Abstract
For xenotransplantation to become a clinical reality, we need to better understand the mechanisms of graft rejection or acceptance. We examined pathologic changes in α1,3-galactosyltransferase gene-knockout pig kidneys transplanted into baboons that were treated with a protocol designed to induce immunotolerance through thymic transplantation (n=4) or were treated with long-term immunosuppressants (n=3). Hyperacute rejection did not occur in α1,3-galactosyltransferase gene-knockout kidney xenografts. By 34 days, acute humoral rejection led to xenograft loss in all three xenografts in the long-term immunosuppression group. The failing grafts exhibited thrombotic microangiopathic glomerulopathy with multiple platelet-fibrin microthrombi, focal interstitial hemorrhage, and acute cellular xenograft rejection. Damaged glomeruli showed IgM, IgG, C4d, and C5b-9 deposition. They also demonstrated endothelial cell death, diffuse endothelial procoagulant activation with high expression of tissue factor and vWF, and low expression of the ectonucleotidase CD39. In contrast, in the immunotolerance group, two of four grafts had normal graft function and no pathologic findings of acute or chronic rejection at 56 and 83 days. One of the remaining kidneys had mild but transient graft dysfunction with reversible, mild microangiopathic glomerulopathy, probably associated with preformed antibodies. The other kidney in the immunotolerance group developed unstable graft function at 81 days and developed chronic xenograft glomerulopathy. In summary, the success of pig-to-primate xenotransplantation may necessitate immune tolerance to inhibit acute humoral and cellular xenograft rejection.
The use of animal organs might alleviate the critical worldwide shortage of donor organs for clinical transplantation.1,2 Miniature swine have been proposed as a suitable donor species because of their anatomic and physiologic similarities to human organs, breeding characteristics, and potential for genetic modification.2–4 Transplantation of porcine organs into primates results in hyperacute rejection of the xenografts, with subsequent acute humoral xenograft rejection (AHXR), also known as acute vascular rejection or delayed xenograft rejection.1–6 Both hyperacute rejection and AHXR are triggered by the binding of xenoreactive natural antibodies to a specific epitope (galactose α1-3 galactose [Gal]) on the porcine vascular endothelium.7,8 To date, considerable effort has been applied to the depletion of anti-Gal antibodies and inhibition of complement in pig-to-nonhuman primate models.9–13 However, despite the successful prevention of hyperacute rejection by these methods, with the return or continuing presence of anti-Gal antibodies, all swine xenografts ultimately succumb to AHXR, resulting in graft failure within a few weeks.10–13
To overcome this problem, α1,3-galactosyltransferase gene-knockout (GalT-KO) pigs, which do not express the Gal epitope, have been developed.14–18 We have reported the clinical and immunologic outcomes of our initial trial of life-supporting kidney xenotransplantation from GalT-KO pigs to baboons treated with long-term immunosuppression (immunosuppression protocol) or a protocol designed to induce tolerance though thymic transplantation (immunotolerance protocol).19 None of the grafts succumbed to hyperacute rejection. However, all GalT-KO kidney grafts in animals receiving the immunosuppression protocol failed within 34 postoperative days.19 In contrast, life-supporting kidney grafts in the immunotolerance group survived 56–83 days, with normal or only slightly elevated creatinine levels.19
The present study is an extension to the earlier work and was designed to assess the pathologic characteristics of the GalT-KO kidney xenografts in baboons treated with immunosuppression or immunotolerance protocols.
Results
Clinical Findings in Animals
We have previously reported on the clinical outcomes of GalT-KO kidneys in baboons treated with immunosuppression or an experimental protocol designed to induce immunotolerance.19 In the present study, we examined the detailed pathologic features of kidney samples from the three baboons (B114, B126, and B131) in the immunosuppression group and the four baboons (B113, B135, B118, and B134) in the immunotolerance group; we have previously reported on all the animals.19 Hyperacute rejection did not develop in any of the GalT-KO grafts. However, in the immunosuppressed baboons, graft dysfunction developed at postoperative day 7 in all three grafts that showed complete graft failure by postoperative day 34. In the immunotolerance group, the grafts were all prolonged but showed variable clinical courses. On the basis of clinical outcome, the animals could be divided into three groups: One animal (B113) developed mild graft dysfunction from postoperative days 7 to 40, with gradual improvement and persistent stable renal function thereafter; a second animal (B118) showed mild and gradually progressive graft dysfunction starting on postoperative day 50 and progressing to death on day 81; and two animals (B135 and B134) showed persistently stable graft function beyond postoperative day 50. Baboon B134 survived with normal renal function until postoperative day 83. All four immunotolerant baboons died with functioning kidneys on postoperative days 56, 68, 81, and 83, respectively, from nonrenal causes (Table 1).
Table 1.
Experimental groups, immunosuppression, graft survival, day of biopsies, and pathologic diagnosis
| Animal ID | Protocols (Thymus Transplantation)a | Immunosuppressionb | Graftectomy (Postoperative Day)c | Death (Postoperative Day) | Cause of Death | Morphologic Changes |
|---|---|---|---|---|---|---|
| B114 | IS | PTCD | 33 | AHXR (advanced) | ||
| B126 | IS | TCD-2 | 34 | Arrhythmia (K+ >10 mmol/L) | AHXR (advanced) | |
| B131 | IS | PTCD | 20 | Glomerulopathy (marked) | ||
| B113 | TI (VTL) | TCD-1 | 68 | Anesthetic problem | No obvious rejection | |
| B135 | TI (VTL) | TCD-2 | 56 | Cardiac infarction | No obvious rejection | |
| B118 | TI (TK) | TCD-2 | 81 | Pneumonia | Mild chronic graft injuries | |
| B134 | TI (TK) | TCD-2a | 83 | Heart failure | No obvious rejection |
IS, immunosuppression; PTCD, partial T cell depletion regimen; AHXR, acute humoral xenograft rejection; TC2-2, T cell depletion regimen-2; TI, tolerance-inducing; VTL, vascularized thymic lobe; TCD-1, T cell depletion regimen-1; TK, thymokidney.
Protocols: Life-supporting kidney transplantation was performed with long-term immunosuppression or tolerance-inducing protocols that were mediated by thymic transplantation, vascularized thymic lobe, or thymokidney.
Immunosuppression: partial T cell depletion regimen: three doses of antithymocyte globulin plus three to four doses of rat antibody specific for human CD2 (LoCD2), anti-CD154 mAb, mycophenolate mofetil, and steroids; T cell depletion regimen-1 (thymectomy; splenectomy; and three doses of antithymocyte globulin plus three to four doses of LoCD2, anti-CD154 antibody, mycophenolate mofetil, and steroids); T cell depletion regimen-2 (thymectomy, splenectomy, 100 cGy whole-body irradiation on day −6, two doses of LoCD2, two doses of antithymocyte globulin, anti-CD154 antibody, mycophenolate mofetil, and steroids); and T cell depletion regimen-2a, which was the same as T cell depletion regimen 2 but without steroids.
