Abstract
Insulin resistance in adipose tissue increases the release of free fatty acids into the circulation, which likely contributes to impaired insulin action in liver and skeletal muscle associated with obesity. However, reliable assessment of adipose tissue insulin resistance requires performing a hyperinsulinemic-euglycemic clamp procedure in conjunction with a fatty acid tracer infusion to determine insulin-mediated suppression of lipolytic rate. We developed a simpler method for evaluating adipose tissue insulin resistance in vivo, determined as the product of palmitate rate of appearance into the bloodstream and plasma insulin concentration during basal conditions. We validated our Adipose Tissue Insulin Resistance Index (ATIRI) by comparison with an assessment of adipose tissue insulin resistance determined by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with a palmitate tracer infusion in 47 obese nondiabetic subjects (body mass index: 40.1 ± 9.3 kg/m2). We found the ATIRI correlated closely with adipose tissue insulin resistance assessed during the clamp procedure (r =−0.854, P < 0.001). These results demonstrate that the ATIRI provides a reliable index of adipose tissue insulin resistance in obese subjects.
Keywords: fatty acid kinetics, palmitate rate of appearance, adipose tissue insulin sensitivity, suppression of lipolysis, hyperinsulinemic-euglycemic clamp, stable isotope tracers, gas chromatography-mass spectrometry
Insulin resistance of adipose tissue lipolysis causes increased release of fatty acids into the bloodstream and excessive delivery of free fatty acids (FFAs) to the liver and skeletal muscle, which leads to impaired insulin action in these organs (1). Therefore, the assessment of adipose tissue insulin resistance is important because of its key role in the pathogenesis of insulin resistance. Reliable assessment of adipose tissue insulin resistance requires performing a hyperinsulinemic-euglycemic clamp procedure in conjunction with a fatty acid tracer infusion to determine insulin-mediated suppression of adipose tissue lipolysis (2). However, clamp procedures are most commonly performed to assess skeletal muscle insulin sensitivity, which does not permit a reliable assessment of adipose tissue insulin action, because the rate of insulin infusion needed to achieve submaximal suppression of adipose tissue lipolysis is much lower than that needed to adequately stimulate skeletal muscle glucose uptake (2). Therefore, a multi-stage insulin infusion has been used to assess multi-organ insulin sensitivity (3), which can be a burden to both study participants and the investigator because it involves a prolonged (usually >9 h) procedure.
Here, we report a novel method for assessing adipose tissue insulin resistance that does not require insulin infusion during a clamp procedure. Accordingly, we tested and validated a simple tracer infusion method to determine the Adipose Tissue Insulin Resistance Index (ATIRI), based on the principle of an index used to assess insulin sensitivity in the liver that is derived from basal glucose kinetics and plasma insulin concentration (4).
METHODS
Study participants
A total of 47 obese subjects [body mass index (BMI): 40.1 ± 9.3 kg/m2; age: 42 ± 10 yrs; 10 men and 37 women] who underwent a hyperinsulinemic-euglycemic clamp procedure with low-dose insulin infusion participated in this study. Forty-three of the 47 subjects had clamp procedures performed for other studies (3, 5, 6). All subjects completed a comprehensive medical evaluation, including a 2 h oral glucose tolerance test (OGTT). Among the 47 subjects, 20 had normal glucose tolerance, 14 had impaired fasting glucose, and 13 had impaired glucose tolerance. No subjects had diabetes mellitus or any history or evidence of other serious chronic diseases or were taking any medications known to influence glucose or fatty acid metabolism. Subjects gave their written informed consent before participating in this study, which was approved by the Human Research Protection Office of Washington University School of Medicine.
Experimental protocol
Body composition and adipose tissue insulin sensitivity measurement.
