Abstract
Patients and models of cystic fibrosis (CF) exhibit consistent abnormalities of polyunsaturated fatty acid composition, including decreased linoleate (LA) and docosahexaenoate (DHA) and variably increased arachidonate (AA), related in part to increased expression and activity of fatty acid desaturases. These abnormalities and the consequent CF-related pathologic manifestations can be reversed in CF mouse models by dietary supplementation with DHA. However, the mechanism is unknown. This study investigates this mechanism by measuring the effect of exogenous DHA and eicosapentaenoate (EPA) supplementation on fatty acid composition and metabolism, as well as on metabolic enzyme expression, in a cell culture model of CF. We found that both DHA and EPA suppress the expression and activity of Δ5- and Δ6-desaturases, leading to decreased flux through the n-3 and n-6 PUFA metabolic pathways and decreased production of AA. The findings also uncover other metabolic abnormalities, including increased fatty acid uptake and markedly increased retroconversion of DHA to EPA, in CF cells. These results indicate that the fatty acid abnormalities of CF are related to intrinsic alterations of PUFA metabolism and that they may be reversed by supplementation with DHA and EPA.
Keywords: fatty acid metabolism, gene expression, omega-3 fatty acids, docosapentaenoate, eicosapentaenoate
Cystic fibrosis (CF) is the most commonly inherited genetic disorder among Caucasians. It is caused by mutations in the gene for the cystic fibrosis transmembrane regulator (CFTR) protein (1) that lead to a myriad of phenotypic effects in the gastrointestinal, pulmonary, endocrine, and reproductive systems (2). This causes significant morbidity and mortality, reducing the average lifespan of CF patients in the United States to 37 years (2). Among the phenotypic manifestations of CF patients are abnormalities in blood and tissue polyunsaturated fatty acid (PUFA) levels that are independent of absorption and nutritional status (reviewed in Refs. 3–5). The most consistent of these abnormalities are decreased linoleate (LA; 18:2n-6) and docosahexaenate (DHA; 22:6n-3). In addition, some studies have shown increased arachidonate (AA; 20:4n-6), palmitoleate (16:1n-7), oleate (18:1n-9), and Mead acid (20:3n-9). Similar findings are seen in animal (6–9) and cell culture (10, 11) models of CF. The magnitude of fatty acid alterations in CF patients correlates with disease severity, suggesting a role for fatty acid metabolism in CF pathophysiology (12–17).
There is increasing evidence that these abnormalities are due to differences in fatty acid metabolism in CF. PUFAs of the n-3 and n-6 series are metabolized in a stepwise fashion along parallel pathways (reviewed in Ref. 18). A common set of desaturase and elongase enzymes catalyzes the conversion of LA through multiple steps to AA and ultimately to docosapentaenoate (DPA; 22:5n-6). The same enzymes convert linolenate (LNA; 18:3n-3) to eicosapentaenoate (EPA; 20:5n-3) and subsequently to DHA. Multiple studies have demonstrated increased conversion of AA to eicosanoids (19–22), stimulating increased metabolism of LA to maintain AA (7, 10, 11) and accounting for the decreased LA and increased AA levels seen in CF.
Recent studies in our laboratory have uncovered a potential mechanism accounting for these metabolic changes (23). In cell culture models of CF, decreased LA and increased AA and EPA levels in CF cells correlated with increased expression and activity of the Δ5- and Δ6-desaturase enzymes that catalyze conversion of LA to AA and LNA to EPA. Similar increases in Δ9-desaturase and elongase-6 enzymes are associated with increases in palmitoleate, oleate, and Mead acid (24). CF cells have lower levels of DHA and DPA with decreased metabolism from their precursors, EPA and AA, respectively. We originally hypothesized that this was due to metabolic shunting of EPA and AA away from DHA and DPA toward eicosanoid production.
An important advance in understanding the role of these metabolic abnormalities in the pathogenesis of CF has come from mouse models. Studies in two different mouse models of CF (6, 8) demonstrated normalization of LA and AA levels in lung, intestine, and pancreas after dietary supplementation with DHA. Furthermore, this treatment corrected the phenotypic manifestations of CF, decreasing ileal villus height, pancreatic duct diameter, and pulmonary inflammation stimulated by lipopolysaccharide (6, 25). Similarly, CF patients treated with DHA exhibited significant decreases in plasma AA levels (26–28), accompanied in some cases by increased LA (29, 30). Despite moving the fatty acid alterations in CF toward normal, the clinical outcomes in these small studies have been inconsistent. DHA supplementation of CF patients, often for short periods of time at low doses, reduced markers of inflammation but did not convincingly improve symptoms in most studies (reviewed in Refs. 3–5).
The mechanism by which DHA corrects these n-6 fatty acid abnormalities in CF is unknown. As detailed above, recent studies show that these abnormalities are associated with increased expression and activity of Δ5- and Δ6-desaturase (23), but they do not establish a causal relationship. This requires demonstrating that the fatty acid abnormalities can be corrected by reversing the observed changes in enzyme activity. As these enzymes are regulated primarily at the transcriptional level (31) and as DHA plays a significant role in this regulation in metabolic tissues, such as liver (32), we hypothesized that suppression of AA production by DHA in CF is due to downregulation of these enzymes. The current study tests this hypothesis by examining the effects of DHA and the related n-3 fatty acid EPA on the composition and metabolism of PUFAs in CF cells and on the expression and activity of fatty acid desaturases. The results establish a mechanistic link between enzyme expression and fatty acid composition alterations in CF.
MATERIALS AND METHODS
Materials
Radiolabeled fatty acids, including [1-14C]LNA (55 mCi/mmol), [1-14C]LA (55 mCi/mmol), [1-14C]EPA (55 mCi/mmol), and [1-14C]AA (55 mCi/mmol), were purchased from American Radiolabeled Chemicals. Free fatty acids and fatty acid methyl ester (FAME) standards were purchased from NuChek Prep (Elysian, MN). All HPLC grade solvents were purchased from Fisher Scientific (Pittsburgh, PA), and IN-flow 2:1 liquid scintillation cocktail was purchased from IN/US Systems (Tampa, FL).
Cell culture
Sense (WT) and antisense (CF) human bronchial epithelial cells (16HBE cells) were a kind gift of Dr. Pamela Davis (Case Western University, Cleveland, OH). The cells were grown in culture flasks precoated with LHC Basal media (Invitrogen, Carlsbad, CA) containing a mixture of 3 μg/ml vitrogen (Angiotech Biomaterials, Palo Alto, CA), 10 μg/ml human fibronectin (Sigma-Aldrich, St. Louis, MO), and 0.1 mg/ml BSA (BSA; Sigma-Aldrich). The cells were maintained at 37°C in 5% CO2 in MEM + glutamax (Invitrogen), supplemented with 10% horse serum (Omega Scientific, Tarzana, CA), penicillin (100 U/ml), and streptomycin (100 μg/ml). Horse serum was used because of its higher concentration of LA, which allows fatty acid changes in CF cells to be manifested (11). The medium was changed three times per week.
Fatty acid supplementation
For each experiment, WT and CF cells were seeded onto 6-well plates at 3 × 105 and 1 × 105 cells/well respectively. The cells were allowed to grow until 1 day postconfluence (typically 6 days), the point at which fatty acid changes are maximized (11), after which they were supplemented with 0, 5, 10, or 20 μM fatty acid for 24 h without prior serum deprivation. The supplementation medium was made by using free fatty acid dissolved in chloroform-methanol (2:1). The fatty acids were dried under nitrogen, and medium containing 10% reduced-lipid FBS or horse serum was added to the tube and sonicated three times for 5 s each, allowing binding by serum albumin.