Graftectomy was performed when recipients demonstrated clinical evidence of graft rejection or severe deterioration of clinical condition, as evident by uremia (BUN >100 mg/dl).
Acute Humoral Xenograft Rejection in Immunosuppressed Baboons
In the early posttransplantation period at postoperative day 12, IgM was clearly positive and IgG was weakly positive in glomeruli, with only minimal deposition noted in the peritubular capillaries (Figure 1). During the progression of graft failure by postoperative day 34, deposition of IgM, IgG, C4d, and C5b-9 increased in glomeruli, small arteries, and peritubular capillaries. On postoperative day 12, focal and segmental glomerulopathy was noted with terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate-biotin nick end-labeling (TUNEL)+ dead cells and CD41+ platelets, but interstitial and small arterial injuries were minimal, with only a few TUNEL+ cells and CD41+ platelets (Figures 1 and 2). These findings indicated that the glomeruli were the first site of anti-nonGal antibody deposition and complement-mediated graft injury in AHXR in GalT-KO kidneys. Progression of AHXR was characterized by diffuse, global, and moderate-to-severe thrombotic microangiopathic glomerulopathy in all graftectomy samples by postoperative day 34. The interstitium showed focal cellular infiltration and focal hemorrhage. TUNEL+ cells and CD41+ platelets were prominent in the glomeruli, and a few were noted in the peritubular capillaries and arteries (Figures 1 and 2).
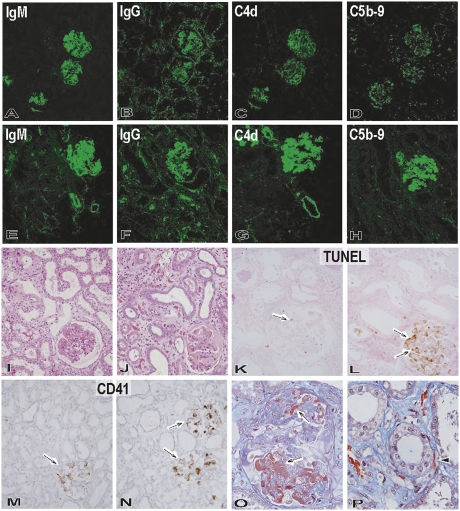
Figure 1.
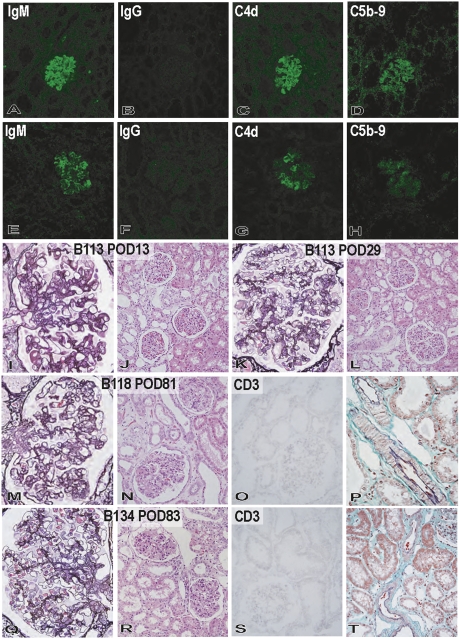
Pathologic features of the grafts during progression of graft failure in the immunosuppression protocol. Immunofluorescence studies of IgM (A and E: original magnification, ×200), IgG (B and F: original magnification, ×200), C4d (C and G: original magnification, ×200), and C5b-9 (D and H: original magnification, ×200) in the grafts (B114) at postoperative days 12 (A–D) and 33 (E–H) showed progression of immunoglobulin and complement deposition in glomeruli and small arteries and focally along peritubular capillaries. Development of graft failure, from postoperative days 12 (I, K, and M) to 33 (J, L, N–P), was associated with the development of thrombotic microangiopathic glomerulopathy (I and J: hematoxylin and eosin stain; original magnification, ×200) with TUNEL+ dead cells (arrow) (K and L: TUNEL stain, original magnification, ×200), CD41+ activated platelets (arrow) (M and N: CD41 stain; original magnification, ×200), and fibrin exudation (arrow) (O: Masson stain; original magnification, ×600). Interstitial edema and focal hemorrhage (J) and fibrin thrombi (arrowhead) in peritubular capillaries (P: Masson stain; original magnification, ×600) were also evident in the interstitium at postoperative day 33.
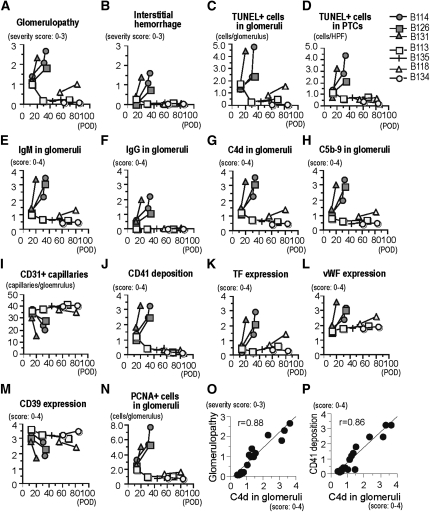
Figure 2.
Pathologic differences between immunosuppression (B114, B126, and B131) and immunotolerance (B113, B135, B118, and B134) protocols. The pathologic characteristics were evaluated semiquantitatively for the severity of glomerulopathy (A); the degree of interstitial hemorrhage (B); the number of TUNEL+ dead cells in glomeruli (C) and peritubular capillaries (PTCs) (D); the glomerular deposition of IgM (E), IgG (F), C4d (G), and C5b-9 (H); the number of CD31+ glomerular capillaries (I); the deposition of CD41 platelets (J); the expression of tissue factor (K), vWF (L), and CD39 (M); and the number of PCNA+ proliferating glomerular cells (N). Correlation between C4d deposition in glomeruli and the severity of glomerulopathy (O) or CD41 deposition (P) was also evaluated. POD, postoperative day.
In the immunosuppression animals, thrombotic microangiopathic glomerulopathy was the characteristic pathologic feature from the early post-transplantation period through the progression of AHXR (Figures 2–4). Development of glomerulopathy was characterized by a rapid increase in IgM, IgG, C4d, and C5b-9 deposition in glomeruli, along with multiple thrombi formation, fibrin exudation, loss of capillaries with dead and activated endothelial cells, mesangiolysis, and mesangial proliferative lesions (Figures 2–4). The multiple thrombi seen in injured glomeruli were CD41+ platelet-rich fibrin thrombi present in glomerular capillaries, together with extensive deposition of IgM and C4d (Figure 3). The severity of glomerulopathy and the extent of CD41+ platelet deposition correlated significantly with the deposition of C4d, indicating that thrombotic microangiopathic glomerulopathy is a characteristic feature of AHXR (Figure 2).
