent of MeOH/H2O/HAc (80/20/0.01 by volume), at a flow rate of 1 ml/min, with on-line UV detection (Agilent 1100 series diode array detector) and radioactive monitoring (Radiomatic Flo-One). B: Reactions of soluble 8R-LOX (21) (∼50 μg/ml) with unlabeled 20:3ω6 and 20:4ω6 (50 μM) were conducted in 1 ml UV cuvettes in 50 mM Tris pH 8 containing 500 mM NaCl, 2 mM CaCl2, and 0.01% Emulphogene detergent at room temperature. The transformations were observed in the UV by repetitive scanning from 350 to 200 nm. When about half of the initially formed 8R-HPETrE was consumed (at 30 min), the reactions were stopped, extracted, and analyzed by RP-HPLC with UV detection as outlined above. The UV profiles at 205 nm are illustrated.
We also tested the soluble 76-kDa 8R-LOX from P. homomalla, which was available in limited quantities from the original purification (21). It reacted very similarly to the recombinant 8R-LOX from the AOS-LOX fusion protein. The substrates 20:4ω6 and 20:3ω6 were comparable for oxygenation to the corresponding 8R-hydroperoxide; however, 8R-HPETrE was converted to further products at over twice the rate of 8R-HPETE. When reactions with identical amounts of enzyme were analyzed and stopped at the same time (with half of the 20:3ω6 hydroperoxide consumed), subsequent RP-HPLC analysis confirmed the more extensive metabolism of 8R-HPETrE and the appearance of a single prominent, more polar peak detected at 205 nm, with no comparable prominent product from 8R-HPETE (Fig. 3B). This polar product from 20:3ω6 was identified as the same epoxyalcohol identified earlier by its identical UV profile and cochromatography on both RP-HPLC and SP-HPLC with the epoxyalcohol formed by the recombinant 8R-LOX.
Retention of hydroperoxy oxygens in the epoxyalcohol
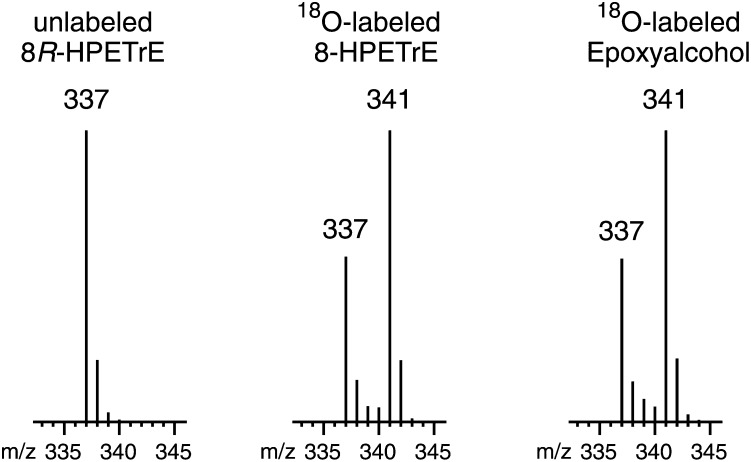
When 8R-HPETrE containing an ∼1:2 mixture of 216O and 218O in the hydroperoxide group was reacted with the recombinant 8R-LOX, the 18O contents of the substrate and epoxyalcohol product were almost indistinguishable (Fig. 4). Close inspection indicated 98% retention of both hydroperoxy oxygens in the epoxyalcohol, pointing to a mechanism involving close control of the transformation by the 8R-LOX enzyme.
Fig.4.
Mass spectrometric analysis of 8R-LOX-catalyzed transformation of [18O]8R-HPETrE to epoxyalcohol. Epoxyalcohol formed by recombinant 8R-LOX from 18O-labeled 8R-HPETrE (comprised of a ∼1:2 ratio of 216O to 218O). The ion abundances were measured by LC-MS (details in Methods) for the unlabeled species (left), the hydroperoxy substrate (middle), and epoxyalcohol product (side). A ∼2% increase in relative abundance in the channel representing 18O-16O ions (m/z 339) corresponds to a very minor loss of one of the oxygens of 18O-18O labeled substrate. Overall, the results show ∼98% retention of both hydroperoxy oxygens in the epoxyalcohol product.
