Abstract
Biomarkers of oxidative stress and inflammation predict cardiovascular events in maintenance hemodialysis patients. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) reduce cardiovascular mortality in the general population, but their benefit in maintenance hemodialysis patients is not fully explored. To test whether ACE inhibitors and ARBs differentially affect markers of oxidative stress, inflammation, and fibrinolysis during hemodialysis, we conducted a randomized, double-blind, placebo-controlled 3×3 crossover study. We randomly assigned 15 participants undergoing hemodialysis to placebo, ramipril (5 mg/d), and valsartan (160 mg/d) for 7 days, with a washout period of 3 weeks in between the treatments. On the morning of the seventh day of drug treatment, participants underwent serial blood sampling during hemodialysis. Neither ramipril nor valsartan affected BP during hemodialysis. Ramipril increased IL-1β concentrations (P=0.02) and decreased IL-10 concentrations (P=0.04) compared with placebo. Valsartan and ramipril both lowered IL-6 levels during dialysis (P<0.01 for each compared with placebo). Valsartan increased F2-isoprostane levels, and ramipril suggested a similar trend (P=0.09). Valsartan and ramipril both lowered D-dimer levels (P<0.01 for both), whereas only ramipril seemed to prevent a rise in vWf levels (P=0.04). In summary, during hemodialysis, valsartan induces a greater anti-inflammatory effect compared with ramipril, although ramipril seems to prevent dialysis-induced endothelial dysfunction as measured by levels of vWf. A prospective clinical trial is necessary to determine whether ACE inhibitors and ARBs also differ with respect to their effects on cardiovascular mortality in this population.
In ESRD patients, cardiovascular death accounts for >50% of overall mortality and the risk of cardiovascular death is 30 times higher than in the general population.1,2 Moreover, patients undergoing maintenance hemodialysis (MHD) have a 5-year survival rate of 10% after acute myocardial infarction.3 Traditional risk factors, as used in Framingham risk scoring, only explain half of the excessive cardiovascular mortality observed in ESRD patients,4 and it is thought that other factors contribute to the high cardiovascular mortality among ESRD patients.
Increased oxidative stress and inflammation may contribute to accelerated atherosclerosis and cardiovascular events in patients with MHD.5,6 Markers of oxidative stress, such as F2-isoprostanes, are increased in MHD patients.7,8 Inflammatory markers, particularly IL-1, IL 6, and TNFα, are also elevated in MHD patients and are associated with increased risk of mortality.9 Both underlying uremia itself and recurrent hemodialysis can contribute to inflammation.10,11 Hemodialysis induces an acute increase in white blood cellular transcription and circulating concentrations of inflammatory markers.12,13 Inflammatory cytokines, in turn, increase the expression of prothrombotic factors, such as plasminogen activator inhibitor-1 (PAI-1),14 the major inhibitor of fibrinolysis by inactivating the tissue plasminogen activator (tPA). Increased PAI-1 antigen levels and activity in MHD patients correlate with increased risk of coronary artery disease.15
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) reduce cardiovascular mortality in the general population,16,17 but a single prospective clinical trial of the ACE inhibitor fosinopril in MHD patients showed no protective effect on cardiovascular events.18 This trial may have been underpowered to detect an effect of ACE inhibition. Another trial in MHD patients with chronic heart failure showed that adding the ARB telmisartan to their medication regimen decreased all-cause mortality, cardiovascular mortality, and duration of hospitalization.19 In a recent retrospective observational study in MHD patients, initiation of an ACE inhibitor plus other antihypertensive medication was associated with increased mortality compared with initiation of an ARB and other antihypertensive medication.20 ACE inhibitors and ARBs both reduce angiotensin II activity but they differ in their effects on bradykinin. ACE inhibitors, but not ARBs, increase bradykinin bioavailability by reducing its degradation.21 Bradykinin increases tPA and inhibits platelet aggregation,22,23 but also increases oxidative stress and inflammation.24–27 Hemodialysis also stimulates activation of the kallikrein-kinin system and the production of bradykinin.28 Endogenous bradykinin contributes to increases in PAI-1 and monocyte chemoattractant protein-1 after hemodialysis.29 Thus, any intervention that increases bradykinin, such as ACE inhibitors, may increase the inflammatory response to hemodialysis in MHD patients.
In this study, we tested the hypothesis that short-term administration of ACE inhibitors and ARBs would differ in their effects on oxidative stress, inflammation, and fibrinolysis in response to hemodialysis in MHD patients. We conducted a randomized, double-blind, placebo-controlled 3×3 crossover study comparing the effect of 1-week treatment with the ACE inhibitor ramipril, the ARB valsartan, and placebo on the responses to hemodialysis.
Results
Patient Characteristics
Table 1 shows baseline patient characteristics. The causes of ESRD were hypertension (9 of the 15 patients), diabetes mellitus (4 patients), nephropathy induced by nonsteroidal anti-inflammatory drugs (1 patient), and unknown (1 patient).