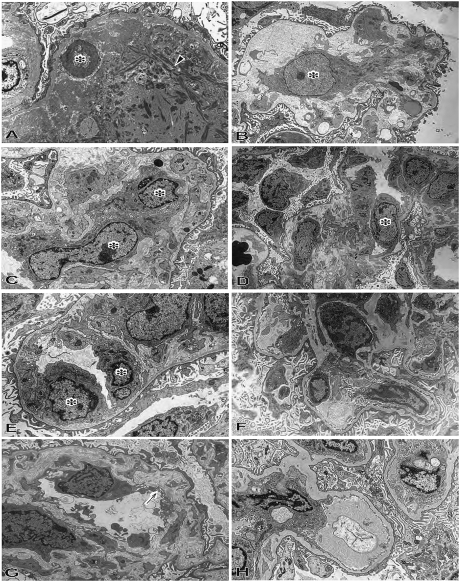
Figure 4.
Electron microscopic findings of xenografts of the immunosuppression and immunotolerance protocols. In thrombotic microangiopathic glomerulopathy at postoperative day 33 in B114 in the immunosuppression protocol, the glomerular capillary structure was lost with apoptotic cell (asterisk) and fibrin deposition (A: original magnification, ×5000), mesangial matrix-lysis and activated and enlarged mesangial cells (asterisk) (B: original magnification, ×4000), and activated and enlarged endothelial cells (asterisk) (C: original magnification, ×6000). In reversible microangiopathic glomerulopathy in the immunotolerant baboon (B113), swelling of endothelial cells (asterisk) was noted in glomeruli with partial dilatation of subendothelial space and mesangiolytic changes at postoperative day 13 (D: original magnification, ×2000; E; original magnification, ×7000). However, in the immunotolerance group, these findings recovered to almost intact glomerular structure by postoperative day 29 (B113: F; original magnification, ×2000). The glomeruli in the graft with unstable graft function from postoperative day 50 showed double contour of glomerular basement membrane (arrow in G), mesangial proliferation, and effacement of foot processes of podocytes at postoperative day 81 (B118: G; original magnification, ×7000). The glomeruli in the graft with stable graft function showed almost intact glomerular capillaries with preservation of foot process of podocytes at postoperative day 83 (B134: H; original magnification, ×5000).
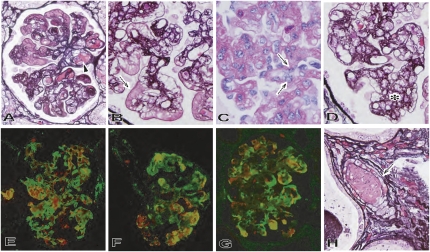
Figure 3.
Development of thrombotic microangiopathic glomerulopathy and arteriolopathy in the immunosuppression protocol (A, F, and G: B114, postoperative day 33; B and H: B131, postoperative day 20; C and D: B126, postoperative day 34). Thrombotic microangiopathic glomerulopathy developed with multiple thrombi (arrowhead in A: periodic acid-methenamine [PAM] stain; original magnification, ×600), fibrin exudation (arrow in B: PAM stain; original magnification, ×800), enlarged endothelial cells (arrow in C: PAS stain; original magnification, ×800) and mesangial proliferative lesion (asterisk in D: PAM stain; original magnification, ×800). Almost all CD41+ platelet-rich thrombi were detected with fibrin; within vessels they were detected with deposition of IgM and C4d (double immunostaining against CD41 [red] and green of fibrin [E], IgM [F] or C4d [G]; original magnification, ×600). Thrombosis (arrow) was also evident in small arteries (H: PAM stain; original magnification, ×800).
Lack of Acute Humoral Xenograft Rejection in Immunotolerance Baboons
In B113, an animal with initial mild graft dysfunction, the deposition of IgM (but not IgG) was evident at postoperative day 13, together with the deposition of C4d and C5b-9 (Figure 5). Mild microangiopathic glomerulopathy developed in the kidney. Cellular infiltration was not prominent, and no obvious alterations were seen in the tubulointerstitium or arteries. Although the deposition patterns of immunoglobulins and complement at postoperative day 29 were similar to those noted at postoperative day 13, recovery of thrombotic microangiopathic glomerulopathy was noted, together with repair of glomerular capillaries in damaged glomeruli. In B118, an animal with late unstable graft function, the deposition of IgM, C4d, and C5b-9, but not IgG, was noted at postoperative 55, with mild microangiopathic glomerulopathy but no obvious cellular infiltration or arterial injury (Figures 4 and 5). At postoperative day 81 in B118, focal chronic transplant glomerulopathy characterized by focal segmental double contour of glomerular basement membrane and mesangial proliferative lesions developed (Figures 4 and 5), with minimal IgM, C4d, and C5b-9 deposition. Focal interstitial fibrosis was noted, but arterial changes and acute cellular xenograft rejection (ACXR) were not detected. The presence of chronic transplant glomerulopathy and focal interstitial fibrosis indicated chronic graft injury with late unstable graft function by postoperative day 81. In B134 and B135, animals that had stable graft function, no obvious thrombotic microangiopathic glomerulopathy developed; rather, the changes were limited to focal IgM, C4d, and C5b-9 deposition in glomeruli (Figures 4 and 5). No IgG deposition was detected. Cellular rejection and arterial injury were not seen in the grafts. These pathologic findings demonstrated that AHXR, ACXR, and chronic xenograft rejection did not develop in these grafts.
Figure 5.
Pathologic changes in the grafts of the immunotolerance protocol. In reversible glomerular injury in B113, deposition of IgM (A), C4d (C), and C5b-9 (D), but not IgG (B), occurred by postoperative day 13 (A–D: original magnification, ×200), and mild thrombotic microangiopathic glomerulopathy developed (I: periodic acid-methenamine [PAM] stain; original magnification, ×600). Cellular rejection and arteriolar changes were not detected at postoperative day 13 in B113 (J: hematoxylin and eosin stain; original magnification, ×200). However, these glomerular alterations recovered to almost their structure by postoperative day 29 in B113 (K: PAM stain; original magnification, ×600). Cellular rejection, arteriolar changes, and interstitial fibrosis did not develop at postoperative day 29 in B113 (L: hematoxylin and eosin stain; original magnification, ×200). In the graft with unstable graft function (B118), focal mesangial proliferation with double contour of glomerular basement membrane were seen at postoperative day 81 (M: PAM stain; original magnification, ×600). Focal interstitial fibrosis was noted, but cellular rejection and arterial changes were not detected (N: hematoxylin and eosin stain; original magnification, ×200; O: CD3 stain; original magnification, ×200; P: elastica-Masson Goldner stain; original magnification, ×600). In the graft with stable graft function (B134) at postoperative day 83, focal and weak deposition of IgM (E), C4d (G), and C5b-9 (H) was seen, but no deposition of IgG (F) was noted (E–H: original magnification, ×200). The glomeruli showed no obvious glomerulopathy at postoperative day 83 in B134 (Q: PAM stain; original magnification, ×600) with no cellular rejection or vascular changes (R: hematoxylin and eosin stain; original magnification, ×200; S: CD3 stain; original magnification, ×200; T: elastica-Masson Goldner stain; original magnification, ×200). POD, postoperative day.