Percent body fat mass was determined by using dual energy X-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA) (3). Adipose tissue insulin sensitivity was measured by using the hyperinsulinemic-euglycemic clamp procedure in conjunction with a stable isotopically labeled palmitate tracer infusion, and hepatic glucose production was measured by infusing a glucose tracer during the basal stage of the clamp (7). Subjects were admitted to the Clinical Research Unit at Washington University School of Medicine in the afternoon on the day before the clamp procedure. The following morning, after subjects fasted overnight, a catheter was inserted into a forearm vein to infuse stable isotopically labeled palmitate and glucose (purchased from Cambridge Isotope Laboratories, Andover, MA), dextrose, and insulin. A second catheter was inserted into the contralateral radial artery to obtain blood samples; if radial artery cannulation was not successful, a catheter was inserted into a hand vein, which was heated to 55°C by using a thermostatically controlled box to obtain arterialized blood samples (8). At 0600 h, a primed, continuous infusion of [6,6-2H2]glucose (priming dose 22.5 μmol/kg body weight; infusion rate 0.25 µmol/kg body weight/min) was started and continued for 3.5 h (until the beginning of the insulin infusion). At 0800 h, a continuous infusion of [2,2-2H2]palmitate (infusion rate 0.035 μmol/kg body weight/min) bound to 25% albumin was started and continued for 4.5 h. At 0930 h, 1.5 h after starting the palmitate tracer infusion, a one-stage hyperinsulinemic-euglycemic clamp procedure was started and continued for 3 h. Insulin was infused at low rates (7–20 mU/m2/min) to submaximally suppress lipolysis of adipose tissue triglycerides. Euglycemia was maintained at a blood concentration of ∼5.6 mmol/l (100 mg/dl) by infusing 20% dextrose at variable rates.
Blood samples were obtained before the start of the tracer infusion to determine background tracer-to-tracee ratio of plasma glucose and palmitate and every 10 min during the final 30 min of the basal period and the insulin infusion to determine plasma glucose, FFA, and insulin concentrations and palmitate kinetics. Samples were placed on ice and plasma was separated by centrifugation within 30 min of collection. Plasma samples were stored at −80°C until final analyses were performed.
Analyses of samples.
Plasma glucose concentration was measured by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH). Plasma insulin concentration was measured by using a chemiluminescent immunoassay method (Immulite 1000, Diagnostic Products Corporation, Los Angeles, CA). Plasma FFA concentrations were determined by using gas chromatography (5890-II; Hewlett-Packard, Palo Alto, CA) (9). Plasma glucose and palmitate tracer-to-tracee ratio were determined by using electron impact ionization gas chromatography-mass spectrometry (MSD 5973 system with capillary column; Hewlett-Packard) (10).
Calculations.
Isotopic steady-state conditions were achieved for plasma palmitate and glucose during the final 30 min of the basal period and for plasma palmitate during the final 30 min of the insulin infusion, so Steele's equation for steady-state conditions was used to calculate palmitate and glucose kinetics (11, 12). The ATIRI was calculated as the product of palmitate rate of appearance (Ra) (in µmol/min to provide an index of whole body lipolytic rate) (11, 12) and plasma insulin concentration (in mU/L) obtained during the basal period. Adipose tissue insulin resistance was also directly assessed as the relative decrease (from basal conditions) in palmitate Ra during insulin infusion, adjusted for the relative change in plasma insulin concentration (i.e., percent decrease in palmitate Ra divided by the percent increase in plasma insulin). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting plasma insulin (in mU/l) and glucose (in mg/dl) concentrations divided by 22.5 (13).
Statistical analyses
All data sets were tested for normality according to the Shapiro-Wilk test, and nonnormally distributed variables (ATIRI, palmitate Ra percent suppression) were ranked for analyses. Pearson correlation analysis was used to assess the relationships between the ATIRI and insulin-mediated suppression of palmitate Ra during insulin infusion. Multiple stepwise linear regression analysis (with age, sex, BMI, percent body fat, insulin concentration during the clamp, and ATIRI as independent variables) was performed to identify significant independent predictors of adipose tissue insulin resistance measured during the clamp procedure (suppression of palmitate Ra). Results are presented as means ± SD for normally distributed variables and medians and quartiles for nonnormally distributed variables. A P-value ≤ 0.05 was considered statistically significant. Analyses were performed by using SPSS 17.0 (SPSS Inc., Chicago, IL).
