Abstract
Mannose receptor 2 (Mrc2) expresses an extracellular fibronectin type II domain that binds to and internalizes collagen, suggesting that it may play a role in modulating renal fibrosis. Here, we found that Mrc2 levels were very low in normal kidneys but subsets of interstitial myofibroblasts and macrophages upregulated Mrc2 after unilateral ureteral obstruction (UUO). Renal fibrosis and renal parenchymal damage were significantly worse in Mrc2-deficient mice. Similarly, Mrc2-deficient Col4α3−/− mice with hereditary nephritis had significantly higher levels of total kidney collagen, serum BUN, and urinary protein than Mrc2-sufficient Col4α3−/− mice. The more severe phenotype seemed to be the result of reduced collagen turnover, because procollagen III (α1) mRNA levels and fractional collagen synthesis in the wild-type and Mrc2-deficient kidneys were similar after UUO. Although Mrc2 associates with the urokinase receptor, differences in renal urokinase activity did not account for the increased fibrosis in the Mrc2-deficient mice. Treating wild-type mice with a cathepsin inhibitor, which blocks proteases implicated in Mrc2-mediated collagen degradation, worsened UUO-induced renal fibrosis. Cathepsin mRNA profiles were similar in Mrc2-positive fibroblasts and macrophages, and Mrc2 genotype did not alter relative cathepsin mRNA levels. Taken together, these data establish an important fibrosis-attenuating role for Mrc2-expressing renal interstitial cells and suggest the involvement of a lysosomal collagen turnover pathway.
CKD is caused by two distinct pathogenetic events: a unique initiation phase triggered by one of numerous congenital or acquired disorders, and a common progression phase characterized by renal parenchymal destruction due to excessive accumulation of interstitial extracellular matrix proteins, especially fibrillar collagens.1 At the center of the fibrogenic response is a population of unique interstitial fibroblasts that appear de novo in response to chronic injury, where they synthesize significant quantities of collagen in response to local fibrogenic signals (especially TGF-β). Left unchecked, the adjacent tubules eventually lose their regenerative capacity and succumb to death pathways, apoptosis, and possibly autophagy.
The primary endogenous defense pathway that minimizes fibrosis-associated renal parenchymal destruction is matrix degradation by specialized proteases. Despite a wealth of data on the substrate specificity of matrix-degrading proteases, it has proven challenging to translate in vitro observations into renal fibrogenesis because it occurs in vivo; the identity of the primary endogenous proteases that regulate matrix accumulation rates and preserve nephron integrity remain elusive. This is likely because of the fact that most of these proteases are multifunctional, with effects that extend beyond matrix proteins within extracellular regions to cleavage-dependent activation of several other molecules, and to cellular effects ranging from interactions with cellular receptors, cytoplasmic signaling pathways, and even intracellular localization.2–6 The matrix metalloproteinases (MMPs)—a large family of zinc-dependent enzymes, each with preferred primary matrix substrates—were assumed to be the primary regulators of renal matrix degradation until studies in genetically engineered mice yielded some surprising results. For example, renal MMP-2 activity is remarkably increased in response to chronic injury such as ureteral obstruction,7 yet when mice were genetically engineered to express high MMP-2 levels in proximal tubules, they spontaneously developed interstitial fibrosis, presumably due to detrimental effects on the epithelium-like cells.8 Genetic MMP-9 deficiency actually reduced renal fibrosis severity in mice with ureteral obstruction.9 MMP-7, or matrilysin, is another family member that is upregulated and implicated in the genesis of fibrotic disorders.10 Plasmin, a serine protease that activates several MMPs in vitro, and its own activator, tissue-type plasminogen activator, have paradoxically been associated with fibrosis progression in mouse CKD models.11,12 Although a systematic interrogation of all MMPs is far from complete, which MMPs (if any) serve a protective role during renal fibrosis remains unknown. Yet, it is clear that renal interstitial collagen does remodel and that early renal fibrosis may even be reversed.13–15 Elucidating these endogenous matrix turnover pathways would open new therapeutic avenues.