Glomerular Endothelial Cell Death and Activation, Associated with AHXR, in Immunosuppression and Immunotolerance Protocols
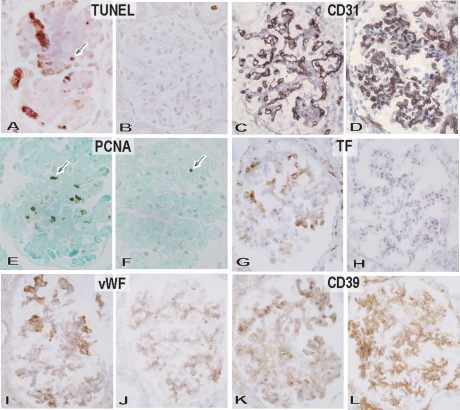
We next examined glomerular cell death and endothelial activation during the development of thrombotic microangiopathic glomerulopathy in the immunosuppression and immunotolerance protocols (Figures 2 and 6). The moderate to marked thrombotic microangiopathic glomerulopathy observed in the immunosuppression protocol was characterized by the presence of many TUNEL+ cells, loss of CD31+ glomerular capillaries, proliferating cell nuclear antigen (PCNA)+ proliferating cells, increased expression of tissue factor and vWF, and decreased expression of CD39 on glomerular endothelial cells. In thrombotic microangiopathic glomerulopathy, death of glomerular endothelial cells, loss of glomerular capillaries, and activation with procoagulation status of remaining endothelial cells developed in the AHXR. In contrast, in the immunotolerance protocol, the glomeruli contained no obvious TUNEL+ cells or PCNA+ cells, no increased expression of tissue factor or vWF, and intact CD31+ glomerular capillaries, together with CD39 expression on endothelia cells.
Figure 6.
Immunopathological characterization of thrombotic microangiopathic glomerulopathy. TUNEL+ dead cells (A and B: original magnification, ×600), CD31+ glomerular capillaries (C and D: original magnification, ×600), PCNA+ proliferating cells (E and F: original magnification, ×600), tissue factor expression (G and H: original magnification, ×600), vWF expression (I and J: original magnification, ×600), and CD39 expression (K and L: original magnification, ×600) in glomeruli of the immunosuppression (A, C, E, G, I, and K: B114, postoperative day 34) and immunotolerance (B, D, F, H, J, and L: B134, postoperative day 83) baboons. In the immunosuppression protocol, thrombotic microangiopathic glomerulopathy developed at postoperative day 34 in B114 with many TUNEL+ dead cells (arrow) (A), loss of CD31+ glomerular capillaries (C), proliferating PCNA+ cells (arrow) (E), increased expression of tissue factor (G) and vWF (I), and decreased expression of CD39 (K). In contrast, the glomeruli of the immunotolerance baboon (B134) at postoperative day 83 were characterized by preservation of CD31+ glomerular capillaries (D) with rare TUNEL+ dead cells (B) and PCNA+ cells (F), no upregulation of tissue factor (H) and vWF expression, and persistent CD39 expression (L).
Cellular Infiltration in Grafts of Immunosuppression and Immunotolerance Baboons
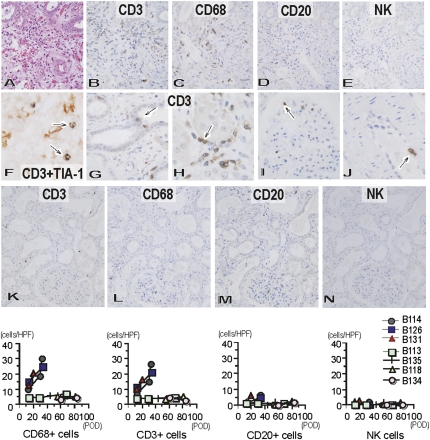
ACXR developed in the grafts of the immunosuppression protocol (Figure 7). During the early posttransplantation period, the cellular infiltrate was composed of polymorphonuclear leukocytes and CD68+ macrophages, which might be associated with AHXR, in addition to a small number of CD3+ T cells. In all grafts in the immunosuppression protocol, the number of infiltrating cells increased after transplantation, and the infiltrating cells comprised CD3+ T cells and CD68+ macrophages, as well as a small number of CD20+ B cells and NK cells. Two-color immunohistochemistry identified the presence of TIA-1+ cytotoxic granules in many infiltrating CD3+ T cells, indicating that they were cytotoxic T cells. Furthermore, CD3+ T cells infiltrated the tubules, peritubular capillaries, and glomerular capillaries and were seen underneath the endothelial cells of small arteries, with findings of tubulitis, capillaritis, acute glomerulitis, and endothelialitis. In contrast, in the immunotolerance protocol, only a few interstitial mononuclear cells were detected and no or little tubulitis, capillaritis, glomerulitis, or endothelialitis was observed.
Figure 7.
Cellular infiltration within Gal knockout kidneys in baboons treated with chronic immunosuppression or immunotolerance protocol. Cellular infiltration in immunosuppression (A–J: B114, postoperative day 34) and immunotolerance (K–N: B134, postoperative day 83) protocols. Cellular infiltration developed at postoperative day 34 (B114) (A: hematoxylin and eosin stain; original magnification, ×400) in the immunosuppression protocol with infiltration of CD3+ T cells (B: original magnification, ×400) and CD68+ macrophages (C: original magnification, ×400), although with little infiltration of CD20+ B cells (D: original magnification, ×400) and NK cells (E: original magnification, ×400). Many CD3+ (brown) T cells contained TIA-1+ (blue) cytotoxic granules (arrow) (F: original magnification, ×900), capillaritis, acute glomerulitis, and endothelialitis developed with CD3+ T cells (arrow) in tubules (G: original magnification, ×800), peritubular capillaries (H: original magnification, ×800), glomeruli (I: original magnification, ×800), and arteries (J: original magnification, ×800). In contrast, only small numbers of CD3+ T cells (K: original magnification, ×200) and CD68+ macrophages (L: original magnification, ×200), and very few CD20+ B cells (M: original magnification, ×200) and NK cells (N: original magnification, ×200) were present in the grafts at postoperative day 83 in B134 in the immunotolerance protocol. Analysis of the number of infiltrating CD3+ T cells, macrophages, CD20+ B cells, and NK cells in the grafts (cells/single field of 0.0625 mm2) indicated increased infiltration of T cells and macrophages in the grafts of the immunosuppression protocol, but not those of the immunotolerance protocol. HPF, high-power field; POD, postoperative day.