DISCUSSION
Hydroperoxide isomerase activity
The typical dioxygenase activity of lipoxygenase enzymes involves activation of the resting ferrous enzyme to the ferric form, then cycling of the ferric enzyme as it catalyzes reaction with polyunsaturated fatty acid and O2 (27). By contrast, the epoxyalcohol biosynthesis we characterize here fits the criteria for a LOX enzyme acting as a hydroperoxide isomerase (28, 29). In this case, the reaction cycle is initiated by the ferrous enzyme. Several lines of evidence suggest that a lack of access of molecular oxygen within the active site promotes hydroperoxide isomerase activity (30). If present, molecular oxygen reacts readily with radical intermediates, thus intercepting and blocking hydroperoxide isomerase cycling. Furthermore, molecular oxygen promotes enzyme activation to the ferric form, also inhibiting isomerase activity (29, 31). Therefore, one can deduce that the 8R-HPETrE is an acceptable substrate for interaction with the ferrous iron and that O2 is excluded from intercepting the radical intermediates. With the arachidonic acid-derived 8R-hydroperoxide, the overall rate of reaction is comparatively sluggish, and very little epoxyalcohol product is formed. The main products are dihydroperoxides or leukotriene A-related diols, both of which are products of the ferric enzyme. This suggests that the selective reaction with the 20:3 8R-hydroperoxide is facilitated by exclusion of O2 within a critical part of the active site and that this does not occur with binding of the arachidonate analog.
Assignment of the 10S (erythro, anti) configuration
In postulating a mechanism for the hydroperoxide cycling with 8R-HPETrE, there is some difficulty in accounting for the erythro configuration of the epoxyalcohol product (discussed in the following subsection), and therefore it is imperative that the structural assignment is secure. For trans-epoxy epoxyalcohols, there are empirical rules that reliably allow assignment of the erythro or threo configuration. These rules relate to their relative polarity on TLC, relative retention time on GC, and both the relative chemical shifts and coupling constants on NMR (22, 32, 33). However, for cis-epoxy products there are fewer closely analogous examples in the literature (e.g., all fatty acid-related epoxyalcohols with data available are trans epoxides), and the differences for erythro and threo on NMR are small or nonexistent (34). Our assignment is founded on the well precedented threo product in Sharpless’ hydroxyl-directed epoxidation of Z-allylic alcohols with Ti(OiPr)4 (see supplementary Scheme I, epoxidation of 10R-3) (34–37). This allowed assignment of the two epoxide diastereomers (erythro and threo) obtained via Sharpless asymmetric epoxidation (see supplementary Scheme I). Indeed, the latter assignment shows good agreement with precedent using closely related model compounds (34, 35). For example, the asymmetric epoxidation (using L-(+)-diisopropyl tartrate) of 3-hydroxy-4Z-undecenol yields an unreactive 3R enantiomer with 2:3 ratio of erythro:threo products (35); our results concur exactly with this precedent and others (34, 36, 37).