Fatty acid composition analysis
After 24 h incubation with or without fatty acid supplementation, cells were washed twice in ice-cold PBS, scraped, and transferred to a glass tube, and 10 μg heptadecanoate was added as an internal standard. Lipids extraction was performed using a modification of the method of Folch et al. (33). Specifically, the cells were pelleted by centrifugation (100 g, 8 minutes) and dissolved in 6 vol of chloroform-methanol (2:1 v/v). They were then incubated on ice for 10 min, vortexed, and centrifuged (1,100 g, 10 minutes). The lower organic phase was transferred to a new glass tube and dried completely under nitrogen. Fatty acids were methylated using boron trifluoride (BF3; 14% in methanol; Sigma Aldrich) and a methanolic-base reagent (34) by adding 0.5 ml 0.5 N methanolic NaOH (Acros Organics, Geel, Belgium) to the sample, vortexing, and heating at 100°C for 3 min, followed by addition of 0.5 ml BF3 at 100°C for 1 min. FAMEs were extracted by sequential addition of 1 ml hexane and 6.5 ml saturated NaCl solution. The sample was then vortexed and centrifuged (500 g, 4 minutes). The upper hexane layer was used for quantification of FAMEs by gas chromatography (GC) using an Agilent 7980A GC system (Agilent Technologies, Santa Clara, CA) equipped with a Supelcowax SP-10 capillary column (Supelco, Bellefonte, PA) coupled to a mass spectrometer (model 5975C, Agilent Technologies). FAME mass was determined by comparing areas of unknown FAMEs to that of the internal standard. Results were expressed as the molar percentage (mol%) of each FAME relative to the total FAME mass of the sample, as previously described (10).
Fatty acid labeling experiments
After a 24 h incubation with or without fatty acid supplementation, the cells were incubated with medium containing 4.1 μM [1-14C]LA, LNA, AA, or EPA for 4 h and then harvested. Lipids were extracted and methylated as above, except that the final hexane layer was dried under nitrogen and reconstituted in 50 μl acetonitrile for HPLC analysis. Samples were separated by reverse-phase HPLC using a 4.6 × 250 mm, 5 μm Agilent Zorbax Eclipse XDB-C18 column on an Agilent 1200 series instrument. A guard column (4.6 × 12.5 mm, 5 μm) was paired with the analytical column. For separation, a gradient system consisting of solvent A (HPLC grade H20 + 0.02% H2PO4) and solvent B (100% HPLC grade acetonitrile) was used, with a flow rate of 1 ml/min. For n-3 fatty acids, the following program was used: 76% B for 0.5 min, 76–86% B for 10 min, hold for 20 min, 86–100% B for 2 min, hold for 18 min, and then reconstitution of the starting conditions. For n-6 fatty acids, the program was 58% B for 25 min, 58–61% B for 2 min, hold for 8 min, 61–100% B for 15 min, hold for 20 min, and then reconstitution of the original conditions. Peaks were identified by UV detection at 205 nm, and their identity was determined by comparing peak retention time with that of unlabeled FAME standards. Radiolabeled peaks were quantified using a β-RAM scintillation detector (counting efficiency >90% for 14C with 5 CPM background) coupled to the HPLC instrument. Results are expressed as percentage of total measured counts in the sample.
Quantitative real-time PCR
Total RNA was isolated from sense and antisense cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples were treated with DNase I (DNA-free kit; Ambion, Inc., Austin, TX), and first strand cDNA was synthesized from 2 μg total RNA with random hexamer primers, using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). The qPCR reaction was carried out in a total volume of 20 μl, using 10 μl 2× Power SYBR Green PCR Master Mix (Applied Biosystems), 50 ng of reverse-transcribed total RNA, and 156 nM forward and reverse primers. Primer design and sequences were as previously described (23). All reactions were performed in triplicate in 96-well plates on a CFX96 system (Bio-Rad), and the data were analyzed using CFX Manager software (Bio-Rad). The relative amount of mRNA was calculated using the comparative CT method, with RPLP0 mRNA serving as an invariant control.
Statistical analysis
Quantitative data for multiple groups (WT and CF cells, with or without fatty acid supplementation) were compared using two-way ANOVA with Bonferroni posttest for pair-wise comparisons using Prism 5 (GraphPad Software, La Jolla, CA).
RESULTS
Experiments were performed in a cell culture model of CF, 16HBE human bronchial epithelial cells stably transfected with a plasmid expressing the first 131 nucleotides of the CFTR gene in either the sense (WT cells) or antisense (CF cells) orientation (35). Compared with WT cells, CF cells exhibit significant loss of both CFTR expression and function (35), and they exhibit fatty acid abnormalities similar to those of CF patients (11).
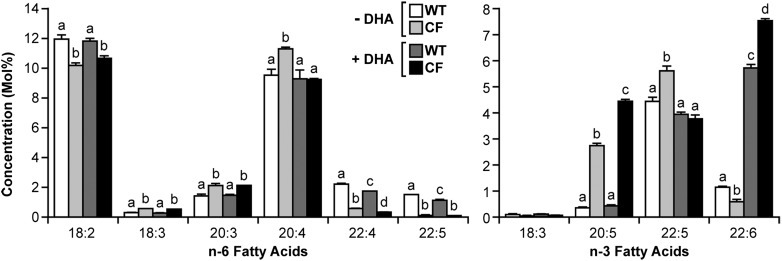
To ascertain the effect of DHA treatment on total fatty acid profiles, WT and CF cells were incubated in the presence or absence of 20 μM DHA for 24 h prior to measurement of relative fatty acid concentrations (Fig. 1). In the absence of DHA, the fatty acid profiles were similar to those in prior studies (10, 11, 23). In particular, LA levels were decreased in CF cells, with increased levels of 18:3n-6, 20:3n-6, AA, and EPA, indicative of increased metabolism of LA to AA and of LNA to EPA. The fatty acid profiles were also indicative of decreased metabolism of AA and EPA to downstream products in CF cells. Both 22:4n-6 and DPA were significantly reduced in CF cells, as was DHA. Levels of 22:5n-3 remained higher in CF cells, but this is more likely due to markedly higher substrate (EPA) levels than to increased metabolism. In fact, 22:5n-3/EPA ratios were significantly lower in CF than WT cells (2.0 ± 0.03 versus 10.5 ± 0.6; P < 0.001) at baseline, suggesting decreased metabolism of EPA to 22:5 in the n-3 pathway.
Fig. 1.
Fatty acid composition of WT and CF cells with or without DHA supplementation. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 20 μM DHA. After 24 h, the cells were harvested, and the total fatty acid composition was measured by GC-MS as described in Materials and Methods. Data are expressed as the molar percentage (mol%) of the total fatty acid mass. Bars represent mean ± SEM (n = 3). Unlike letters indicate significant differences in pair-wise comparisons. The findings are representative of at least two independent experiments.