Acute and Chronic Rejection Score in Banff Classification 2007
In the xenografts (B114, B126, and B131) in the immunosuppression protocol, AHXR and ACXR developed by postoperative day 34, with a high acute rejection score according to Banff classification 2007 (Table 2).20 C4d deposition of peripheral tubular cells was also evident because the C4d sore was 2–3. On the other hand, in immunotolerance protocol, although one graft (B118) with unstable graft function had a mild chronic rejection score, the other three grafts with initial mild graft dysfunction (B113) and stable graft function (B135 and B134) had only a minimal acute and chronic rejection score.
Table 2.
Banff classification 2007 score of graftectomy samples
| Acute Rejection Score | Chronic Rejection Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal ID | i | t | g | v | ptc | C4d | ci | ct | cg | mm | cv |
| B114 | 1 | 1 | 2 | 3a | 3 | 3 | 2 | 2 | 0 | 3 | 0 |
| B126 | 1 | 1 | 1 | 3a | 2 | 2 | 1 | 1 | 0 | 2 | 0 |
| B131 | 1 | 1 | 1 | 3a | 3 | 2 | 0 | 1 | 0 | 2 | 0 |
| B113 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| B135 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| B118 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 |
| B134 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
i, mononuclear cell interstitial inflammation score (i0–i3); t, tubulitis score (t0–t3); g, early type of allograft glomerulitis score (g0–g3); v, intimal arteritis score (v0–v3); ptc, peritubular margination of inflammatory cells score (ptc0–ptc3); C4d, C4d score (C4d0–C4d3); ci, interstitial fibrosis score (ci0–ci3); ct, tubular atrophy score (ct0–ct3); cg, allograft glomerulopathy score (cg0–cg3); mm, mesangial matrix score (mm0–mm3); cv, fibrous intimal thickening score (cv0–cv3).
Presence of interstitial hemorrhage.
Discussion
We examined the differences in the pathologic findings of swine renal xenografts in long-term immunosuppression or immunotolerance protocols. We used GalT-KO Massachusetts General Hospital miniature pigs that do not express the Gal epitope as donors, thereby abrogating anti-Gal antibody–mediated rejection. All three GalT-KO kidneys under the long-term immunosuppression protocol were rejected by AHXR and ACXR by postoperative day 34; they were characterized pathologically by thrombotic microangiopathic glomerulopathy, focal interstitial hemorrhage, and cellular infiltration. In contrast, two of four kidneys from the immunotolerance protocol maintained normal graft function at postoperative days 56 and 83, without pathologic findings of AHXR, ACXR, or other signs of chronic xenograft rejection.
In our long-term immunosuppression protocol, AHXR and ACXR could not be overcome, despite intensive treatment. During the progression of graft failure, IgM, IgG, C4d, and C5b-9 deposits appeared in the grafts by postoperative day 34, and the increase in C4d deposition correlated strongly with platelet-fibrin thrombi formation and the development of thrombotic microangiopathic glomerulopathy. Focal interstitial hemorrhage was also evident. These findings indicate that AHXR develops even when the donor is the GalT-KO swine. Although the exact mechanism of AHXR is not yet clear, it is likely that anti-nonGal antibodies were involved.21–24 In the present study, anti-nonGal antibodies were primarily directed against endothelial cells because immunoglobulin was deposited in the capillary walls. In this regard, several reports have recently suggested that the epitopes on pig grafts recognized by human anti-nonGal antibodies include the swine leukocyte antigen, N-glycolyl neuraminic acid (NeuGc) epitopes (Hanganatziu-Deicher antigen, although this would not be a target in baboons because these animals also express this antigen), and the Forssman antigen.25 Further experiments are necessary to identify new target epitopes of these antibodies before consideration of strategies to prevent anti-nonGal antibody–mediated rejection, including the deletion or modification of nonGal epitopes by genetic engineering of the pig. Another possibility is suggested by several recent studies demonstrating that Gal could not be completely eliminated by using current GalT-KO technology because the enzyme iGb3 synthase can also synthesize Gal in GalT-KO mice and pigs.21,26,27 However, because AHXR did not develop in the two GalT-KO kidney grafts with stable graft function in the present study, we consider it likely that their tissue expressed no Gal.
In thrombotic microangiopathic glomerulopathy, which is similar to the glomerulopathy seen in human decay accelerating factor-transgenic swine–to–baboon kidney transplantation,28 both cell death and activation of endothelial cells were noted and are probably related to immunoglobulin deposition and complement activation. Cell death and activation of glomerular endothelial cells with the presence of TUNEL+ cells and PCNA+ cells indicated that increased expression of vWF and tissue factor, and decreased expression of CD39, may have contributed to thrombotic formation because these changes are associated with an inevitable shift from an anticoagulant to a procoagulant state, further compounded by intrinsic molecular barriers in thromboregulation.1 This type of glomerulopathy is considered the next major hurdle for xenotransplantation.29
The characterization of cellular rejection in vascularized xenograft models is still limited by the rapid development of AHXR. In our immunosuppression protocol, cellular infiltration developed in the xenografts. In addition to neutrophil and CD68+ macrophage infiltration, which may be associated with AHXR, many CD3 and TIA-1+ cytotoxic T cells were also present in the infiltrate with tubulitis, peritubular capillaritis, acute glomerulitis, and endothelialitis. These findings suggest that T cell–dependent immunologic pathways play an important role in xenograft rejection. Recent studies also demonstrated the involvement of T cells in kidney xenograft rejection in miniature swine or human decay accelerating factor pig–to–baboon model and in heart xenografts in GalT-KO swine–to–baboon transplantation.28,30–32 In vitro assays also showed T cell sensitization and T cell–dependent cytotoxic antibody production after transplantation in recipients of the long-term immunosuppression protocol.19,33 As is seen with allotransplantation, ACXR may be a more important and common type of xenograft rejection, and humoral rejection might be avoided by various strategies directed at T- and B-cell immunity.
In clinical kidney transplantation, chronic allograft glomerulopathy is a characteristic pathologic finding of chronic rejection.34,35 Ample evidence suggests that chronic rejection of allografts is caused by a combination of chronic immune and nonimmune injury, and that the immune mechanism involves both cellular and humoral rejection.34,35 In addition, in the immunotolerance protocol in pig allokidney transplantation, induction of unstable tolerance of kidney grafts is associated with late progression of chronic rejection.36,37 In the present study, chronic xenograft glomerulopathy developed in the graft at postoperative day 81 in B118, with late unstable graft function in the immunotolerance protocol. The pathologic features of this glomerulopathy are similar to those seen with chronic allograft glomerulopathy in clinical kidney allotransplantation.35 Progressive AHXR and ACXR were not detected in any graft from baboons receiving the immunotolerance protocol. However, before transplantation, small amounts of cytotoxic anti-nonGal antibodies were present in most of the baboons.19 Indeed, one (B113) kidney showed mild and transient graft dysfunction from postoperative days 7 to 40 in association with mild thrombotic microangiopathic glomerulopathy involving deposition of IgM and complements.