RESULTS
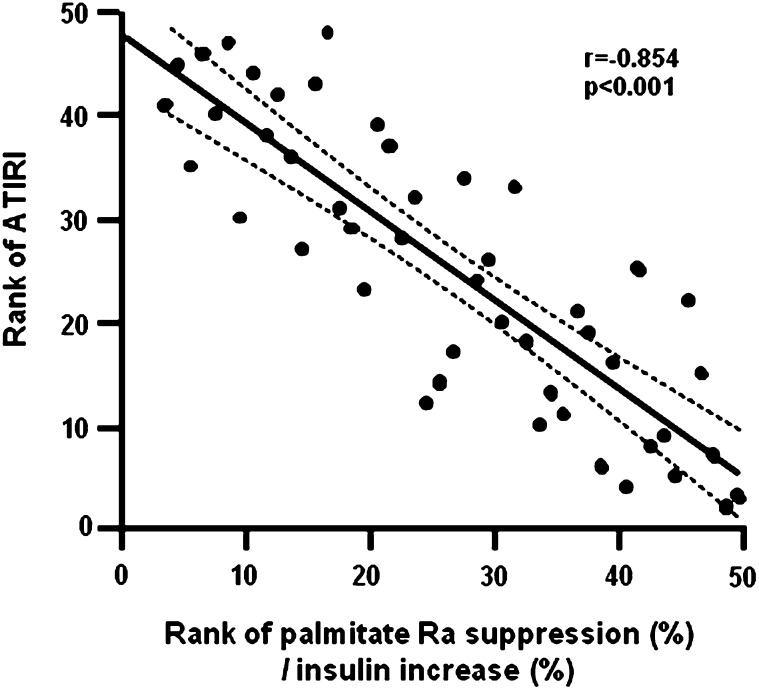
The metabolic characteristics of the study subjects are shown in Table 1. Although all subjects were obese, they had a wide range in BMI and percent body fat. There was also a large range in fasting plasma glucose, insulin, and lipid concentrations. Plasma insulin concentration during the clamp procedure (when adipose tissue insulin sensitivity was evaluated) ranged from 32 to 52 mU/L (mean value: 43.0 ± 6.3 mU/L). Insulin infusion resulted in a 7-fold range in percent suppression of palmitate Ra and there was more than a 10-fold range in ATIRI values (Table 2), which enhanced our ability to evaluate the relationship between the ATIRI and adipose tissue insulin resistance derived from the clamp procedure. There was a negative correlation between the ATIRI and the percent suppression of palmitate Ra during insulin infusion adjusted for the percent change in insulin concentration during the clamp (r = −0.854, P < 0.001) (Fig. 1). A similar strong negative correlation between percent palmitate suppression and ATIRI was also observed in men and women analyzed separately (men: r = −0.940, P < 0.001, women: r = −0.828, P < 0.001). To evaluate the validity of the ATIRI across different levels of insulin sensitivity, we divided the subjects into tertiles based on hepatic glucose production. The correlation between the ATIRI and percent suppression of palmitate Ra was very strong in each group (1st tertile: r = −0.868; 2nd tertile: r = −0.782; 3rd tertile: r = −0.841, p < 0.001 for all). Multivariate linear regression analyses, which included age, sex, BMI, percent body fat, insulin concentration during the clamp, and ATIRI as independent variables, indicated that the ATIRI was the best predictor of palmitate Ra suppression, accounting for 73% of the total variance; insulin concentration during the clamp was the only other significant independent predictor, accounting for an additional 7% of the total variance.
TABLE 1.