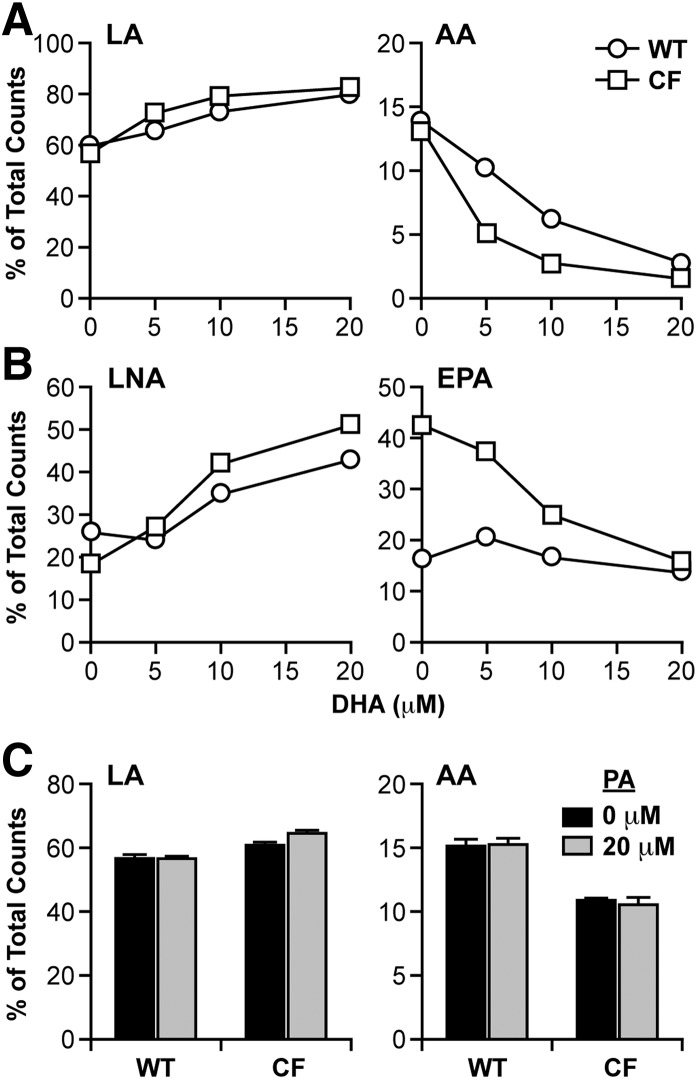
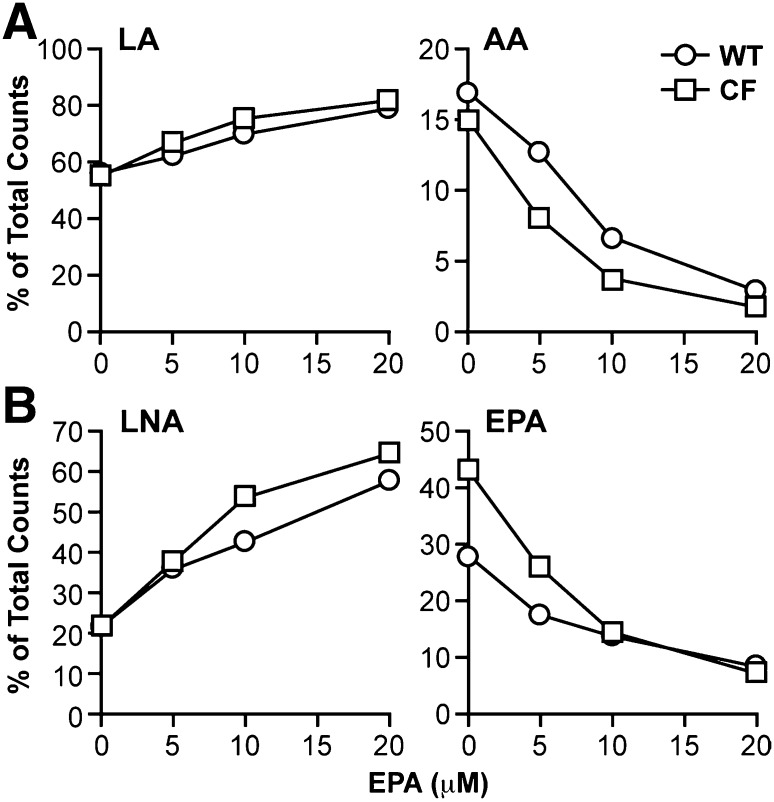
As expected, DHA treatment increased DHA levels in both types of cells, although the increase was much higher in CF cells (6.9 mol%) than in WT cells (4.6 mol%), such that DHA levels were significantly higher in DHA-treated CF cells compared with their WT cell counterparts. DHA treatment also caused a significant change in AA levels in CF cells, which were reduced to WT cell levels. To determine whether this change was due to decreased metabolism of LA to AA, CF and WT cells were incubated with increasing concentrations of DHA for 24 h, followed by [14C]LA for 4 h. Fatty acids were extracted, and conversion of radiolabeled LA to AA was measured as described in Materials and Methods. The results showed a dose-dependent decrease in production of labeled AA corresponding to increased LA (Fig. 2A), indicating suppression of LA to AA metabolism by DHA. This decrease was observed in both CF and WT cells, although CF cells appeared to be more sensitive to DHA treatment, with greater suppression of labeled AA production, particularly at 5 μM and 10 μM concentrations, shifting the curve to the left.
Fig. 2.
LA and LNA metabolism through the n-6 and n-3 pathways in WT and CF cells supplemented with DHA. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 5, 10, or 20 μM DHA (A, B) or 20 μM PA (C). After 24 h, the medium was replaced with reduced-lipid cell culture medium containing either 4.1 μM [1-14C]LA (A, C) or 4.1 μM LNA (B). Cells were incubated for an additional 4 h and harvested. Levels of labeled LA and AA (A, C) or LNA and EPA (B) were determined by HPLC as described in Materials and Methods. Data are expressed as percentage of total counts (dpm). Each point represents mean ± SEM (n = 3). The findings are representative of at least three independent experiments.
Unlike AA, EPA levels in CF cells were significantly increased by DHA treatment, with no change in WT cells (Fig. 1). It is unlikely that this is due to increased production of EPA from LNA, as LNA levels are so low. Furthermore, labeling experiments with [14C]LNA (Fig. 2B) indicated that conversion of LNA to EPA, which was markedly higher in CF cells at baseline, was reduced to almost WT levels by increasing concentrations of DHA. A second possibility is that increased EPA levels are due to retroconversion of DHA to EPA, as has been previously described (36, 37). In fact, calculated retroconversion [ΔEPA/(ΔEPA+ΔDHA)] (38) is much higher in CF cells (20%) than in WT cells (1%).
To determine the specificity of these effects, cells were incubated with saturated and monounsaturated fatty acids. Incubation of WT and CF cells with 20 μM palmitate (PA; 16:0) (Fig. 2C) or oleate (OA; 18:1n-9) (data not shown) had no effect on the metabolism of LA to AA.