Table 1.
Baseline characteristics
| Parameter | Value |
|---|---|
| Age (yr) | 50.5±3.1 |
| Sex (male/female) | 7:8 |
| Race (African-American/Caucasian/Hispanic) | 11:2:2 |
| Smoker (yes/no) | 5:10 |
| Hypertension (yes/no) | 15:0 |
| ACE inhibitor or ARB use (yes/no) | 3:12 |
| BMI (kg/m2) | 30.2±2.0 |
| Calcium × phosphate product (mg2/dl2) | 53.3±3.3 |
| Hemoglobin (g/dl) | 11.9±0.3 |
| Erythropoietin dose (units) | 5111.1±1488.4 |
| Heparin dose (units) | 4800±579.0 |
Data are presented as mean ± SEM or ratio.
Effects of Hemodialysis and Treatment on Hemodynamics and Renin-Angiotensin System Parameters
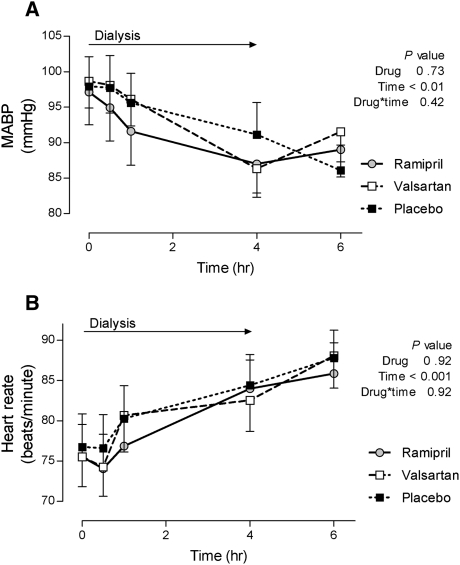
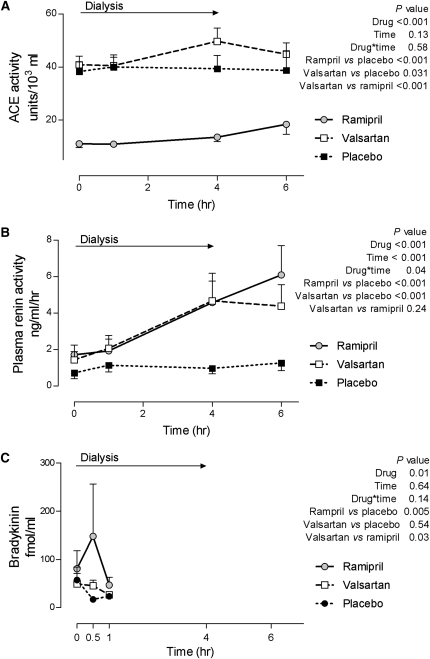
Baseline mean arterial BP (MABP) was comparable among the treatment arms and MABP decreased significantly and similarly during hemodialysis (from 97.91±3.65 at the beginning to 88.16±3.43 mmHg at the end of dialysis, Figure 1) among all three treatments. Heart rate increased significantly and similarly during dialysis but there was no effect of treatment (Figure 1). ACE activity was significantly lower during ramipril treatment compared with during placebo or valsartan treatment (Figure 2), and did not change during hemodialysis. Valsartan increased ACE activity when compared with placebo at the end of hemodialysis (Figure 2). Plasma renin activity (PRA) was significantly higher during treatment with ramipril or valsartan compared with placebo. PRA increased during dialysis with ramipril and valsartan treatments but not placebo (P=0.04 for time × drug interaction, Figure 2). We detected no evidence for a carry-over effect on ACE activity or PRA. Bradykinin concentrations were increased during ramipril treatment compared with during placebo or valsartan (Figure 2).
Figure 1.
Effect of hemodialysis and study interventions on hemodynamic parameters. Changes in MABP (A) and heart rate (B) are depicted during hemodialysis after 1-week treatment with ramipril, valsartan, and placebo.
Figure 2.
Effect of hemodialysis and study interventions on the renin-angiotensin-aldosterone system and bradykinin levels. Plasma ACE activity (A), PRA (B), and bradykinin (C) concentrations are depicted during hemodialysis after 1-week treatment with ramipril, valsartan, and placebo.