Proposed catalytic cycle
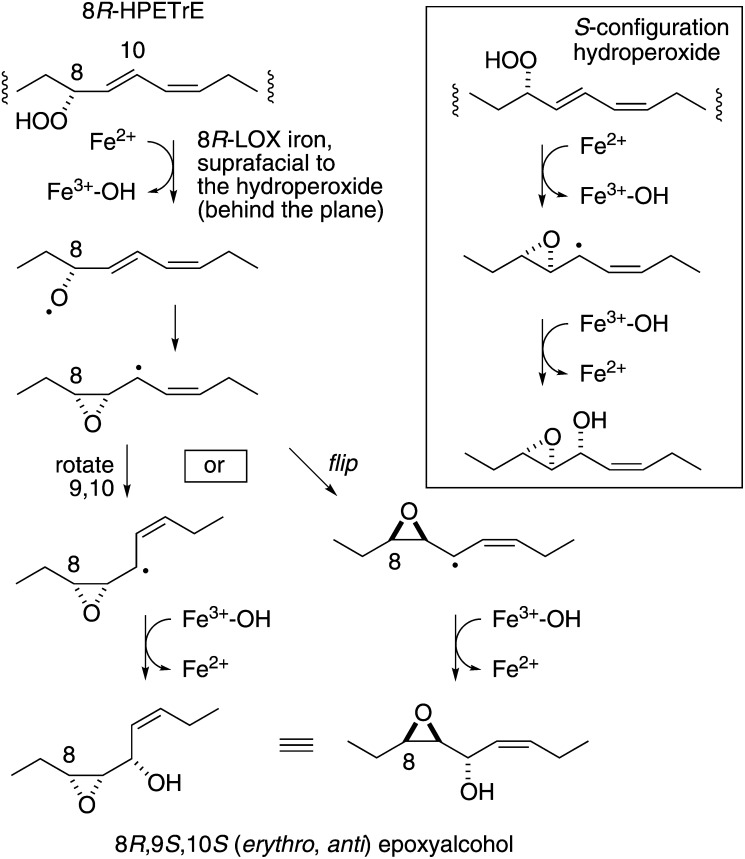
The reaction is catalyzed and controlled by the active site iron, which must first cleave the hydroperoxide and subsequently catalyze an oxygen rebound and hydroxylate the intermediate epoxyallylic radical while both hydroperoxy oxygens are retained in the epoxyalcohol product (Fig. 5). This is easy to conceptualize for the reactions of S-configuration fatty acid hydroperoxides because all steps occur on the same face of the reacting molecule, allowing formation of a trans epoxide and threo alcohol (Fig. 5, box). Our results with the R-configuration hydroperoxide indicate not only formation of a cis-epoxide, which itself presents no conceptual problem, but also the erythro configuration of the alcohol. Assuming the iron is in control, this necessitates either a 9,10 bond rotation before hydroxylation or flipping over of the reacting epoxyallylic radical intermediate (Fig. 5, right and left options). Perhaps the 8R-hydroperoxide sits partly turned away from square so that the epoxyallylic intermediate, when formed, further rotates to expose the opposite face of the intermediate for hydroxylation. We note too that the formation of cis-epoxides may be a characteristic of 8R-LOX because the activity in P. homomalla extracts was shown to convert 5S-HPETE to cis-epoxy LTA4, not to the well known trans-epoxy leukotriene A4 (38). Although the mechanisms of epoxyalcohol and LTA4 synthesis differ, the reactions being initiated by the ferrous and ferric enzymes, respectively, the substrate conformation that predisposes to cis-epoxide formation is dictated by binding in the active site and thus could be dictated in similar fashion by an enzyme that favors R versus S oxygenation.
Fig.5.
Mechanism of 8R-LOX-catalyzed epoxyalcohol synthesis from 8R-HPETrE. The scheme accounts for the stereochemistry of the epoxyalcohol (8,9-cis-epoxy, 9,10 erythro) and the complete retention of the hydroperoxy oxygens. Assuming that the active site iron cleaves the hydroperoxide and momentarily binds the distal hydroperoxy oxygen, the epoxyallylic radical intermediate must either rotate at the 9,10 bond (left) or flip over (right) to produce the epoxyalcohol product. In the box: reaction of S-configuration fatty acid hydroperoxide forms a trans-epoxy threo-hydroxy epoxyalcohol.