We think that in this protocol chronic xenograft glomerulopathy may be associated with unstable induction of immunotolerance, graft injury from preformed antibodies, or persistent nonimmunologic graft injury, potentially involving the deregulation of coagulation and platelet activation in the xenogeneic vasculature; these changes are similar to what is observed in allokidney transplantation.36,37 Previous studies have also reported the presence of variable amounts of preformed anti-nonGal antibodies in humans.38 Additional induction therapy to reduce the levels of preformed anti-nonGal antibodies and to prevent initial graft injury associated with preformed anti-nonGal antibodies may be required to ensure long-term xenograft survival. Transgenic modification of swine to allow overexpression of human anticoagulant and thromboregulatory factors such as CD39 may be also further required for long-term xenograft survival.1
Clinical application of xenotransplantation will first require the demonstration of its efficacy in a nonhuman primate model. In this study, the response to GalT-KO kidney xenografts in baboons was not controlled by our long-term immunosuppression protocol, and AHXR and ACXR developed in the grafts by postoperative day 34. In contrast, the immunotolerance protocol prevented AHXR, ACXR, and chronic xenograft rejection in two of four GalT-KO kidney grafts by postoperative days 56 and 83. Unfortunately, in this study, all the animals in the immunotolerance protocol died of complications not associated with rejection. We believe that improved strategies for rapid and stable induction of xenogeneic T cell tolerance and prevention of side effects of immunosuppression drugs, such as thrombotic complications and infection, may be required for long-term xenograft survival. Ongoing studies in our center are designed to induce immunotolerance toward GalT-KO miniature swine organs in nonhuman primates by bone marrow transplantation and donor vascularized thymus grafting and by additional transgenesis to protect the renal vasculature.1,39
Concise Methods
Animals
Seven life-supporting GalT-KO kidney transplantations were performed in baboons (Papio anibus; Manheimer Foundation, Homestead, FL) weighing 7–12 kg; GalT-KO miniature swine weighing 9–27 kg were used as donors.19 All GalT-KO donors were individually generated by nuclear transfer from GalT-KO fibroblasts from Massachusetts General Hospital MHC-inbred miniature swine.15 All animal care procedures were performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health. All protocols were approved by the MGH Subcommittee on Research Animal Care.
Pig-to-Baboon Xenotransplantation
In the immunotolerance protocol, we performed 11 experimental transplants of kidney plus vascularized thymus.19 In the present study, however, 3 of 11 transplants were excluded because of early termination (days 4, 13, and 33) with cessation of immunosuppression (n=2) or cerebral infarction (n=1). The other four experimental animals were also excluded because these animals with stable renal function died of causes unrelated to the transplant by day 31. The pathologic features of the grafts in these four animals on days 16, 18, 26, and 31 were similar to those of the grafts with long-term survival (n=4), but the chronic period of the grafts could not be examined. Therefore, we focused on 4 of the 11 transplants in the immunotolerance protocol that survived more than 50 days and could thus be characterized long term. In our immunotolerance protocol, kidney transplantation was performed together with vascularized thymus as a thymokidney or a vascularized thymic lobe.33,40,41 In two cases of thymokidney transplantation (B118 and B134), composite thymokidney grafts were prepared from GalT-KO miniature pigs by the implantation of autologous thymic tissue under the kidney capsule 6–8 weeks before transplantation to allow thymic revascularization prior to transplantation.19,42 In two cases (B113 and B135), baboon recipients received an orthotopic kidney and a donor-matched vascularized thymic lobe using the procedure described in detail elsewhere.19,43 Finally, in the three cases from the immunosuppression protocol (B114, B126, and B131), recipients received only an orthotopic kidney without vascularized thymic tissue.
Immunosuppression
Our immunotolerance and immunosuppression protocols were described in detail previously.19 Immunotolerance in our baboon recipients (n=4) was induced by thymectomy, splenectomy, and T cell depletion, first with anti-thymocyte globulin (ATG; 50 mg/kg per day intravenously) for 3–4 days (Pharmacia/Upjohn). The T cell count was reduced to <200 cells/mm3 with 12 mg of LoCD2/kg intravenously (rat anti-primate CD2b, Immerge, BioTherapeutics, Charlestown, MA). Furthermore, three of the four baboons received 100 rads of whole-body irradiation on day −6, followed by reductions in the doses of ATG (100 mg/kg) and LoCD2 (8 mg/kg). Maintenance therapy consisted of human anti-human CD154 mAb(ABI793, Novartis Pharma, Basel, Switzerland) administered intravenously at 20–25 mg/kg on day −1 and continued every 2–5 days thereafter, and mycophenolate mofetil (Roche, Nutley, NJ), which was administered by continuous intravenous infusion at 110 mg/kg per day from day −7. All animals, except B134, received methylprednisolone, 2 mg/kg per day intravenously (Upjohn, Kalamazoo, MI), starting on the day of transplantation; the dose was tapered slowly thereafter. In the immunosuppression protocol, one baboon (B126) received the same immunotolerance induction regimen as the experimental animals. The two other animals (B114 and B131) underwent immunosuppression protocol at our center, which included 700-cGy thymic irradiation on day −2, anti-CD154 mAb, mycophenolate mofetil, and steroids, but did not undergo thymectomy. All recipients received cobra venom factor daily for the first 2 weeks after transplantation to protect against the effects of natural anti-nonGal antibodies and of nonspecific complement activity.
Histologic and Immunohistochemical Examination
Kidney graft samples were taken during open biopsies and from graftectomies (Table 1). For light microscopic examination, tissue was fixed in 10% buffered formalin and embedded in paraffin. Sections were examined after hematoxylin and eosin, periodic acid-Schiff, Masson trichrome, periodic acid-methenamine silver, and elastica-Masson Goldner staining. Tissues for electron microscopy were fixed in 2.5% glutaraldehyde plus 2% paraformaldehyde, postfixed with 1% osmium tetroxide, and embedded in Epon 812. Ultrathin sections were stained with lead citrate. Frozen tissue sections were stained by direct immunofluorescence using fluorescein isothiocyanate (FITC)-conjugated rabbit polyclonal antibody to human IgG (1:30), IgM (1: 20), C3 (1:10), and fibrinogen (1:20) (all from Dako, Carpinteria, CA); for indirect immunofluorescence, antihuman C4d mAb (Quidel, San Diego, CA), polyclonal rabbit anti-human C4d antibody (American Research Products, Inc., Belmont, MA) and anti-human C5b-9 mAb (Dako) were used. The C4d antibody does not stain pig C4d, so it would not detect residual donor C4d.