Effect of Hemodialysis and Treatment on Inflammation and Oxidative Stress
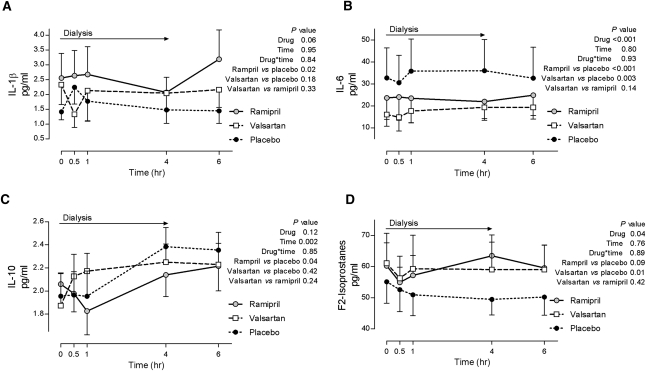
IL-1β levels were significantly higher during ramipril treatment compared with placebo (Figure 3). Serum IL-6 and IL-8 concentrations were lower during valsartan and ramipril treatment compared with placebo, and did not change with dialysis (Figure 3 and Table 2). IL-10, an anti-inflammatory cytokine, increased during dialysis (Figure 3); however, IL-10 concentrations initially decreased and remained significantly lower during ramipril treatment compared with placebo (Figure 3). Plasma F2-isoprostanes concentrations were significantly higher during valsartan treatment compared with placebo and there was a trend toward higher levels during ramipril treatment compared with placebo (Figure 3).
Figure 3.
Effect of 1-week treatment with ramipril, valsartan, and placebo on markers of inflammation and oxidative stress in response to hemodialysis.
Table 2.
Effects of hemodialysis and treatment on markers of inflammation
| Time | P Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 30 min | 1 h | 4 h | 6 h | Time | Drug | |
| IL-8 (pg/ml) | 0.01 | 0.002 | |||||
| placebo | 40.2±6.0 | 27.5±4.0a | 28.9±5.1a | 34.1±6.3a | 35.0±5.5 | ||
| ARB | 38.2±5.9 | 25.8±3.7a | 30.4±6.2a | 30.5±4.9a | 30.8±4.2a | ||
| ACE inhibitor | 31.8±3.6 | 26.4±3.5a | 25.3±4.0a | 26.9±3.0a | 30.1±3.3 | ||
| MCP-1 (pg/ml) | <0.001 | 0.51 | |||||
| placebo | 415±113 | 277±65a | 264±59a | 332±104a | 387±87 | ||
| ARB | 364±108 | 273±61a | 280±56a | 344±79 | 378±108 | ||
| ACE inhibitor | 353±74 | 286±48 | 257±53a | 333±58 | 370±83 | ||
Data are presented as mean ± SEM. MCP-1, monocyte chemoattractant protein-1.
P<0.05 versus baseline.
Effect of Hemodialysis and Treatment on Markers of Coagulation, Fibrinolysis, and Endothelial Injury
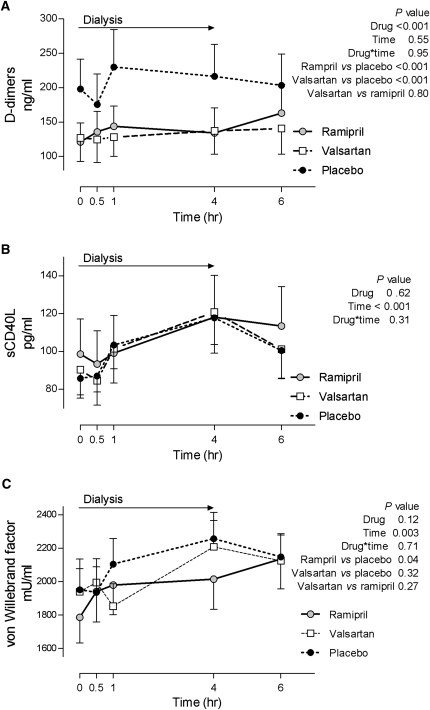
Concentrations of tPA increased during dialysis and were similar during all three treatments (Table 3). PAI-1 concentrations were also similar during all three treatments. D-dimers, a product of fibrinolysis and marker of thrombosis, were significantly lower during valsartan and ramipril treatment compared with placebo (Figure 4). Soluble CD40 ligand (sCD40L) is a platelet-derived cytokine important for thrombi stabilization.30 sCD40L concentrations increased throughout dialysis during all treatments (from 91.4±8.5 to 118.9±11.2 ng/ml, beginning to end of dialysis, respectively; Figure 4) and there was no difference among the treatment arms. In contrast, plasma vWf, a marker of endothelial injury, increased during dialysis, and only ramipril treatment significantly attenuated this effect (Figure 4).
Table 3.
Effects of hemodialysis and treatment on markers of fibrinolysis
| Time | P Value | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 30 min | 1 h | 4 h | 6 h | Time | Drug | |
| tPA (ng/ml) | <0.001 | 0.64 | |||||
| placebo | 6.6±0.7 | 7.3±0.9 | 7.4±1.0a | 10.7±1.4a | 8.7±1.0a | ||
| ARB | 6.6±0.8 | 7.2±0.9 | 7.7±1.0 | 9.9±1.4a | 8.3±1.1a | ||
| ACE inhibitor | 7.3±0.8 | 7.9±1.0 | 8.4±0.9 | 10.6±1.1a | 8.7±1.0a | ||
| PAI-1 (ng/ml) | |||||||
| placebo | 4.5±0.8 | 4.6±0.6 | 5.8±0.9 | 5.2±0.8 | 4.3±0.8 | 0.31 | 0.73 |
| ARB | 4.1±0.9 | 4.9±1.0 | 4.5±1.0 | 6.1±1.0 | 4.9±1.0 | ||
| ACE inhibitor | 5.1±1.0 | 4.4±0.7 | 5.8±0.9 | 5.2±1.0 | 4.4±0.9 | ||
Data are presented as mean ± SEM.