Other biosyntheses of cis-epoxyalcohols
Although heretofore only trans-epoxyalcohols have been reported from lipoxygenase catalysis (e.g., 23, 28, 39–41), other enzymes can make the cis-epoxides. The majority of these are mechanistically quite distinct, however, because the epoxide is formed via oxygen transfer. The epoxyalcohol synthase activities in the fish parasitic fungus Saprolegnia parasitica (42) and in potato leaves and beetroot (43, 44) catalyze oxygen transfer from the hydroperoxy fatty acid to the adjacent conjugated diene; the original hydroperoxide moiety is reduced to an alcohol, while the transferred oxygen produces trans- or cis-epoxidation of the trans and cis double bonds, respectively. In the case of plant peroxygenases, epoxidation may occur via intermolecular or intramolecular oxygen transfer from a fatty acid hydroperoxide to a cis double bond (45, 46). More similar to our reaction, but forming the threo product, is the conversion of 13S-hydroperoxylinoleic acid to the 11S-threo-hydroxy-12R,13S-cis-epoxide by a cytochrome P450 in the amphioxus Branchiostoma floridae (26). Notably, the oxygen rebound step in P450 catalysis is very fast (∼10−9 s), tending to favor suprafacial hydroxylation of the intermediate, forming the threo epoxyalcohol. By comparison, the equivalent intermediate in the hydroperoxide isomerase activity of lipoxygenases can diffuse out of the active site or be subject to interception by molecular oxygen, an event that promotes lipoxygenase activation to the ferric form (30). Accordingly, one might expect there is more time in the 8R-LOX reaction for the rotation required to form the observed erythro epoxyalcohol product (Fig. 5).
Wrap-up of a historical issue
The striking and unexpected difference between 20:4ω6 and 20:3ω6 metabolism in P. homomalla was detected in the original investigations of prostaglandin biosynthesis by Corey and Ensley, and the prominent extra product from 20:3ω6 was partially characterized (17). For example, it was shown to exhibit only weak end absorbance in the UV, to not react with sodium borohydride, to contain two double bonds and an alcohol and a possible epoxy functionality, and to have a molecular formula as the methyl ester of C21H36O4, all a perfect match for the epoxyalcohol we identify. Furthermore, the reported mass spectrum of the hydrogenated product as the methyl ester TMS derivative [listed in tabular form in the thesis (17)] contains all the major ions and similar ion abundances as reported in our Results section. There is little doubt that this product and our epoxyalcohol are the same compound. The existence of 8R-LOX metabolism in P. homomalla was not uncovered until the mid-1980s, a decade after these early biosynthetic studies (8), and it was only around the years 1995–2000 that the origin of the coral prostaglandins via cyclooxygenase was firmly established (6, 7, 47–49).
Supplementary Material
Acknowledgments
The authors thank Prof. Thomas M. Harris and Donald F. Stec, Ph.D., for help with the NMR analyses.
Footnotes
Abbreviations:
- H(P)ETE
- hydro(pero)xyeicosatetraenoic acid
- H(P)ETrE
- hydro(pero)xyeicosatrienoic acid
- LOX
- lipoxygenase
- RP-HPLC
- reverse phase high-pressure liquid chromatography
- SP-HPLC
- straight phase high-pressure liquid chromatography
This work was supported by National Institutes of Health grants GM-15431 and GM-74888 (ARB) and NSF grant CHE0615604 (JKC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of data and references, a scheme, two tables, and two figures.
REFERENCES
- 1.Weinheimer A. J., Spraggins R. L. 1969. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla. Tetrahedron Lett. 59: 5185–5188. [DOI] [PubMed] [Google Scholar]