Largely overlooked until recently, new evidence suggests that intracellular processes may make an important contribution to collagen turnover. Surprisingly, these pathways may even exist within fibroblasts, cells that are traditionally viewed as the perpetrators of fibrosis. In vitro studies dating back 30 years first reported that fibroblasts could degrade collagen.16 More recent studies suggested that collagen phagocytosis via the α2β1 integrin was the primary cellular pathway.17 However, a series of in vitro studies published since 2000 report that the fibronectin type II domain of the mannose receptor 2 (Mrc2) functions as an endocytic receptor for soluble collagens using clathrin-coated pits to deliver collagen cargos to endolysosomes to be degraded.18 Mrc2 is one of four members of the mannose receptor family, each a constitutive recycling receptor, but with distinct ligands.19 The other members are mannose receptor 1,20 the M-type phospholipase A2 receptor,21 and dendritic cell DEC-205/LY75.22 Cultured Mrc2+/+ fibroblasts were shown to internalize collagens I, VI, and V; it has been predicted that additional collagens may also be degraded via this pathway.18,23–25 Inhibition by E64d suggests that the collagenolytic lysosomal cathepsins are involved.25–27 One physiologic function of Mrc2 seems to be in bone formation.28,29 Mrc2 almost certainly has additional functions. Indeed, three independent groups of investigators first identified new receptors while pursuing diverse interests and each was subsequently shown to be identical to Mrc2. In 1990, Isacke et al. identified it as the target 180-kD antigen of an antifibroblast antibody in 1990 (p180, or Endo180)30,31; in 1993, Behrendt et al. reported it as a protein associated with the urokinase receptor (uPAR)32; and in 1996, Wu et al. identified it as a C-type lectin receptor.33 Mrc2 expression is typically induced at sites of tissue remodeling in response to injury. At these sites, fibroblasts and myofibroblasts are a major source, although it can also be associated with subsets of macrophages and endothelial cells.
Given our findings that uPAR is upregulated and serves an antifibrotic role in experimental CKD,34–36 we were interested in the expression and function of its co-receptors. Despite its impressive ability to degrade soluble collagen in vitro, evidence that Mrc2 serves this role in vivo during solid organ fibrosis is lacking. In this study, which is based primarily on the unilateral ureteral obstruction (UUO) model of CKD, we report upregulated Mrc2 expression by myofibroblasts and macrophages and significantly worse fibrosis in Mrc2 knockout mice. Significantly worse fibrosis and renal functional impairment was also observed in Mrc2−/− mice with hereditary nephritis compared with their Mrc2+/+ littermates.
Results
Mrc2 Is Expressed in Experimental Models of CKD
Baseline Mrc2 levels are very low in normal mouse kidneys. In response to chronic injury induced by UUO, protein levels increased eight- to 10-fold (Figure 1). By immunostaining, Mrc2 was shown to be expressed by numerous cells throughout the interstitium. In two less aggressive models of chronic kidney injury, induced by two injections of nephrotoxic serum (NTS)37 or caused by a genetic defect in the basement membrane protein collagen α3 (IV),38 Mrc2 protein was also upregulated and localized to interstitial cells (Figure 2). Dual staining confocal microscopy determined that a subset of platelet-derived growth factor receptor-β (PDGFR-β) positive myofibroblasts (14% and 15%), α−smooth muscle actin (αSMA) positive myofibroblasts (15% and 17%), and F4/80+ macrophages (16% and 4%) expressed Mrc2 7 and 14 days after UUO, respectively (Figure 3).