P<0.05 versus baseline.
Figure 4.
Effect of 1-week treatment with ramipril, valsartan, and placebo on markers of coagulation, fibrinolysis, and endothelial function during hemodialysis.
Discussion
This randomized study tested the hypothesis that ACE inhibitors and ARBs differ in their effects on oxidative stress, inflammation, and fibrinolysis in MHD patients. The major findings are that although short-term treatment with an ACE inhibitor or ARB decreased IL-6 and IL-8, ramipril also exerted a proinflammatory effect during hemodialysis, as measured by an increase in IL-1β and a decrease in the anti-inflammatory cytokine IL-10. Both ramipril and valsartan decreased the marker of thrombosis, D-dimers, but ramipril also prevented a rise in vWf, a marker of endothelial damage, during hemodialysis. These effects are not attributable to an antihypertensive effect of ramipril or valsartan, because neither drug affected BP in the MHD studied participants.
Increased oxidative stress and inflammation are commonly observed in MHD patients. The etiology of inflammation is complex and includes underlying uremia and the continuous contact between blood and dialyzer/dialysate. Indeed, the hemodialysis procedure results in increased leukocyte transcript levels of many proinflammatory cytokines13 and circulating cytokines increase and remain high at least 2 hours after the end of dialysis.12 In our study, valsartan and ramipril reduced the levels of IL-6 and IL-8 during hemodialysis. Angiotensin II stimulates IL-6 production and release31–33; therefore, ramipril and valsartan have likely reduced IL-6 by preventing the formation or action of angiotensin II. The finding that ramipril treatment increased levels of IL-1β and decreased IL-10, an anti-inflammatory cytokine, however, suggests that ramipril had an additional proinflammatory effect. One potential explanation for the differential effects on the inflammatory response between ACE inhibitors and ARBs may relate to their effect on bradykinin metabolism. ACE inhibition enhances bradykinin effects by decreasing its breakdown, whereas ARB does not. In addition, hemodialysis increases bradykinin levels,28 albeit to lesser extent with polysulfone membranes than polyacrylonitrile membranes. Supporting a proinflammatory role of bradykinin is the observation that bradykinin B2-receptor blockade increases circulating IL-10 in an animal model of ischemia/reperfusion.34 Furthermore, in MHD patients, bradykinin B2-receptor blockage raises IL-10 levels during hemodialysis.29 In this study, we found that bradykinin concentrations were increased during ramipril treatment compared with during placebo or valsartan. Taken together, these data suggest that increased endogenous bradykinin accounts for lower IL-10 levels during ramipril treatment. The relation between bradykinin and IL-1β is complex. Bradykinin increases the release and gene expression of IL-1β35–37 and may enhance the effects of IL-1β.38 On the other hand, IL-1β induces the expression of bradykinin receptors.39
Inflammatory cytokines predict cardiovascular endpoints in ESRD patients. For example, IL-6 predicts mortality and correlates with the severity of carotid atherosclerosis.40,41 On the other hand, decreased levels of IL-10 have been described in MHD patients with carotid atherosclerotic plaques.42 Furthermore, a genotype associated with lower IL-10 production predicts cardiovascular death in MHD patients.43 These data support the hypothesis that ARBs may be preferable to ACE inhibitors in limiting cardiovascular events in MHD patients. Indeed, a recent study in MHD patients with chronic heart failure showed that the addition of telmisartan to standard therapies significantly reduces all-cause mortality, cardiovascular death, and heart failure hospital stays.19
Contrary to our initial hypothesis, F2-isoprostanes were increased during both ramipril and valsartan treatment. In contrast, a previous study showed that oxidative stress levels decreased in hemodialysis patients after 6-week treatment with valsartan.44 The discrepancy with our study may reside in the duration of the treatment. Although this study does not address the mechanism for the effect of valsartan and ramipril on F2-isoprostanes, we observed that increasing blood flow increases F2-isoprostanes in the human forearm vasculature.26 Further experiments may explain the relation between angiotensin II, bradykinin, and oxidative stress during hemodialysis.