- 2.Schneider W. P., Hamilton R. D., Rhuland L. E. 1972. Occurrence of esters of (15S)-prostaglandin A2 and E2 in coral. J. Am. Chem. Soc. 94: 2122–2123. [DOI] [PubMed] [Google Scholar]
- 3.Weinheimer A. J. 1973. Prostaglandins from Plexaura homomalla: Ecology, utilization and conservation of a major medical marine resource. Univ. of Miami Press, Coral Gables, FL. [Google Scholar]
- 4.Corey E. J., Ensley H. E., Hamberg M., Samuelsson B. 1975. Disparate pathways of prostaglandin biosynthesis in coral and mammalian systems. J. Chem. Soc. Chem. Commun. 277–278. [Google Scholar]
- 5.Brash A. R., Baertschi S. W., Ingram C. D., Harris T. M. 1987. On non-cyclooxygenase prostaglandin synthesis in the sea whip coral Plexaura homomalla: An 8(R)-lipoxygenase pathway leads to formation of an α-ketol and a racemic prostanoid. J. Biol. Chem. 262: 15829–15839. [PubMed] [Google Scholar]
- 6.Koljak R., Järving I., Kurg R., Boeglin W. E., Varvas K., Valmsen K., Ustav M., Brash A. R., Samel N. 2001. The basis of prostaglandin synthesis in coral. Molecular cloning and expression of a cyclooxygenase from the Arctic soft coral Gersemia fruticosa. J. Biol. Chem. 276: 7033–7040. [DOI] [PubMed] [Google Scholar]
- 7.Valmsen K., Järving I., Boeglin W. E., Varvas K., Koljak R., Pehk T., Brash A. R., Samel N. 2001. The origin of 15R-prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. USA. 98: 7700–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bundy G. L., Nidy E. G., Epps D. E., Mizsak S. A., Wnuk R. J. 1986. Discovery of an arachidonic acid C-8 lipoxygenase in the gorgonian coral Pseudoplexaura porosa. J. Biol. Chem. 261: 747–751. [PubMed] [Google Scholar]
- 9.Corey E. J., Matsuda S. P. T., Nagata R., Cleaver M. B. 1988. Biosynthesis of 8-R-HPETE and preclavulone A from arachidonate in several species of Caribbean coral. A widespread route to marine prostanoids. Tetrahedron Lett. 29: 2555–2558. [Google Scholar]
- 10.Schneider C., Brash A. R. 2002. Lipoxygenase-catalyzed formation of R-configuration hydroperoxides. Prostaglandins Other Lipid Mediat. 68–69: 291–301. [DOI] [PubMed] [Google Scholar]
- 11.Fischer J. 2009. Autosomal recessive congenital ichthyosis. J. Invest. Dermatol. 129: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y., Yin H., Boeglin W. E., Elias P. M., Crumrine D., Beier D. R., Brash A. R. 2011. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: A proposed role in releasing ω-hydroxyceramide for construction of the corneocyte lipid envelope. J. Biol. Chem. 286: 24046–24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey E. J., d'Alarcao M., Matsuda S. P. T., Lansbury P. T., Jr 1987. Intermediacy of 8-(R)-HPETE in the conversion of arachidonic acid to pre-clavulone A by Clavularia viridis. Implications for the biosynthesis of marine prostanoids. J. Am. Chem. Soc. 109: 289–290. [Google Scholar]
- 14.Kikuchi H., Tsukitani Y., Iguchi K., Yamada Y. 1982. Clavulones, new type of prostanoids from the stolonifer Clavularis viridis Quoy and Gaimard. Tetrahedron Lett. 23: 5171–5174. [Google Scholar]
- 15.Bundy G. L. 1985. Non-mammalian sources of eicosanoids. Adv. Prostaglandin Thromboxane Leukot. Res. 14: 229–262. [PubMed] [Google Scholar]
- 16.Corey E. J., Lansbury P. T., Jr, Yamada Y. 1985. Identification of a new eicosanoid from in vitro biosynthetic experiments with Clavularia viridis. Implications for the biosynthesis of clavulones. Tetrahedron Lett. 26: 4171–4174. [Google Scholar]
- 17.Ensley H. E. 1976. I. Asymmetric synthesis via the Diels-Alder reaction. II. The stereoselective conversion of prostaglandin A2 to prostaglandin E2. III. Studies on the biosynthesis of prostaglandin A2 by Plexaura homomalla. Harvard, Cambridge, MA. [Google Scholar]