Characteristics of the study subjects
| Minimum | Maximum | Mean ± SD | |
| Weight (kg) | 72 | 218 | 113 ± 28 |
| BMI (kg/m ) | 30 | 70 | 40.1 ± 9.3 |
| Body fat (%) | 28 | 52 | 43 ± 7 |
| Glucose (mg/dl) | 83 | 117 | 97 ± 9 |
| Insulin (mU/l) | 7.1 | 36.6 | 17 ± 8 |
| HOMA-IR score | 1.6 | 10.3 | 4.1 ± 2.1 |
| FFA (µmol/l) | 278 | 816 | 532 ± 130 |
| Total cholesterol (mg/dl) | 63 | 245 | 173 ± 35 |
| LDL-cholesterol (mg/dl) | 50 | 151 | 99 ± 25 |
| HDL-cholesterol (mg/dl) | 28 | 103 | 46 ± 14 |
| Triglyceride (mg/dl) | 45 | 395 | 148 ± 72 |
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance.
TABLE 2.
Adipose tissue fatty acid kinetics and insulin resistance
| Minimum | Maximum | Mean ± SD | |
| Fatty acid kinetics during basal conditions: | |||
| Palmitate Ra (µmol/min) | 56 | 246 | 142 ± 42 |
| Palmitate Ra (µmol/kgBW/min) | 0.7 | 2.0 | 1.3 ± 0.3 |
| Fatty acid kinetics during insulin infusion: | |||
| Palmitate Ra (µmol/min) | 17 | 155 | 55 ± 30 |
| Palmitate Ra (µmol/kgBW/min) | 0.2 | 1.1 | 0.5 ± 0.2 |
| Palmitate Ra suppression (%) | 37 | 85 | 64 (54,71)a |
| Palmitate Ra suppression (% decrease in palmitate Ra/% increase in plasma insulin) | 0.2 | 1.5 | 0.3 (0.2, 0.6)a |
| Adipose Tissue Insulin Resistance Index (ATIRI) | 595 | 6816 | 2123 (1138, 3652)a |
Ra, rate of appearance; BW, body weight. ATIRI is calculated as palmitate Ra × insulin concentration [µmol/ min × mU/l]).
Median and quartiles.
Fig.1.
Relationship between the Adipose Tissue Insulin Resistance Index (ATIRI) and adipose tissue insulin sensitivity, evaluated as the percent suppression of palmitate rate of appearance (Ra) in plasma during insulin infusion adjusted for changes in insulin concentration. The lines represent the linear correlation and 95th percentile confidence interval. Data were ranked for analysis.
DISCUSSION
Impaired insulin-mediated suppression of adipose tissue triglyceride lipolysis and excessive release of FFA into plasma are likely involved in the pathogenesis of hepatic and skeletal muscle insulin resistance in obese people (1, 14, 15). Therefore, assessment of adipose tissue insulin resistance is important for understanding the metabolic complications associated with obesity. However, reliable evaluation of adipose tissue insulin sensitivity is difficult, because it requires performing a hyperinsulinemic-euglycemic clamp procedure. In the present study, we evaluated the use of a simplified method to assess adipose tissue insulin action, obtained by measuring basal FFA kinetics and plasma insulin concentration. Our data demonstrate that our approach, which we have called the “Adipose Tissue Insulin Resistance Index”, correlates closely with insulin-mediated suppression of adipose tissue lipolytic activity, and, therefore, provides a reliable index of adipose tissue insulin resistance in obese nondiabetic subjects.
The ATIRI used in our study is based on the reciprocal of the Hepatic Insulin Sensitivity Index, proposed by Matsuda and DeFronzo (4), to assess hepatic insulin sensitivity. The Hepatic Insulin Sensitivity Index is derived from the reciprocal of the product of basal endogenous glucose production rate and plasma insulin concentration. Accordingly, we hypothesized that a similar principle could be used to assess adipose tissue insulin resistance by measuring the product of basal palmitate Ra and plasma insulin concentration, because both basal hepatic glucose production rate and adipose tissue lipolytic rate are primarily regulated by circulating insulin (2, 16). We enrolled obese subjects who had a large range in adipose tissue insulin sensitivity to enhance our ability to evaluate the reliability of this approach. The values for the ATIRI were compared with those obtained by using a low-dose insulin infusion hyperinsulinemic-euglycemic clamp procedure in the same subjects. Our data show that the ATIRI is negatively correlated with insulin-mediated suppression of lipolytic rate in obese subjects. Moreover, multiple stepwise linear regression analysis (with age, sex, BMI, percent body fat, insulin concentration during the clamp, and ATIRI as independent variables) found the ATIRI was the best predictor of insulin-mediated suppression of lipolysis, accounting for 73% of the variability in palmitate Ra suppression.