Consistent with previous studies,29 we found that tPA antigen increased during dialysis. Although bradykinin stimulates the release of tPA from the endothelium, we observed no effect of ramipril on tPA concentrations during dialysis. This is in agreement with the recent results of Marney et al., who reported no effect of bradykinin B2-receptor blockade on tPA levels during hemodialysis.29 The lack of effect of bradykinin or ACE inhibitors on tPA concentrations may reflect endothelial dysfunction, commonly observed in MHD patients,45,46 because endothelial dysfunction attenuates bradykinin-stimulated tPA release.47,48
ESRD patients exhibit hypercoagulability, resulting in elevated D-dimer levels.49 Plasma D-dimers are increased in MHD, although their diagnostic value has been questioned.50 In MHD patients, D-dimers have been correlated with bleeding propensity rather than hypercoagulability51; however, D-dimers predict mortality in ESRD patients.52 The finding that either ramipril or valsartan reduced D-dimers levels in the MHD patients confirms data from clinical trials in patients without ESRD, such as the Perindopril-Thrombosis Inflammation, Endothelial Dysfunction and Neurohormonal Activation Trial (PERTINENT) and the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors.53,54 In the PERTINENT study, perindopril also improved the endothelial function, and specifically decreased vWf levels.55 This study extends this observation to demonstrate that ACE inhibition decreases acute endothelial injury during dialysis.
sCD40L is a proinflammatory and procoagulant molecule, released from platelets upon activation. sCD40L ligand concentrations are associated with an increased risk of cardiovascular events in the general population56 and are increased in patients with MHD.57 We found that sCD40L increased during dialysis and there was no difference among the treatment arms. Koh et al. also reported that 2 months of treatment with candesartan reduced the plasma levels of sCD40L in hypertensive patients.58 In contrast, studies in patients with either heart failure or atrial fibrillation failed to show a change in plasma levels of sCD40L during either ACE inhibitor or ARB treatment.59,60
This study has some limitations. It was designed to evaluate the short-term effect of valsartan and ramipril on the acute inflammatory response to hemodialysis and does not address the long-term effect of these drugs. Nevertheless, the data support a greater anti-inflammatory effect of valsartan compared with ramipril, whereas only ramipril prevented dialysis-induced endothelial dysfunction, as measured by levels of vWf. The differences between the effects of the two drugs may be attributed to bradykinin, which has proinflammatory as well as endothelial protective effects. These data suggest that results from randomized clinical trials of ACE inhibitors in MHD patients cannot be readily extrapolated to the effects of ARBs. Our results suggest that there may be specific subgroups of MHD patients that might benefit from each drug differently and emphasize the need for a long-term randomized clinical trial to compare the effects of ARBs and ACE inhibitors on cardiovascular endpoints in patients on MHD.
Concise Methods
Study Population
This study was approved by the Vanderbilt University Institutional Review Board and performed according to the Declaration of Helsinki. Approximately 100 patients were prescreened for eligibility, 20 of whom were approached and provided written informed consent and agreed to participate in this study. Three participants were excluded on the basis of the exclusion criteria after screening (hyperkalemia, hypotension, and uncontrollable hypertension), one withdrew from the study (moved to another city), and one patient was withdrawn from the study because of stroke. (The stroke was not felt to be study related by the Data and Safety Monitoring Committee for this study.) Therefore, 15 participants completed this study. All of them underwent adequate hemodialysis (Kt/V >1.2) three times per week for at least 6 consecutive months. Hemodialysis was performed for 4 hours using the Fresenius Optiflux 180 dialyzer (Fresenius Medical Care, Waltham, MA) with a polysulphone membrane. Dialysate quality was within the Association for the Advancement of Medical Instrumentation standards for endotoxin concentrations. In addition, dialysis was performed using Diasafe filters (Fresenius Medical Care) to ensure further purity of the dialysate. Patients were clinically stable with predialysis potassium levels <5.5 mmol/L.
Participants with a history of functional transplant <6 months before the study or anticipated live donor kidney transplant were excluded. Patients were excluded from the study if they had history of active connective tissue disease, acute infection within 1 month before the study, advanced liver disease, gastrointestinal dysfunction requiring parental nutrition, or active malignancy. Use of the following medications were also exclusion criteria: anti-inflammatory medication other than aspirin <325 mg/d, immunosuppressive drugs within 1 month before the study, vitamin E >60 IU/d, or vitamin C >500 mg/d. Participants with a history of myocardial infarction or cerebrovascular event within 3 months before the study or with an ejection fraction <40% were excluded from this study. Other exclusion criteria were a history of ACE inhibitor–associated angioedema or cough, inability to discontinue ACE inhibitors or ARBs, pregnancy, breastfeeding, or child-bearing potential.