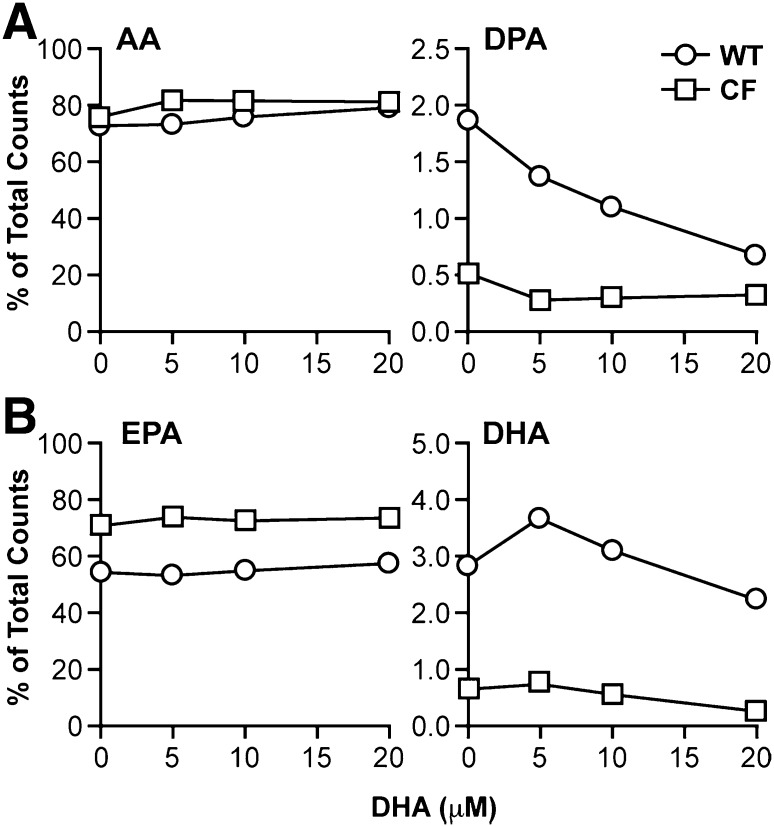
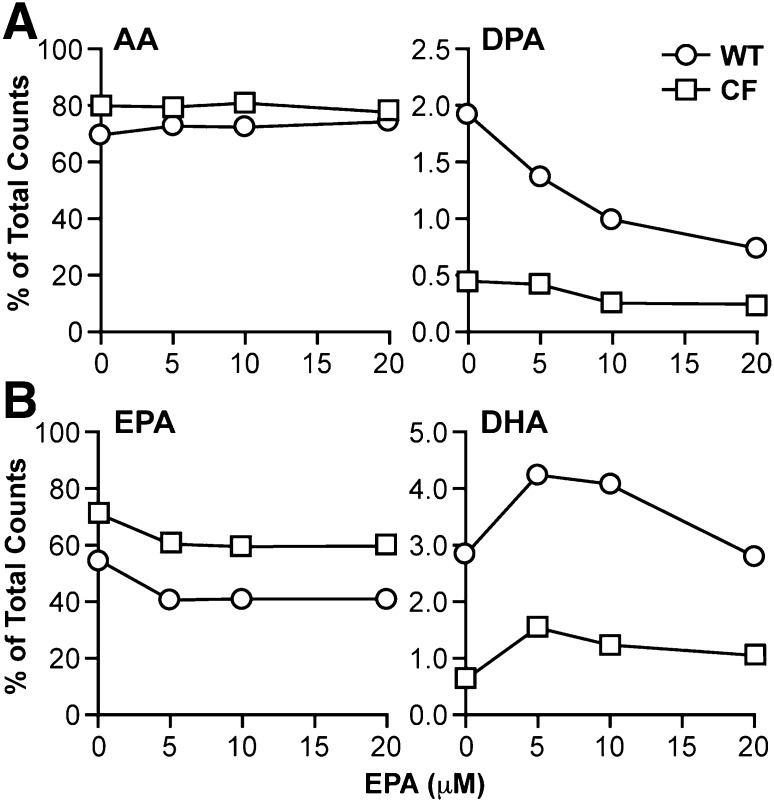
DHA treatment further decreased metabolism of AA in the n-6 pathway. Levels of the AA metabolite 22:4n-6 were significantly reduced in both WT and CF cells (Fig. 1), whereas DPA levels were decreased in WT cells only. DPA levels in CF cells, already very low, were decreased but not by a statistically significant margin. Labeling studies with [14C]AA confirmed a marked decrease in AA to DPA metabolism (Fig. 3A) in WT cells. This conversion was markedly reduced at baseline in CF cells and showed a modest decline with DHA supplementation.
Fig. 3.
AA and EPA metabolism through the n-6 and n-3 pathways in WT and CF cells supplemented with DHA. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 5, 10, or 20 μM DHA. After 24 h, the medium was replaced with reduced-lipid cell culture medium containing either 4.1 μM [1-14C]AA (A) or 4.1 μM EPA (B). Cells were incubated for an additional 4 h and harvested. Levels of labeled AA and DPA (A) or EPA and DHA (B) were determined by HPLC as described in Materials and Methods. Data are expressed as percentage of total counts (dpm). Bars represent mean ± SEM (n = 3). The findings are representative of at least three independent experiments.
The effect of DHA on EPA metabolism was complex. Levels of the immediate elongation product of EPA, 22:5n-3, were reduced in CF cells with DHA treatment, despite the significant increase in EPA levels (Fig. 1). This diminution is confirmed by labeling studies that show a 60% decrease in conversion of [14C]EPA to DHA in CF cells (Fig. 3B). Levels of 22:5n-3 were essentially unchanged in DHA-treated WT cells (Fig. 1). Labeled DHA production from EPA actually increased at lower DHA concentrations (5 μM and 10 μM) and then showed a minimal decline from baseline at 20 μM DHA (Fig. 3B).
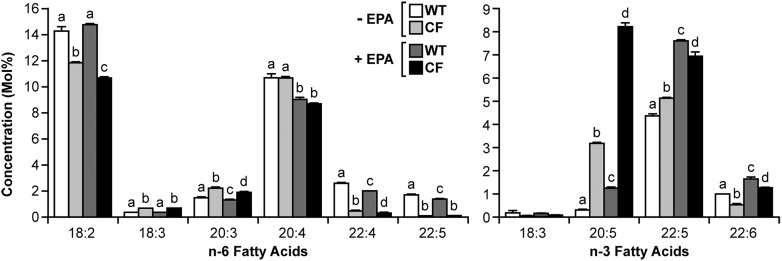
To determine whether the effect of DHA was specific, WT and CF cells were also incubated with EPA, and the effects on fatty acid composition and metabolism were measured. Fig. 4 shows the fatty acid composition of WT and CF cells with and without 24 h incubation with 20 μM EPA. Similar to what was observed for DHA treatment, CF cells demonstrated a greater increase in EPA (5.0 mol%) than WT cells (0.9 mol%), further increasing the EPA disparity between the cells.
Fig. 4.
Fatty acid composition of WT and CF cells with or without EPA supplementation. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 20 μM EPA. After 24 h, the cells were harvested, and the total fatty acid composition was measured by GC-MS as described in Materials and Methods. Data are expressed as the molar percentage (mol%) of the total fatty acid mass. Bars represent mean ± SEM (n = 3). Unlike letters indicate significant differences in pair-wise comparisons. The findings are representative of multiple independent experiments.
The effects of EPA on fatty acid metabolism were also similar to those of DHA. EPA treatment led to decreased levels of both 20:3n-6 and AA in WT and CF cells, corresponding to a dose-dependent decrease in labeled LA incorporation into AA (Fig. 5A). There was a similar decrease in conversion of LNA to EPA (Fig. 5B), although the effects of this change on EPA levels (Fig. 4) are obscured by incorporation of exogenous EPA. In both cases, the effect of EPA appeared to be greater in CF than in WT cells. These data indicate that EPA suppresses metabolism of LA and LNA, similar to DHA.
Fig. 5.