- 18.Corey E. J., Washburn W. N., Chen J. C. 1973. Studies on the prostaglandin A2 synthetase complex from Plexaura homomalla. J. Am. Chem. Soc. 95: 2054–2055. [DOI] [PubMed] [Google Scholar]
- 19.Koljak R., Boutaud O., Shieh B-H., Samel N., Brash A. R. 1997. Identification of a naturally occurring peroxidase-lipoxygenase fusion protein. Science. 277: 1994–1996. [DOI] [PubMed] [Google Scholar]
- 20.Boutaud O., Brash A. R. 1999. Purification and catalytic activities of the two domains of the allene oxide synthase-lipoxygenase fusion protein of the coral Plexaura homomalla. J. Biol. Chem. 274: 33764–33770. [DOI] [PubMed] [Google Scholar]
- 21.Brash A. R., Boeglin W. E., Chang M. S., Shieh B-H. 1996. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J. Biol. Chem. 271: 20949–20957. [DOI] [PubMed] [Google Scholar]
- 22.Mercier J., Agoh B. 1974. Comportement d'hydroperoxydes allyliques a longue chaine en presence de complexes de certains metaux de transition. II. Structure des époxy-alcools formés à partir d'hydroperoxydes d'octadécène-9 oates de méthyle cis et trans en présence d'acétylacétonate de vanadyle. Chem. Phys. Lipids. 12: 239–248. [Google Scholar]
- 23.Pace-Asciak C. R., Reynaud D., Demin P. M. 1995. Hepoxilins: a review on their enzymatic formation, metabolism and chemical synthesis. Lipids. 30: 107–114. [DOI] [PubMed] [Google Scholar]
- 24.Song W-C., Baertschi S. W., Boeglin W. E., Harris T. M., Brash A. R. 1993. Formation of epoxyalcohols by a purified allene oxide synthase. Implications for the mechanism of allene oxide biosynthesis. J. Biol. Chem. 268: 6293–6298. [PubMed] [Google Scholar]
- 25.Gao B., Boeglin W. E., Zheng Y., Schneider C., Brash A. R. 2009. Evidence for an ionic intermediate in the transformation of fatty acid hydroperoxide by a catalase-related allene oxide synthase from the cyanobacterium Acaryochloris marina. J. Biol. Chem. 284: 22087–22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D. S., Nioche P., Hamberg M., Raman C. S. 2008. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature. 455: 363–368. [DOI] [PubMed] [Google Scholar]
- 27.Schilstra M. J., Veldink G. A., Vliegenthart J. F. G. 1994. The dioxygenation rate in lipoxygenase catalysis is determined by the amount of iron(Iii) lipoxygenase in solution. Biochemistry. 33: 3974–3979. [DOI] [PubMed] [Google Scholar]
- 28.Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. 2003. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. USA. 100: 9162–9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y., Brash A. R. 2010. Dioxygenase activity of epidermal lipoxygenase-3 unveiled: Typical and atypical features of its catalytic activity with natural and synthetic polyunsaturated fatty acids. J. Biol. Chem. 285: 39866–39875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y., Brash A. R. 2010. On the role of molecular oxygen in lipoxygenase activation: Comparison and contrast of epidermal lipoxygenase-3 with soybean lipoxygenase-1. J. Biol. Chem. 285: 39876–39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanov I., Saam J., Kuhn H., Holzhutter H. G. 2005. Dual role of oxygen during lipoxygenase reactions. FEBS J. 272: 2523–2535. [DOI] [PubMed] [Google Scholar]
- 32.Mihelich E. D. 1979. Vanadium-catalyzed epoxidations. 1. New selectivity pattern for acyclic allylic alcohols. Tetrahedron Lett. 49:4729–4732. [Google Scholar]
- 33.Bernart M. W., Gerwick W. H. 1994. Eicosanoids from the tropical red alga Murrayella periclados. Phytochemistry. 36: 1233–1240. [Google Scholar]
- 34.Adam W., Corma A., Reddy T. I., Renz M. 1997. Diastereoselective catalytic epoxidation of chiral allylic alcohols by the TS-1 and Ti-beta zeolites: Evidence for a hydrogen-bonded, peroxy-type loaded complex as oxidizing species. J. Org. Chem. 62: 3631–3637. [Google Scholar]