Study Protocol
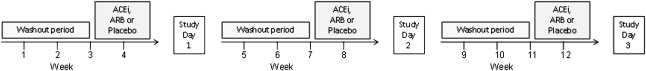
This study used a randomized, double-blind, placebo-controlled 3×3 crossover design (Figure 5). Participants were randomized to treatment with ramipril (King Pharmaceuticals, Bristol, TN), valsartan (Novartis Pharmaceuticals, East Hanover, NJ), or placebo for 1 week. Patients receiving either ACE inhibitors or ARBs before the study discontinued that medication for 3 weeks (washout period); during this period, BP was closely monitored. If, at any time, systolic BP exceeded 160 mmHg or diastolic BP exceeded 100 mmHg, other antihypertensive medications were maximized. If BP remained high, the protocol called for the addition of either amlodipine or clonidine; only one participant required the addition of amlodipidine to control BP. After any initial washout period, participants were randomly assigned to one of the six study sequences for a 3×3 orthogonal Latin square design specified in Jones and Kenward.61 Each patient underwent three different 7-day treatment arms as follows: ramipril (initiated at 2.5 mg/d for 2 days, followed by 5 mg/d), valsartan (80 mg/d for 2 days, followed by 160 mg/d), and matching placebo. On the seventh day of treatment, patients took their medication early in the morning and reported to the Vanderbilt General Clinical Research Center for hemodialysis, blood sampling, and hemodynamic monitoring. During hemodialysis, BP was measured every 5 minutes in the arm contralateral to the vascular access using an automated device (Dinamap; Critikon, Carlsbad, CA). Blood samples were collected at the beginning of hemodialysis, 30 minutes and 1 hour after the initiation of dialysis, at the end of dialysis, and 2 hours after the completion of dialysis. After the first study day, participants entered a second washout period and completed two more cycles of treatment.
Figure 5.
Randomized double blind 3×3 crossover study. Doses are specified in the text.
Laboratory Procedures
Blood was centrifugated immediately after collection and plasma or serum was stored at −80°C until analysis. Serum IL-1β, IL-6, IL-8, IL-10, IL-12p70, and IL-17 were measured using Luminex immunoassay technology. Monocyte chemoattractant protein-1 concentrations were measured using commercially available kits (Linco Research, St. Charles, MO). F2-isoprostanes were measured in plasma separated from EDTA-anticoagulated blood using negative ion gas-chromatography mass spectroscopy as previously described.62 PAI-1, tPA, D-dimers, vWf, and sCD40L were measured in plasma from blood anticoagulated with 0.105 M sodium citrate. D-dimer levels were measured using a commercial kit (TintElize; Biopool, Berkeley Heights, NJ). PAI-1 and tPA antigens were determined using commercial ELISA kits (TriniLIZE; Trinity Biotech, Berkeley Heights, NJ). ELISA was also used to determine the levels of vWf (American Diagnostica Inc, Stamford, CT) and sCD40L (Quantikine; R&D Systems, Minneapolis, MN). PRA and aldosterone were determined using RIA; ACE activity was measured by kinetic analysis, as previously described.63 Blood for measurement of bradykinin was drawn into cold anhydrous ethanol and centrifuged after 1 hour; the supernatant was saved at −80°C until the time of assay. Bradykinin concentrations were determined by use of a commercially available enzyme immunoassay (Bachem; Peninsula Laboratories, San Carlos, CA).
Statistical Analyses
Data are presented as mean ± SEM. Using a coefficient of variation of 0.39 in IL-6 concentrations and a correlation of 0.7 between measurements taken in the same patient, the sample size was calculated to give 90% power to detect a 20% difference in the effect of ACE inhibitors and ARBs on IL-6 in this crossover study, on the basis of our previously reported data.12 We used general linear model as detailed in Chapter 5 of Jones and Kenward61 to test for a carry-over effect using PRA and ACE activity. Repeated-measurements ANOVA was used to analyze changes in hemodynamic parameters. Linear mixed-effect models were used to evaluate the effect of drug treatment (placebo, ramipril, and telmisartan) and time after initiation of dialysis (0, 0.5, 1, 4, and 6 hours) on biomarkers. Model selection was based in the lower Akaike's information criterion. Sex, race, and age were included as covariates if there was evidence for an interactive effect of these variables. Bradykinin concentrations were natural log-transformed before analysis. Two-sided P<0.05 was considered significant. Data analysis was performed using SPSS (v.17.0; SPSS Inc, Chicago, IL) and SAS for Windows (Version 9; SAS Institute, Cary, NC) software.
Disclosures
None.
Acknowledgments
The authors thank Ms. Delia Woods for assistance with patient recruitment, carrying out the research protocol, and data entry. We also thank Mr. Anthony DeMatteo, Zuofei Wang, and Jeff Petro for laboratory assistance.