The following primary antibodies were used for staining by the standard avidin-biotin-peroxidase complex technique:28 (1) anti-swine CD31 (PCAM1) mAb (Serotec, Raleigh, NC) to detect capillary endothelium in swine grafts; (2) polyclonal rabbit anti-tissue factor antibody (the cross-reactive antiporcine tissue factor), provided by Professor Yale Nemerson (Mount Sinai School of Medicine, New York, NY),44,45 to detect tissue factor on porcine activated endothelial cells; (3) anti-pig CD39 mAb,46 to detect nucleoside triphosphate diphosphohydrolase (a thromboregulatory factor) on porcine endothelial cells; (4) anti-human CD41 mAb (5B12: Dako) to detect baboon platelets; (5) polyclonal rabbit anti-human vWF (Dako), which detects vWF in endothelial cells and thrombi; (6) anti-PCNA mAb (PC10: Dako), which detects proliferating cells; (7) anti–TIA-1 mAb (granule membrane protein of 17 kD; Coulter Immunology, Hialeah, FL) to detect cytotoxic granule protein in T and NK cells; and (8) polyclonal rabbit anti-human CD3 antibody (A0452; Dako), anti-human CD20 mAb (B cells, L26; Dako), anti-human CD68 mAb (macrophages, KP-1; Dako), anti-human NKB1 mAb (NK cells, DX9; BD Biosciences, San Jose, CA), and anti-human CD56 mAb (neural cell adhesion molecules/NK cells, 1B6; Nitirei), which were used to determine the phenotype of infiltrating cells.
To detect platelet-fibrin thrombi in xenografts, two-color immunohistochemistry for CD41 (Texas Red) and fibrinogen (FITC) was performed using frozen sections. The relationship between CD41+ platelet aggregation and the deposition of immunoglobulin or complement was assessed in frozen sections using two-color immunohistochemistry for CD41 (Texas Red) and IgM (FITC) or C4d (FITC). To detect cytotoxic T cells in xenografts, two-color immunohistochemical staining against TIA-1 (alkaline phosphatase, blue) and CD3 (3, 3′-diaminobenzidine, tetrahydrochloride, brown) was performed in paraffin-embedded sections. In histologic sections, fragmented nuclear DNA associated with apoptosis was labeled by the terminal deoxynucleotidyl transferase–mediated TUNEL method.31
Quantification of Histologic Findings
In each kidney sample, 40 randomly selected interstitial fields (0.065 mm2) or 40 glomerular cross-sections were assessed at a magnification of ×400, without prior knowledge of the clinical or histologic findings, for the following measures: (1) thrombotic microangiopathic glomerulopathy: the mean semi-quantitative severity score of glomerulopathy, per glomerular cross-section in periodic acid-Schiff–stained sections (scores 0–3: 0, no glomerulopathy; 1, segmental and mild glomerulopathy; 2, segmental and moderate glomerulopathy; 3, global and severe glomerulopathy); (2) interstitial hemorrhage: the mean semiquantitative severity score of interstitial hemorrhage, per interstitial field in hematoxylin and eosin–stained sections (scores 0 to 3: 0, no hemorrhage; 1, focal and mild hemorrhage; 2, focal and moderate hemorrhage; 3, diffuse and marked hemorrhage); (3) evaluation of deposition of IgM and IgG, complement (C4d and C5b-9), and CD41, and expression of tissue factor, vWF, CD39: mean semiquantitative staining score of IgM, IgG, C4d, C5b-9, CD41, tissue factor, vWF, or CD39, respectively, per glomerular cross-section (scores 0–4: 0, no localized increase in staining; 1, little increase in staining [deposition in two or three glomerular capillaries]; 2, moderate increase in staining [involving <25% of the glomerular capillaries]; 3, intense increase in staining [involving <50% of capillaries]; and 4, maximal increase in staining [50% capillary involvement]);28 (5) glomerular capillaries: the mean number of glomerular capillary lumina surrounded by CD31+ cells, per glomerular cross-section; (6) the frequency of PCNA+ cells in glomeruli: the mean number of PCNA+ cells per glomerular cross-section; and (7) the number of CD3+, CD68+, CD20+, and NK cells in the interstitium: the mean number of CD3+, CD68+, CD20+, and NKB-1+ cells in selected interstitial fields (0.065 mm2). Correlations between the deposition of C4d and the severity of glomerulopathy or CD41 deposition were computed and analyzed using a Pearson test. In the graftectomy samples, acute and chronic rejection scores were evaluated using Banff classification 2007.20
Renal Function
Arterial blood sampling was performed twice daily to measure serum creatinine and blood BUN. The endpoint for renal xenograft survival was rejection, as indicated by plasma creatinine levels >9 mg/dl or by severe deterioration of clinical condition due to uremia (BUN >100 mg/dl).
Disclosures
None.
Acknowledgments
The authors thank Drs. John and Isabel Hanekamp and Dr. Leo Buhler for critical review of the manuscript.