LA and LNA metabolism through the n-6 and n-3 pathways in WT and CF cells supplemented with EPA. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 5, 10, or 20 μM EPA. After 24 h, the medium was replaced with reduced-lipid cell culture medium containing either 4.1 μM [1-14C]LA (A) or 4.1 μM LNA (B). Cells were incubated for an additional 4 h and harvested. Levels of labeled LA and AA (A) or LNA and EPA (B) were determined by HPLC as described in Materials and Methods. Data are expressed as percentage of total counts (dpm). Bars represent mean ± SEM (n = 3). The findings are representative of at least three independent experiments.
EPA also reduced further metabolism of AA in the n-6 pathway. Levels of the AA metabolites 22:4n-6 and DPA were reduced in EPA-treated WT and CF cells (Fig. 4) in a pattern almost identical to that of DHA treatment (Fig. 1). Labeling studies with [14C]AA confirmed a marked decrease in AA to DPA metabolism (Fig. 6A) in WT cells. This conversion was markedly reduced at baseline in CF cells and showed a modest decline at 20 μM EPA.
Fig. 6.
AA and EPA metabolism through the n-6 and n-3 pathways in WT and CF cells supplemented with EPA. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 5, 10, or 20 μM EPA. After 24 h, the medium was replaced with reduced-lipid cell culture medium containing either 4.1 μM [1-14C]AA (A) or 4.1 μM EPA (B). Cells were incubated for an additional 4 h and harvested. Levels of labeled AA and DPA (A) or EPA and DHA (B) were determined by HPLC as described in Materials and Methods. Data are expressed as percentage of total counts (dpm). Bars represent mean ± SEM (n = 3). The findings are representative of at least three independent experiments.
As expected, increased EPA after treatment led to increases in the EPA metabolites 22:5n-3 and DHA (Fig. 4). However, the increases were more significant in WT than CF cells. Conversion rates [calculated as Δproduct / (Δsubstrate + Δproduct), where Δ represents the fatty acid level after DHA treatment subtracted from the baseline level] were much higher in WT than CF cells for both 22:5n-3 (78% versus 27%) and DHA (42% versus 11%). Consequently, DHA levels remained significantly lower in CF than WT cells. These findings correlate with much lower conversion of labeled EPA to DHA in CF cells at all concentrations of EPA (Fig. 6B). For both CF and WT cells, this conversion was increased at lower concentrations of EPA (5 μM and 10 μM) and then returned to roughly baseline levels at 20 μM (Fig. 6B), similar to the effect of DHA supplementation (Fig. 3B).
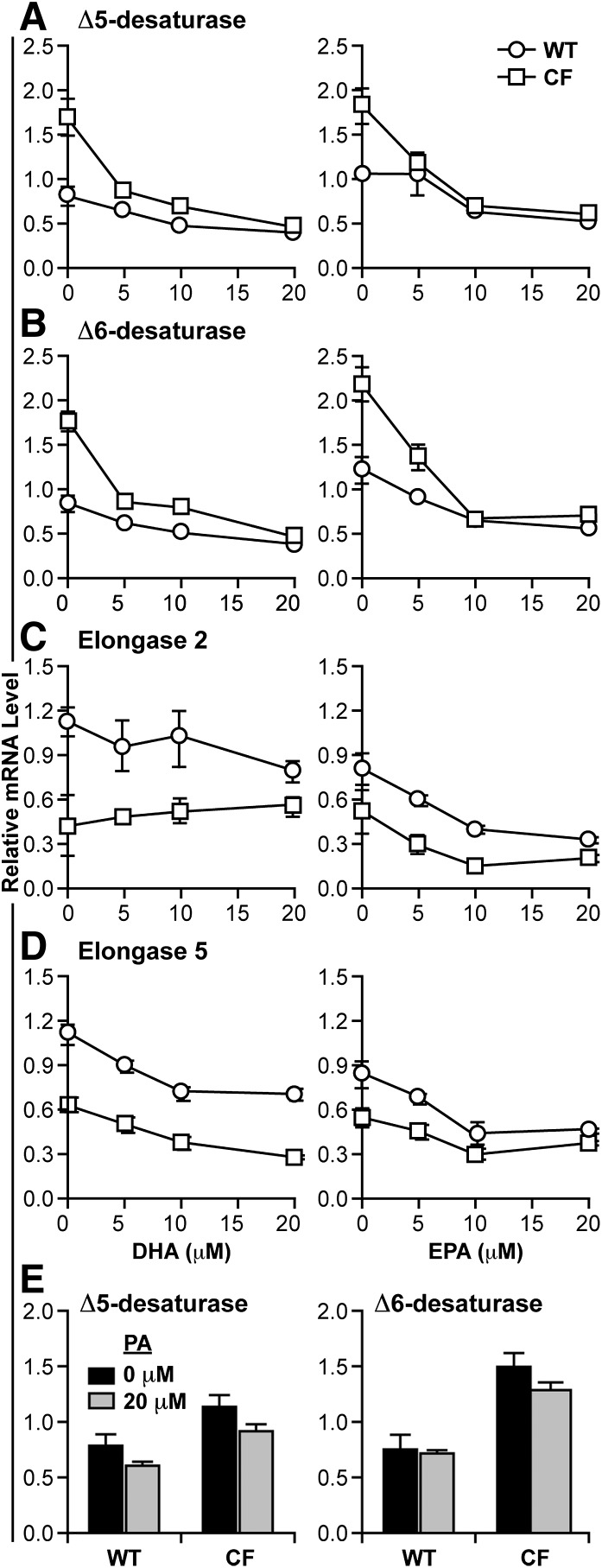
Previous studies (23) have shown that the differences in LA and LNA metabolism in CF cells are associated with increased expression of Δ5- and Δ6-desaturase enzymes, which are primarily regulated at the transcriptional level (31). Therefore, we hypothesized that the effects of DHA and EPA on metabolism may be due to changes in the expression of these enzymes. To test this, we measured the expression of the four major enzymes in the n-3 and n-6 metabolic pathways (Δ5- and Δ6-desaturases, plus elongases 2 and 5) by qRT-PCR in WT and CF cells after incubation with varying concentrations of DHA and EPA (Fig. 7). At baseline, mRNA levels of Δ5- and Δ6-desaturases were significantly higher (approximately 2-fold) in CF than WT cells (P < 0.05 for each comparison). Incubation with as little as 5 μM DHA or EPA reduced expression of these genes in CF cells to the level of WT cells (Fig. 7A, B). Expression of elongases 2 and 5 was either slightly decreased or unchanged (Fig. 7C, D). These data suggest that the reduction of fatty acid metabolism observed with both DHA and EPA incubation is due to downregulation of desaturase gene expression.
Fig. 7.
Changes in Δ5- and Δ6-desaturase mRNA expression with DHA and EPA supplementation. WT and CF cells were cultured in complete medium for 6 days, after which medium was replaced with either unsupplemented medium or medium containing 5, 10, or 20 μM DHA (A–D, left) or EPA (A–D, right). After 24 h, the cells were harvested, and total RNA isolated as described in Materials and Methods. qRT-PCR was performed using primers for the mRNA sequences of Δ5-desaturase (FADS1) (A), Δ6-desaturase (FADS2) (B), elongase 2 (ELOVL2) (C), or elongase 5 (ELOVL5) (D). As a control, Δ5- and Δ6-desaturase mRNA levels were measured after exposure to 20 μM PA for 24 h (E). Relative expression was determined by the ΔΔCT method using ribosomal protein RPLP0 as a control. Data points represent mean ± SEM (n = 3). The findings are representative of at least three independent experiments.