This work was supported by NIH/NHLBI Grant R01 HL065193, NIH/NIDKK Grant K24 DK62849, a grant from Vanderbilt University's Clinical and Translational Science Award (CTSA) program (UL1 RR024975 from the NCRR/NIH), and NIGMS/NIH Grant T32 GM007569.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Crossroad of RAAS Modulation, Inflammation, and Oxidative Stress in Dialysis Patients: Light at the End of the Tunnel?” on pages 189–191.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, LeveyA S: Epidemiology of cardiac disease in dialysis patients. Semin Dial 12: 69–76, 1999 [Google Scholar]
- 3.Herzog CA, Ma JZ, Collins AJ: Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med 339: 799–805, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C, Tripepi G, Mallamaci F: Predictors of cardiovascular death in ESRD. Semin Nephrol 25: 358–362, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Himmelfarb J: Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial 22: 636–643, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J: Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol 58: 190–197, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB: Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int 59: 1960–1966, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himmelfarb J, Hakim RM: Oxidative stress in uremia. Curr Opin Nephrol Hypertens 12: 593–598, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Morena M, Delbosc S, Dupuy AM, Canaud B, Cristol JP: Overproduction of reactive oxygen species in end-stage renal disease patients: A potential component of hemodialysis-associated inflammation. Hemodial Int 9: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Caglar K, Peng Y, Pupim LB, Flakoll PJ, Levenhagen D, Hakim RM, Ikizler TA: Inflammatory signals associated with hemodialysis. Kidney Int 62: 1408–1416, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Friedrich B, Alexander D, Janessa A, Häring HU, Lang F, Risler T: Acute effects of hemodialysis on cytokine transcription profiles: Evidence for C-reactive protein-dependency of mediator induction. Kidney Int 70: 2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Macfelda K, Weiss TW, Kaun C, Breuss JM, Zorn G, Oberndorfer U, Voegele-Kadletz M, Huber-Beckmann R, Ullrich R, Binder BR, Losert UM, Maurer G, Pacher R, Huber K, Wojta J: Plasminogen activator inhibitor 1 expression is regulated by the inflammatory mediators interleukin-1α, tumor necrosis factor-α, transforming growth factor-β and oncostatin M in human cardiac myocytes. J Mol Cell Cardiol 34: 1681–1691, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Segarra A, Chacón P, Martinez-Eyarre C, Argelaguer X, Vila J, Ruiz P, Fort J, Bartolomé J, Camps J, Moliner E, Pelegrí A, Marco F, Olmos A, Piera L: Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: Biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol 12: 1255–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. The Heart Outcomes Prevention Evaluation Study Investigators: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 342: 145–153, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. CHARM Investigators and Committees: Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 362: 777–781, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Zannad F, Kessler M, Lehert P, Grünfeld JP, Thuilliez C, Leizorovicz A, Lechat P: Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70: 1318–1324, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Cice G, Di Benedetto A, D’Isa S, D’Andrea A, Marcelli D, Gatti E, Calabrò R: Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol 56: 1701–1708, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Chan KE, Ikizler TA, Gamboa JL, Yu C, Hakim RM, Brown NJ: Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int 80: 978–985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ: Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med 339: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Murphey LJ, Malave HA, Petro J, Biaggioni I, Byrne DW, Vaughan DE, Luther JM, Pretorius M, Brown NJ: Bradykinin and its metabolite bradykinin 1-5 inhibit thrombin-induced platelet aggregation in humans. J Pharmacol Exp Ther 318: 1287–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Vanhoutte PM: Endothelium and control of vascular function. State of the art lecture. Hypertension 13: 658–667, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Brunius G, Domeij H, Gustavsson A, Yucel-Lindberg T: Bradykinin upregulates IL-8 production in human gingival fibroblasts stimulated by interleukin-1β and tumor necrosis factor α. Regul Pept 126: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Santos DR, Calixto JB, Souza GE: Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats. Br J Pharmacol 139: 271–278, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong P, Stafforini DM, Brown NJ, Pretorius M: Increased blood flow induces oxidative stress through an endothelium- and nitric oxide-independent mechanism. Free Radic Biol Med 49: 301–305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD: Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 29: 739–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renaux JL, Thomas M, Crost T, Loughraieb N, Vantard G: Activation of the kallikrein-kinin system in hemodialysis: Role of membrane electronegativity, blood dilution, and pH. Kidney Int 55: 1097–1103, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Marney AM, Ma J, Luther JM, Ikizler TA, Brown NJ: Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol 20: 2246–2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aukrust P, Damas JK, Solum NO: Soluble CD40 ligand and platelets: Self-perpetuating pathogenic loop in thrombosis and inflammation? J Am Coll Cardiol 43: 2326–2328, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ: Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension 48: 1050–1057, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Luft FC: Angiotensin, inflammation, hypertension, and cardiovascular disease. Curr Hypertens Rep 3: 61–67, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kranzhöfer R, Schmidt J, Pfeiffer CAH, Hagl S, Libby P, Kübler W: Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1623–1629, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Souza DG, Pinho V, Pesquero JL, Lomez ES, Poole S, Juliano L, Correa A, Jr, de A Castro MS, Teixeira MM: Role of the bradykinin B2 receptor for the local and systemic inflammatory response that follows severe reperfusion injury. Br J Pharmacol 139: 129–139, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiffany CW, Burch RM: Bradykinin stimulates tumor necrosis factor and interleukin-1 release from macrophages. FEBS Lett 247: 189–192, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Pan ZK, Ye RD, Christiansen SC, Jagels MA, Bokoch GM, Zuraw BL: Role of the Rho GTPase in bradykinin-stimulated nuclear factor-kappaB activation and IL-1β gene expression in cultured human epithelial cells. J Immunol 160: 3038–3045, 1998 [PubMed] [Google Scholar]