This work was supported in part by National Institutes of Health Program Project 1PO1 A145897 and 5PO1-A145897 and by a Sponsored Research Agreement between Immerge BioTherapeutics, Inc. and the Massachusetts General Hospital. A.S. was a recipient of a grant from the Japanese Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C20591900). The authors thank Novartis Pharma AG (Basel, Switzerland) for generously providing anti-CD154 mAb and Immerge BioTherapeutics Inc. (Cambridge, MA) for providing LoCD2b mAb and cobra venom factor.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Sachs DH, Sykes M, Robson SC, Cooper DK: Xenotransplantation. Adv Immunol 79: 129–223, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Cascalho M, Ogle BM, Platt JL: Xenotransplantation and the future of renal replacement. J Am Soc Nephrol 15: 1106–1112, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cooper DK, Gollackner B, Sachs DH: Will the pig solve the transplantation backlog? Annu Rev Med 53: 133–147, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Sachs DH: The pig as a potential xenograft donor. Vet Immunol Immunopathol 43: 185–191, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Bach FH, Robson SC, Winkler H, Ferran C, Stuhlmeier KM, Wrighton CJ, Hancock WW: Barriers to xenotransplantation. Nat Med 1: 869–873, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Platt JL: The immunological barriers to xenotransplantation. Crit Rev Immunol 16: 331–358, 1996 [PubMed] [Google Scholar]
- 7.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR: Identification of carbohydrate structures that bind human antiporcine antibodies: Implications for discordant xenografting in humans. Transplant Proc 24: 559–562, 1992 [PubMed] [Google Scholar]
- 8.Alwayn IP, Basker M, Buhler L, Cooper DK: The problem of anti-pig antibodies in pig-to-primate xenografting: current and novel methods of depletion and/or suppression of production of anti-pig antibodies. Xenotransplantation 6: 157–168, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Cozzi E, White DJ: The generation of transgenic pigs as potential organ donors for humans. Nat Med 1: 964–966, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Lambrigts D, Sachs DH, Cooper DK: Discordant organ xenotransplantation in primates: World experience and current status. Transplantation 66: 547–561, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, Monroy R, Xu Y, Awwad M, Colvin RB, Cosimi AB, Robson SC, Fishman J, Spitzer TR, Cooper DK, Sachs DH: Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation 67: 18–30, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Zhong R, Luo Y, Yang H, Garcia B, Ghanekar A, Luke P, Chakrabarti S, Lajoie G, Phillips MJ, Katopodis AG, Duthaler RO, Cattral M, Wall W, Jevnikar A, Bailey M, Levy GA, Grant DR: Improvement in human decay accelerating factor transgenic porcine kidney xenograft rejection with intravenous administration of gas914, a polymeric form of alphaGAL. Transplantation 75: 10–19, 2003 [DOI] [PubMed] [Google Scholar]
- 13.McGregor CG, Teotia SS, Byrne GW, Michaels MG, Risdahl JM, Schirmer JM, Tazelaar HD, Walker RC, Logan JS: Cardiac xenotransplantation: progress toward the clinic. Transplantation 78: 1569–1575, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL: Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299: 411–414, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ: Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A 101: 7335–7340, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsoondar JJ, Macháty Z, Costa C, Williams BL, Fodor WL, Bondioli KR: Production of alpha 1,3-galactosyltransferase-knockout cloned pigs expressing human alpha 1,2-fucosylosyltransferase. Biol Reprod 69: 437–445, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Takahagi Y, Fujimura T, Miyagawa S, Nagashima H, Shigehisa T, Shirakura R, Murakami H: Production of alpha 1,3-galactosyltransferase gene knockout pigs expressing both human decay-accelerating factor and N-acetylglucosaminyltransferase III. Mol Reprod Dev 71: 331–338, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Nottle MB, Beebe LF, Harrison SJ, McIlfatrick SM, Ashman RJ, O’Connell PJ, Salvaris EJ, Fisicaro N, Pommey S, Cowan PJ, d’Apice AJ: Production of homozygous alpha-1,3-galactosyltransferase knockout pigs by breeding and somatic cell nuclear transfer. Xenotransplantation 14: 339–344, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH: Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11: 32–34, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS: The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol 176: 2448–2454, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Buhler L, Xu Y, Li W, Zhu A, Cooper DK: An investigation of the specificity of induced anti-pig antibodies in baboons. Xenotransplantation 10: 88–93, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Lam TT, Paniagua R, Shivaram G, Schuurman HJ, Borie DC, Morris RE: Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation 11: 531–535, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, Liu W, Jevnikar A, Wall W, Cooper DK, Murase N, Dai Y, Wang W, Xiong Y, White DJ, Zhong R: Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 11: 1295–1298, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai HC, Ezzelarab M, Hara H, Ayares D, Cooper DK: Progress in xenotransplantation following the introduction of gene-knockout technology. Transpl Int 20: 107–117, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Naziruddin B, Cui C, Martin MJ, Xu H, Wan H, Lei Y, Harrison C, Yin J, Okabe J, Mathews C, Stark A, Adams CS, Houtz J, Wiseman BS, Byrne GW, Logan JS: Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation 75: 430–436, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU: Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J Biol Chem 275: 25308–25314, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, Sachs DH, Colvin RB: Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol 16: 2732–2745, 2005 [DOI] [PubMed] [Google Scholar]
- 29.O’Connell PJ: Thrombotic microangiopathy: the next big hurdle for xenotransplantation. J Am Soc Nephrol 16: 2529–2530, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu A, Meehan SM, Kozlowski T, Sablinski T, Ierino FL, Cooper DK, Sachs DH, Colvin RB: Acute humoral xenograft rejection: Destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts. Lab Invest 80: 815–830, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, Bradley JR, Thiru S, Davies HF, Wallwork J, White DJ, Goddard M, Friend PJ: Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation 10: 300–310, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Hisashi Y, Yamada K, Kuwaki K, Tseng YL, Dor FJ, Houser SL, Robson SC, Schuurman HJ, Cooper DK, Sachs DH, Colvin RB, Shimizu A: Rejection of cardiac xenografts transplanted from alpha1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant 8: 2516–2526, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachs DH, Sykes M, Yamada K: Achieving tolerance in pig-to-primate xenotransplantation: Reality or fantasy. Transpl Immunol 21: 101–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman JR, O’Connell PJ, Nankivell BJ: Chronic renal allograft dysfunction. J Am Soc Nephrol 16: 3015–3026, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ’05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Shimizu A, Yamada K, Sachs DH, Colvin RB: Persistent rejection of peritubular capillaries and tubules is associated with progressive interstitial fibrosis. Kidney Int 61: 1867–1879, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Shimizu A, Yamada K, Sachs DH, Colvin RB: Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest 82: 673–686, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wong BS, Yamada K, Okumi M, Weiner J, O’Malley PE, Tseng YL, Dor FJ, Cooper DK, Saidman SL, Griesemer A, Sachs DH: Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation 82: 314–319, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Sachs DH, Sykes M, Yamada K: Achieving tolerance in pig-to-primate xenotransplantation: Reality or fantasy. Transpl Immunol 21: 101–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada K, Vagefi PA, Utsugi R, Kitamura H, Barth RN, LaMattina JC, Sachs DH: Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation 76: 530–536, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Kamano C, Vagefi PA, Kumagai N, Yamamoto S, Barth RN, LaMattina JC, Moran SG, Sachs DH, Yamada K: Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A 101: 3827–3832, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barth RN, Yamamoto S, LaMattina JC, Kumagai N, Kitamura H, Vagefi PA, Awwad M, Colvin RB, Cooper DK, Sykes M, Sachs DH, Yamada K: Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 75: 1615–1624, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Lavelle JM, Vagefi PA, Arakawa H, Samelson-Jones E, Moran S, Teranishi K, Kamano C, Fishman J, Awwad M, Neville DM, Shimizu A, Sykes M, Sachs DH, Yamada K: Vascularized thymic lobe transplantation in a pig-to-baboon model: A novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation 80: 1783–1790, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Thiruvikraman SV, Guha A, Roboz J, Taubman MB, Nemerson Y, Fallon JT: In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab Invest 75: 451–461, 1996 [PubMed] [Google Scholar]
- 45.Gollackner B, Goh SK, Qawi I, Buhler L, Knosalla C, Daniel S, Kaczmarek E, Awwad M, Cooper DK, Robson SC: Acute vascular rejection of xenografts: Roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation 77: 1735–1741, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, Bach FH: Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med 185: 153–163, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]