To determine the specificity of these effects, WT and CF cells were incubated with LNA, AA, PA, and OA, all at 20 μM. Both LNA and AA reduced the expression of desaturases, but to a lesser degree compared with DHA and EPA (not shown). For example, LNA and AA reduced Δ6-desaturase mRNA by 48% and 45%, respectively, whereas DHA and EPA reduced expression by 68% and 73%, respectively, at 20 μM. Similar results were seen for Δ5-desaturase. There was little or no significant effect on gene expression after incubation with PA (Fig. 7E) or OA (not shown).
DISCUSSION
While alterations in PUFA levels in CF are a well-established phenomenon, a precise mechanism to explain these changes has not been definitively established. A number of hypotheses have been advanced, including increased AA release and eicosanoid metabolism (4), changes in thiol and phospholipid metabolism (5), and increased flux within PUFA metabolic pathways (10). Work from our laboratory provided correlative data suggesting that these metabolic changes were due to increased expression and activity of fatty acid desaturases (23, 24). The current study demonstrates that treatment of CF cells with exogenous DHA reverses the metabolic changes by suppressing expression of these enzymes. Specifically, DHA decreases expression of Δ5- and Δ6-desaturases, leading to decreased conversion of LA to AA in the n-6 metabolic pathway, thereby reversing the PUFA abnormalities. This study demonstrates that altered desaturase activity plays a role in abnormal fatty acid metabolism in CF. Furthermore, it provides a mechanistic explanation for the observation that DHA therapy reduces AA levels in CF patients and animal models (6, 25–30).
To test the specificity of the DHA effect, all of the experiments were repeated using the related n-3 fatty acid EPA. Interestingly, there was little difference in the effects of EPA versus DHA on PUFA metabolism. Both caused downregulation of desaturase gene expression and reduced metabolism of LA and LNA to AA and EPA, respectively. Both had similar effects on fatty acid composition. LNA, the precursor of all n-3 fatty acids, and AA, an n-6 fatty acid, had similar, although smaller effects. Saturated (PA) and monounsaturated (OA) fatty acids had no effect. These in vitro findings contrast with those in a mouse model (6), which showed a beneficial effect of DHA treatment on CF pathology, but no effect with EPA or LNA treatment. The reason for this difference is unclear. Human trials (39) have shown differences in metabolism using EPA alone or combinations of EPA and DHA (29, 30, 40, 41). However, the individual effects of EPA and DHA are difficult to disaggregate, as supplementation with either of these fatty acids increases concentrations of the other. It is also possible that species differences may play a role.
Unlike previous studies that have focused exclusively on n-6 PUFA metabolism, the current work also examines the n-3 pathway. As indicated above, these are parallel pathways in which the metabolic reactions are catalyzed by a common set of enzymes. Although the metabolic alterations at baseline and with PUFA treatment are similar in direction, there are striking changes in degree that appear to be accentuated in CF. For example, the n-3 pathway appears to be more active than the n-6 pathway, particularly in CF cells, as was reported previously (23) and confirmed in the current study (Figs. 1 and 2). This may reflect a preference of the common desaturase and elongase enzymes for substrates of the n-3 pathway, as has been described by others (42, 43). This difference is apparent in the response to DHA and EPA, as these fatty acids induce a greater decline in LA to AA than in LNA to EPA metabolism and a greater effect in CF than WT cells (Figs. 2 and 5). However, the disparity in EPA levels observed between WT and CF cells has not been consistently described in patients. The most likely explanation for this is that dietary n-3 fatty acids, particularly LNA, are relatively underrepresented in the Western diet (44).
The current DHA supplementation data identify another source of EPA, that generated by retroconversion from DHA. This is a previously described phenomenon wherein DHA is shortened and desaturated by modified β-oxidation in peroxisomes (36, 37). Interestingly, this process appears to be markedly upregulated in CF cells compared with WT cells. This finding suggests that retroconversion may be a second mechanism of increased EPA and decreased DHA levels in these cells. It also raises the possibility that DHA retroconversion is a regulated process, as has been suggested by others (45). This may have implications for the n-3 dietary treatment regimens for many diseases, including CF. Furthermore, CF may be a particularly good system for the study of this poorly understood metabolic pathway.
In addition to their effects on LA and LNA metabolism, DHA and EPA reduce metabolism of AA to DPA, particularly in WT cells. This may also be due to decreased Δ6-desaturase, which is involved in this pathway. It may also be due to suppression of fatty acid elongases, which are also regulated by these fatty acids (46). Interestingly, DHA and EPA did not significantly reduce conversion of EPA to DHA. In fact, at lower concentrations, the activity of this pathway was actually increased. One possible explanation is that the increased concentrations of these fatty acids stimulate activity in their own metabolic pathway, perhaps by mass action, which effectively cancels their effects on enzyme expression.
A final metabolic abnormality highlighted by the current data is a disparity in fatty acid uptake between WT and CF cells. For both DHA and EPA, CF cells appeared to incorporate more exogenous fatty acid than WT cells (compare DHA and EPA levels in Figs. 1 and 4, respectively), consistent with previous findings (10). The causes of this observation are unknown, but it may indicate increased fatty acid transport into CF cells. Recent studies have highlighted the importance of fatty acid transport and its regulation in metabolic diseases (reviewed in Ref. 47). It is possible that the differential effects of DHA and EPA on desaturase gene expression in CF and WT cells may be due to increased uptake of these fatty acids. Future investigation of the mechanism of this disparity in CF may contribute to the understanding of this process.
The complete link between CFTR mutations and the observed PUFA abnormalities in CF remains unknown. However, the identification in this study of a proximal mechanism, upregulation of desaturase expression, is a step toward this ultimate goal. A number of transcription factors are known to participate in the PUFA-mediated regulation of Δ5- and Δ6-desaturase expression (31, 48). Two of the most prominent, sterol regulatory element-binding protein (SREBP)-1 and peroxisome proliferator-activated receptor (PPAR)α, exhibit altered expression and/or activity in CF (49–51). Identification of the relevant transcription factor may identify signaling pathways connecting fatty acid metabolism to CFTR.
An important caveat to this study is that it is restricted to bronchial epithelial cells in culture. In contrast, Mailhot et al. (52) showed no apparent increase in LA to AA metabolism in CFTR-negative intestinal epithelial cells. In addition, Bhura-Bandali et al. (53) demonstrated decreased incorporation of exogenous LA into pancreatic duct cells carrying the ΔF508 mutation. While there are significant methodologic differences between these results and the current study, making direct comparison difficult, it is also possible that the observed alterations are cell-type specific. Future studies using animal models will attempt to reconcile these differences by assessing PUFA metabolism across tissue types.
This study highlights the possible connection between AA and the phenotypic manifestations of CF. AA is a precursor of bioactive oxygenated metabolites known as eicosanoids, which include prostaglandins and leukotrienes. Production of these metabolites is known to be increased in CF, and several studies have drawn a connection between eicosanoid alterations and disease pathogenesis (54–59). It is plausible that the observed benefit of n-3 fatty acid therapy in the CF mouse model (6) and in a small subset of human trials (29, 30) is due to its ability to normalize AA levels and, consequently, reduce excessive production of eicosanoids. In fact, changes in leukotriene metabolism have been noted in several of these trials (39–41). More extensive studies in animal models and CF patients are required to confirm these early observations.
CF remains a deadly disease in need of new therapeutic approaches based on a better understanding of pathophysiology. The current study contributes to a mechanistic explanation for the very consistent PUFA metabolic abnormalities observed in CF and provides a basis for understanding how dietary lipid therapy might be able to modulate these changes. These findings provide an enhanced framework for future studies of the role of lipid metabolism in the pathophysiology and therapy of this disease.