- 35.Martin V. S., Woodard S. S., Katsuki T., Yamada Y., Ikeda M., Sharpless K. B. 1981. Kinetic resolution of racemic allylic alcohols by enantioselective epoxidation - A route to substances of absolute enantiomeric purity. J. Am. Chem. Soc. 103: 6237–6240. [Google Scholar]
- 36.Rossiter B. E., Verhoeven T. R., Sharpless K. B. 1979. Stereoselective epoxidation of acyclic allylic alcohols. A correction of our previous work. Tetrahedron Lett. 49:4733–4736. [Google Scholar]
- 37.Cui M., Adam W., Shen J. H., Luo X. M., Tan X. J., Chen K. X., Ji R. Y., Jiang H. L. 2002. A density-functional study of the mechanism for the diastereoselective epoxidation of chiral allylic alcohols by the titanium peroxy complexes. J. Org. Chem. 67: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 38.Corey E. J., Wright S. W., Matsuda S. P. T. 1989. Stereochemistry and mechanism of the biosynthesis of Leukotriene A4 from 5(S)-hydroperoxy-6(E),8,11,14(Z)-eicosatetraenoic acid. Evidence for an organoiron intermediate. J. Am. Chem. Soc. 111: 1452–1455. [Google Scholar]
- 39.Garssen G. J., Veldink G. A., Vliegenthart J. F. G., Boldingh J. 1976. The formation of threo-11-hydroxy-trans-12: 13-epoxy-9-cis-octadecenoic acid by enzymic isomerisation of 13-L-hydroperoxy-9-cis, 11-trans-octadecadienoic acid by soybean lipoxygenase-1. Eur. J. Biochem. 62: 33–36. [DOI] [PubMed] [Google Scholar]
- 40.Bryant R. W., Schewe T., Rapoport S. M., Bailey J. M. 1985. Leukotriene formation by a purified reticulocyte lipoxygenase enzyme. Conversion of arachidonic acid and 15-hydroperoxyeicosatetraenoic acid to 14,15-leukotriene A4. J. Biol. Chem. 260: 3548–3555. [PubMed] [Google Scholar]
- 41.Nigam S., Patabhiraman S., Ciccoli R., Ishdorj G., Schwarz K., Petrucev B., Kuhn H., Haeggstrom J. Z. 2004. The rat leukocyte-type 12-lipoxygenase exhibits an intrinsic hepoxilin A3 synthase activity. J. Biol. Chem. 279: 29023–29030. [DOI] [PubMed] [Google Scholar]
- 42.Hamberg M., Herman R. P., Jacobsson U. 1986. Stereochemistry of two epoxy alcohols from Saprolegnia parasitica. Biochim. Biophys. Acta. 879: 410–418. [Google Scholar]
- 43.Hamberg M. 1999. An epoxy alcohol synthase pathway in higher plants: biosynthesis of antifungal trihydroxy oxylipins in leaves of potato. Lipids. 34: 1131–1142. [DOI] [PubMed] [Google Scholar]
- 44.Hamberg M., Olsson U. 2011. Efficient and specific conversion of 9-lipoxygenase hydroperoxides in the beetroot. Formation of pinellic acid. Lipids. 46: 873–878. [DOI] [PubMed] [Google Scholar]
- 45.Blée E., Wilcox A. L., Marnett L. J., Schuber F. 1993. Mechanism of reaction of fatty acid hydroperoxides with soybean peroxygenase. J. Biol. Chem. 268: 1708–1715. [PubMed] [Google Scholar]
- 46.Hamberg M., Hamberg G. 1996. Peroxygenase-catalyzed fatty acid epoxidation in cereal seeds (sequential oxidation of linoleic acid into 9(S),12(S),13(S)-trihydroxy-10(E)-octadecenoic acid). Plant Physiol. 110: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varvas K., Koljak R., Järving I., Pehk T., Samel N. 1994. Endoperoxide pathway in prostaglandin biosynthesis in the soft coral Gersemia fruticosa. Tetrahedron Lett. 35: 8267–8270. [Google Scholar]
- 48.Varvas K., Järving I., Koljak R., Valmsen K., Brash A. R., Samel N. 1999. Evidence of a cyclooxygenase-related prostaglandin synthesis in coral. The allene oxide pathway is not involved in prostaglandin synthesis. J. Biol. Chem. 274: 9923–9929. [DOI] [PubMed] [Google Scholar]
- 49.Valmsen K., Boeglin W. E., Järving J., Schneider C., Varvas K., Brash A. R., Samel N. 2004. Structural and functional comparison of 15S- and 15R-specific cyclooxygenases from the coral Plexaura homomalla. Eur. J. Biochem. 271: 3533–3538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.