- 37.Locatelli F, Canaud B, Eckardt K-U, Stenvinkel P, Wanner C, Zoccali C: Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant 18: 1272–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Brechter AB, Lerner UH: Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum 56: 910–923, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Adner M, Cardell LO: IL-1beta-induced transcriptional up-regulation of bradykinin B1 and B2 receptors in murine airways. Am J Respir Cell Mol Biol 36: 697–705, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Kato A, Odamaki M, Takita T, Maruyama Y, Kumagai H, Hishida A: Association between interleukin-6 and carotid atherosclerosis in hemodialysis patients. Kidney Int 61: 1143–1152, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Seyrek N, Karayaylali I, Balal M, Paydas S, Aikimbaev K, Cetiner S, Seydaoglu G: Is there any relationship between serum levels of interleukin-10 and atherosclerosis in hemodialysis patients? Scand J Urol Nephrol 39: 405–409, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Girndt M, Kaul H, Sester U, Ulrich C, Sester M, Georg T, Köhler H: Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 62: 949–955, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Aslam S, Santha T, Leone A, Wilcox C: Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int 70: 2109–2115, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Annuk M, Lind L, Linde T, Fellström B: Impaired endothelium-dependent vasodilatation in renal failure in humans. Nephrol Dial Transplant 16: 302–306, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Annuk M, Zilmer M, Lind L, Linde T, Fellström B: Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol 12: 2747–2752, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Pretorius M, Rosenbaum DA, Lefebvre J, Vaughan DE, Brown NJ: Smoking impairs bradykinin-stimulated t-PA release. Hypertension 39: 767–771, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Brown NJ: Blood pressure reduction and tissue-type plasminogen activator release. Hypertension 47: 648–649, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Ambühl PM, Wüthrich RP, Korte W, Schmid L, Krapf R: Plasma hypercoagulability in haemodialysis patients: Impact of dialysis and anticoagulation. Nephrol Dial Transplant 12: 2355–2364, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Miozzari M, Wahl C: D-dimers in hemodialysis patients. Nephron 88: 278–279, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Yu A, Egberg N, Jacobson SH: Haemostatic complications in haemodialysis patients: Effect of type of vascular access and dialysis filter. Scand J Clin Lab Invest 63: 127–133, 2003 [PubMed] [Google Scholar]
- 52.Hocher B, Ziebig R, Altermann C, Krause R, Asmus G, Richter CM, Slowinski T, Sinha P, Neumayer HH: Different impact of biomarkers as mortality predictors among diabetic and nondiabetic patients undergoing hemodialysis. J Am Soc Nephrol 14: 2329–2337, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Ceconi C, Fox KM, Remme WJ, Simoons ML, Deckers JW, Bertrand M, Parrinello G, Kluft C, Blann A, Cokkinos D, Ferrari R: ACE inhibition with perindopril and biomarkers of atherosclerosis and thrombosis: Results from the PERTINENT study. Atherosclerosis 204: 273–275, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Cesari M, Kritchevsky SB, Atkinson HH, Penninx BW, Di Bari M, Tracy RP, Pahor M: Angiotensin-converting enzyme inhibition and novel cardiovascular risk biomarkers: Results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. Am Heart J 157: 334–, e1–e8., 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ceconi C, Fox KM, Remme WJ, Simoons ML, Bertrand M, Parrinello G, Kluft C, Blann A, Cokkinos D, Ferrari R. EUROPA Investigators PERTINENT Investigators and the Statistical Committee: ACE inhibition with perindopril and endothelial function. Results of a substudy of the EUROPA study: PERTINENT. Cardiovasc Res 73: 237–246, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. CAPTURE Study Investigators: Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 348: 1104–1111, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Lim PS, Wu MY, Chien SW, Wu TK, Liu CS, Hu CY, Chang HC, Pai MA: Elevated circulating levels of soluble CD-40 ligand in haemodialysis patients with symptomatic coronary heart disease. Nephrology (Carlton) 13: 677–683, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK: Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol 108: 96–100, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Ueland T, Aukrust P, Yndestad A, Otterdal K, Frøland SS, Dickstein K, Kjekshus J, Gullestad L, Damås JK: Soluble CD40 ligand in acute and chronic heart failure. Eur Heart J 26: 1101–1107, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Tveit A, Seljeflot I, Grundvold I, Abdelnoor M, Smith P, Arnesen H: Effect of candesartan and various inflammatory markers on maintenance of sinus rhythm after electrical cardioversion for atrial fibrillation. Am J Cardiol 99: 1544–1548, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Jones B, Kenward MG: Design and Analysis of Crossover Trials, 2nd Ed., Boca Raton, FL, CRC Press LLC, 2003, pp 212–213 [Google Scholar]
- 62.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ, 2nd: A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 87: 9383–9387, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurst PL, Lovell-Smith CJ: Optimized assay for serum angiotensin-converting enzyme activity. Clin Chem 27: 2048–2052, 1981 [PubMed] [Google Scholar]