Acknowledgments
The authors thank Eva Henderson and the Vanderbilt Molecular Cell Biology Core Laboratory for primer design and testing and qRT-PCR support.
Footnotes
Abbreviations:
- AA
- arachidonate
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane regulator
- DHA
- docosahexaenoate
- DPA
- docosapentaenoate
- EPA
- eicosapentaenoate
- FAME
- fatty acid methyl ester
- LA
- linoleate
- LNA
- linolenate
- OA
- oleate
- PA
- palmitate
- WT
- wild-type
This work was funded in part by the Edward and Nancy Fody Endowed Chair in Pathology (M.L.) at the Vanderbilt University Medical Center and by the Vanderbilt Physician Scientist Training Program (A.C.S.).
References
- 1.Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. 1989. Identification of the cystic fibrosis gene: genetic analysis. Science. 245: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan B. P., Freedman S. D. 2009. Cystic fibrosis. Lancet. 373: 1891–1904. [DOI] [PubMed] [Google Scholar]
- 3.Al-Turkmani M. R., Freedman S. D., Laposata M. 2007. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids. 77: 309–318. [DOI] [PubMed] [Google Scholar]
- 4.Strandvik B. 2010. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids. 83: 121–129. [DOI] [PubMed] [Google Scholar]
- 5.Innis S. M., Davidson A. G. 2008. Cystic fibrosis and nutrition: linking phospholipids and essential fatty acids with thiol metabolism. Annu. Rev. Nutr. 28: 55–72. [DOI] [PubMed] [Google Scholar]
- 6.Freedman S. D., Katz M. H., Parker E. M., Laposata M., Urman M. Y., Alvarez J. G. 1999. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(-/-) mice. Proc. Natl. Acad. Sci. USA. 96: 13995–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollero M., Laposata M., Zaman M. M., Blanco P. G., Andersson C., Zeind J., Urman Y., Kent G., Alvarez J. G., Freedman S. D. 2006. Evidence of increased flux to n-6 docosapentaenoic acid in phospholipids of pancreas from cftr-/- knockout mice. Metabolism. 55: 1192–1200. [DOI] [PubMed] [Google Scholar]
- 8.Mimoun M., Coste T. C., Lebacq J., Lebecque P., Wallemacq P., Leal T., Armand M. 2009. Increased tissue arachidonic acid and reduced linoleic acid in a mouse model of cystic fibrosis are reversed by supplemental glycerophospholipids enriched in docosahexaenoic acid. J. Nutr. 139: 2358–2364. [DOI] [PubMed] [Google Scholar]
- 9.Zaman M. M., Martin C. R., Andersson C., Bhutta A. Q., Cluette-Brown J. E., Laposata M., Freedman S. D. 2010. Linoleic acid supplementation results in increased arachidonic acid and eicosanoid production in CF airway cells and in cftr-/- transgenic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 299: L599–L606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Turkmani M. R., Andersson C., Alturkmani R., Katrangi W., Cluette-Brown J. E., Freedman S. D., Laposata M. 2008. A mechanism accounting for the low cellular level of linoleic acid in cystic fibrosis and its reversal by DHA. J. Lipid Res. 49: 1946–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson C., Al-Turkmani M. R., Savaille J. E., Alturkmani R., Katrangi W., Cluette-Brown J. E., Zaman M. M., Laposata M., Freedman S. D. 2008. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J. Lipid Res. 49: 1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strandvik B., Gronowitz E., Enlund F., Martinsson T., Wahlstrom J. 2001. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J. Pediatr. 139: 650–655. [DOI] [PubMed] [Google Scholar]
- 13.Olveira G., Dorado A., Olveira C., Padilla A., Rojo-Martinez G., Garcia-Escobar E., Gaspar I., Gonzalo M., Soriguer F. 2006. Serum phospholipid fatty acid profile and dietary intake in an adult Mediterranean population with cystic fibrosis. Br. J. Nutr. 96: 343–349. [DOI] [PubMed] [Google Scholar]
- 14.Van Biervliet S., Vanbillemont G., Van Biervliet J. P., Declercq D., Robberecht E., Christophe A. 2007. Relation between fatty acid composition and clinical status or genotype in cystic fibrosis patients. Ann. Nutr. Metab. 51: 541–549. [DOI] [PubMed] [Google Scholar]
- 15.Maqbool A., Schall J. I., Garcia-Espana J. F., Zemel B. S., Strandvik B., Stallings V. A. 2008. Serum linoleic acid status as a clinical indicator of essential fatty acid status in children with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 47: 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilbault C., Wojewodka G., Saeed Z., Hajduch M., Matouk E., De Sanctis J. B., Radzioch D. 2009. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am. J. Respir. Cell Mol. Biol. 41: 100–106. [DOI] [PubMed] [Google Scholar]
- 17.Ollero M., Astarita G., Guerrera I. C., Sermet-Gaudelus I., Trudel S., Piomelli D., Edelman A. 2011. Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J. Lipid Res. 52: 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura M. T., Nara T. Y. 2003. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot. Essent. Fatty Acids. 68: 145–150. [DOI] [PubMed] [Google Scholar]
- 19.Carlstedt-Duke J., Bronnegard M., Strandvik B. 1986. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc. Natl. Acad. Sci. USA. 83: 9202–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levistre R., Lemnaouar M., Rybkine T., Bereziat G., Masliah J. 1993. Increase of bradykinin-stimulated arachidonic acid release in a delta F508 cystic fibrosis epithelial cell line. Biochim. Biophys. Acta. 1181: 233–239. [DOI] [PubMed] [Google Scholar]
- 21.Berguerand M., Klapisz E., Thomas G., Humbert L., Jouniaux A. M., Olivier J. L., Bereziat G., Masliah J. 1997. Differential stimulation of cytosolic phospholipase A2 by bradykinin in human cystic fibrosis cell lines. Am. J. Respir. Cell Mol. Biol. 17: 481–490. [DOI] [PubMed] [Google Scholar]
- 22.Miele L., Cordella-Miele E., Xing M., Frizzell R., Mukherjee A. B. 1997. Cystic fibrosis gene mutation (deltaF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol. 16: 749–759. [DOI] [PubMed] [Google Scholar]
- 23.Njoroge S. W., Seegmiller A. C., Katrangi W., Laposata M. 2011. Increased Delta5- and Delta6-desaturase, cyclooxygenase-2, and lipoxygenase-5 expression and activity are associated with fatty acid and eicosanoid changes in cystic fibrosis. Biochim. Biophys. Acta. 1811: 431–440. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen K. F., Laposata M., Njoroge S. W., Umunakwe O. C., Katrangi W., Seegmiller A. C. 2011. Increased elongase 6 and Delta9-desaturase activity are associated with n-7 and n-9 fatty acid changes in cystic fibrosis. Lipids. 46: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman S. D., Weinstein D., Blanco P. G., Martinez-Clark P., Urman S., Zaman M., Morrow J. D., Alvarez J. G. 2002. Characterization of LPS-induced lung inflammation in cftr-/- mice and the effect of docosahexaenoic acid. J. Appl. Physiol. 92: 2169–2176. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Still J. D., Powers C. A., Hoffman D. R., Boyd-Trull K., Lester L. A., Benisek D. C., Arterburn L. M. 2006. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized, controlled study. Nutrition. 22: 36–46. [DOI] [PubMed] [Google Scholar]