In conclusion, the ATIRI provides a reliable index of adipose tissue insulin resistance in obese subjects and correlates with results obtained from a low-dose insulin infusion hyperinsulinemic-euglycemic clamp procedure. However, the ATIRI is not a direct measure of adipose tissue insulin resistance, and should not be used to replace the low-dose insulin infusion hyperinsulinemic-euglycemic clamp procedure when a precise measure of insulin action in adipose tissue is needed. In addition, our study was conducted in nondiabetic obese adults, so our findings cannot be extrapolated to other patient populations.
Acknowledgments
The authors thank Freida Custodio and Jennifer Shew for their technical assistance, the staff of the Clinical Research Unit of Washington University School of Medicine in St. Louis for their help in performing the studies, and the study subjects for their participation.
Footnotes
Abbreviations:
- ATIRI
- Adipose Tissue Insulin Resistance Index
- BMI
- body mass index
- HOMA-IR
- homeostasis model assessment of insulin resistance
- Ra
- rate of appearance
This study was supported by the National Institutes of Health grants DK 37948, HD 57796, UL1 RR024992 (Clinical and Translational Science Award), DK 56341 (Nutrition and Obesity Research Center), and RR-00954 (Biomedical Mass Spectrometry Resource). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. No conflicts of interest exist.
REFERENCES
- 1.Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. 1983. Effect of fatty acids on glucose production and utilization in man. J. Clin. Invest. 72: 1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen M. D., Nielsen S. 2007. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 56: 68–76. [DOI] [PubMed] [Google Scholar]
- 3.Korenblat K. M., Fabbrini E., Mohammed B. S., Klein S. 2008. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 134: 1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda M., DeFronzo R. A. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 5.Gregor M. F., Yang L., Fabbrini E., Mohammed B. S., Eagon J. C., Hotamisligil G. S., Klein S. 2009. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 58: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kars M., Yang L., Gregor M. F., Mohammed B. S., Pietka T. A., Finck B. N., Patterson B. W., Horton J. D., Mittendorfer B., Hotamisligil G. S., et al. 2010. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 59: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabbrini E., Magkos F., Mohammed B. S., Pietka T., Abumrad N. A., Patterson B. W., Okunade A., Klein S. 2009. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA. 106: 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen M. D., Heiling V. J. 1991. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 40: 406–409. [DOI] [PubMed] [Google Scholar]
- 9.Patterson B. W., Zhao G., Elias N., Hachey D. L., Klein S. 1999. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J. Lipid Res. 40: 2118–2124. [PubMed] [Google Scholar]
- 10.Patterson B. W., Zhao G., Klein S. 1998. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 47: 706–712. [DOI] [PubMed] [Google Scholar]
- 11.Steele R., Wall J. S., De Bodo R. C., Altszuler N. 1956. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am. J. Physiol. 187: 15–24. [DOI] [PubMed] [Google Scholar]
- 12.Steele R. 1959. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. N. Y. Acad. Sci. 82: 420–430. [DOI] [PubMed] [Google Scholar]
- 13.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 14.Kelley D. E., Mandarino L. J. 2000. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 49: 677–683. [DOI] [PubMed] [Google Scholar]
- 15.Boden G. 1997. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 46: 3–10. [PubMed] [Google Scholar]
- 16.Prager R., Wallace P., Olefsky J. M. 1986. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J. Clin. Invest. 78: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]