- 27.Jumpsen J. A., Brown N. E., Thomson A. B., Paul Man S. F., Goh Y. K., Ma D., Clandinin M. T. 2006. Fatty acids in blood and intestine following docosahexaenoic acid supplementation in adults with cystic fibrosis. J. Cyst. Fibros. 5: 77–84. [DOI] [PubMed] [Google Scholar]
- 28.Van Biervliet S., Devos M., Delhaye T., Van Biervliet J. P., Robberecht E., Christophe A. 2008. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot. Essent. Fatty Acids. 78: 109–115. [DOI] [PubMed] [Google Scholar]
- 29.De Vizia B., Raia V., Spano C., Pavlidis C., Coruzzo A., Alessio M. 2003. Effect of an 8-month treatment with omega-3 fatty acids (eicosapentaenoic and docosahexaenoic) in patients with cystic fibrosis. JPEN J. Parenter. Enteral Nutr. 27: 52–57. [DOI] [PubMed] [Google Scholar]
- 30.Olveira G., Olveira C., Acosta E., Espildora F., Garrido-Sanchez L., Garcia-Escobar E., Rojo-Martinez G., Gonzalo M., Soriguer F. 2010. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch. Bronconeumol. 46: 70–77. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura M. T., Nara T. Y. 2002. Gene regulation of mammalian desaturases. Biochem. Soc. Trans. 30: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 32.Jump D. B., Botolin D., Wang Y., Xu J., Demeure O., Christian B. 2008. Docosahexaenoic acid (DHA) and hepatic gene transcription. Chem. Phys. Lipids. 153: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 34.Alvarez J. G., Storey B. T. 1995. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 42: 334–346. [DOI] [PubMed] [Google Scholar]
- 35.Rajan S., Cacalano G., Bryan R., Ratner A. J., Sontich C. U., van Heerckeren A., Davis P., Prince A. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23: 304–312. [DOI] [PubMed] [Google Scholar]
- 36.Grønn M., Christensen E., Hagve T. A., Christophersen B. O. 1991. Peroxisomal retroconversion of docosahexaenoic acid (22:6(n-3)) to eicosapentaenoic acid (20:5(n-3)) studied in isolated rat liver cells. Biochim. Biophys. Acta. 1081: 85–91. [DOI] [PubMed] [Google Scholar]
- 37.Brossard N., Croset M., Pachiaudi C., Riou J. P., Tayot J. L., Lagarde M. 1996. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am. J. Clin. Nutr. 64: 577–586. [DOI] [PubMed] [Google Scholar]
- 38.Conquer J. A., Holub B. J. 1997. Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores. Lipids. 32: 341–345. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence R. H., Sorrell T. C. 1994. Eicosapentaenoic acid modulates neutrophil leukotriene B4 receptor expression in cystic fibrosis. Clin. Exp. Immunol. 98: 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurlandsky L. E., Bennink M. R., Webb P. M., Ulrich P. J., Baer L. J. 1994. The absorption and effect of dietary supplementation with omega-3 fatty acids on serum leukotriene B4 in patients with cystic fibrosis. Pediatr. Pulmonol. 18: 211–217. [DOI] [PubMed] [Google Scholar]
- 41.Panchaud A., Sauty A., Kernen Y., Decosterd L. A., Buclin T., Boulat O., Hug C., Pilet M., Roulet M. 2006. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo-controlled trial. Clin. Nutr. 25: 418–427. [DOI] [PubMed] [Google Scholar]
- 42.Sayanova O. V., Beaudoin F., Michaelson L. V., Shewry P. R., Napier J. A. 2003. Identification of primula fatty acid delta 6-desaturases with n-3 substrate preferences. FEBS Lett. 542: 100–104. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X., Ding Z., Xu Y., Monroig O., Morais S., Tocher D. R. 2009. Physiological roles of fatty acyl desaturases and elongases in marine fish: characterisation of cDNAs of fatty acyl delta6-desaturase and elovl5 elongase of cobia (Rachycentron canadum). Aquaculture. 290: 122–131. [Google Scholar]
- 44.Blasbalg T. L., Hibbeln J. R., Ramsden C. E., Majchrzak S. F., Rawlings R. R. 2011. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93: 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stark K. D., Holub B. J. 2004. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am. J. Clin. Nutr. 79: 765–773. [DOI] [PubMed] [Google Scholar]
- 46.Jakobsson A., Westerberg R., Jacobsson A. 2006. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. 45: 237–249. [DOI] [PubMed] [Google Scholar]
- 47.Glatz J. F., Luiken J. J., Bonen A. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol. Rev. 90: 367–417. [DOI] [PubMed] [Google Scholar]
- 48.Jump D. B., Botolin D., Wang Y., Xu J., Christian B., Demeure O. 2005. Fatty acid regulation of hepatic gene transcription. J. Nutr. 135: 2503–2506. [DOI] [PubMed] [Google Scholar]
- 49.Reynders V., Loitsch S., Steinhauer C., Wagner T., Steinhilber D., Bargon J. 2006. Peroxisome proliferator-activated receptor alpha (PPAR alpha) down-regulation in cystic fibrosis lymphocytes. Respir. Res. 7: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y., Tertilt C., Krause A., Quadri L. E., Crystal R. G., Worgall S. 2009. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir. Res. 10: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pall H., Zaman M. M., Andersson C., Freedman S. D. 2006. Decreased peroxisome proliferator activated receptor alpha is associated with bile duct injury in cystic fibrosis transmembrane conductance regulator-/- mice. J. Pediatr. Gastroenterol. Nutr. 42: 275–281. [DOI] [PubMed] [Google Scholar]
- 52.Mailhot G., Rabasa-Lhoret R., Moreau A., Berthiaume Y., Levy E. 2010. CFTR depletion results in changes in fatty acid composition and promotes lipogenesis in intestinal Caco 2/15 cells. PLoS ONE. 5: e10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhura-Bandali F. N., Suh M., Man S. F., Clandinin M. T. 2000. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J. Nutr. 130: 2870–2875. [DOI] [PubMed] [Google Scholar]
- 54.Rigas B., Korenberg J. R., Merrill W. W., Levine L. 1989. Prostaglandins E2 and E2 alpha are elevated in saliva of cystic fibrosis patients. Am. J. Gastroenterol. 84: 1408–1412. [PubMed] [Google Scholar]
- 55.Strandvik B., Svensson E., Seyberth H. W. 1996. Prostanoid biosynthesis in patients with cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids. 55: 419–425. [DOI] [PubMed] [Google Scholar]
- 56.Sampson A. P., Spencer D. A., Green C. P., Piper P. J., Price J. F. 1990. Leukotrienes in the sputum and urine of cystic fibrosis children. Br. J. Clin. Pharmacol. 30: 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konstan M. W., Walenga R. W., Hilliard K. A., Hilliard J. B. 1993. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am. Rev. Respir. Dis. 148: 896–901. [DOI] [PubMed] [Google Scholar]
- 58.De Lisle R. C., Meldi L., Flynn M., Jansson K. 2008. Altered eicosanoid metabolism in the cystic fibrosis mouse small intestine. J. Pediatr. Gastroenterol. Nutr. 47: 406–416. [DOI] [PubMed] [Google Scholar]
- 59.De Lisle R. C., Sewell R., Meldi L. 2010. Enteric circular muscle dysfunction in the cystic fibrosis mouse small intestine. Neurogastroenterol. Motil. 22: 341–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]