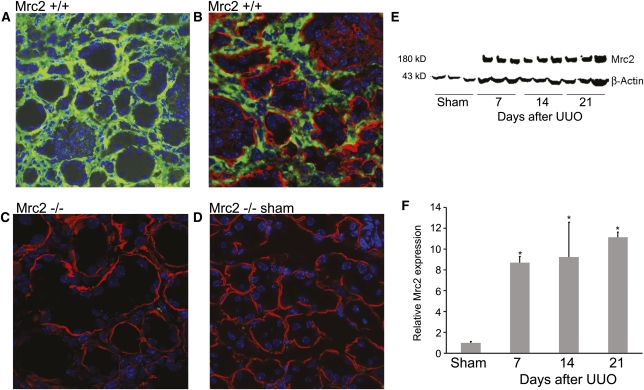
Figure 1.
Mrc2 protein is expressed by interstitial cells in the UUO model of CKD. (A and B) Immunofluorescence confocal microscopy illustrates diffuse Mrc2 protein expression by interstitial cells in wild-type kidneys damaged by 14 days of UUO. Mrc2 was not detected in Mrc2 knockout mice exposed to UUO 14 days (C) or wild-type mice that underwent sham surgery (D). The photomicrographs are magnified ×400 (A) and ×630 (B–D). Staining for the basement membrane protein laminin (red) in B–D highlights that all of the Mrc2+ cells are in the interstitium. Kidney protein levels quantified by Western blotting (E), corrected for protein loading using β-actin, showed significant protein induction in response to UUO (F). The bars in the graph represent results as mean ± SD. *P<0.05 compared with sham levels.
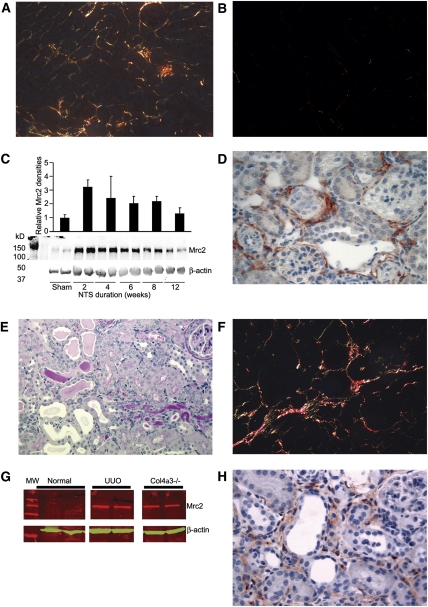
Figure 2.
Mrc2 protein is upregulated in other CKD models. (A) Two doses of nephrotoxic serum nephritis induce proteinuria and interstitial fibrosis as illustrated by picrosirius red staining at 8 weeks. (B) In control kidneys, interstitial picrosirius red+ deposits of mature collagen are minimal. (C) Increased renal Mrc2 protein expression was detected by Western blotting, shown graphically after correctly for protein loading using β-actin band densities. (D) Immunohistochemical staining (brown) at 2 weeks illustrates Mrc2 protein localized to interstitial cells. Hereditary nephritis due to genetic deficiency of IV (α1) procollagen causes slowly progressive renal insufficiency and death at 8–10 months of age in C57BL/6 mice. Kidney tissue examined at 6 months of age showed diffuse interstitial inflammation and tubular damage by periodic acid–Schiff staining (E) and interstitial picrosirius red+ collagen deposits (F). At this time-point, kidney Mrc2 protein levels measured by Western blotting were similar to day 14 UUO levels (G) and immunolocalized to interstitial cells (H). Bar graphs are mean band densities expressed as mean ± SD. The photomicrographs are magnified ×400.
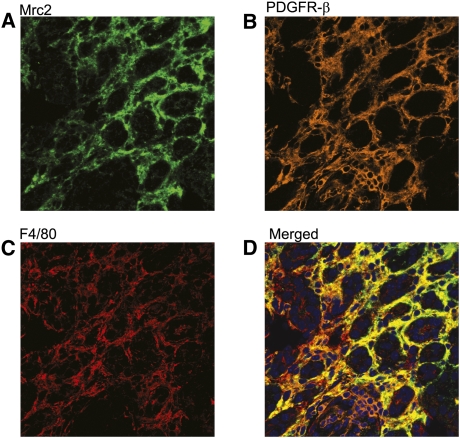
Figure 3.
Triple staining multi-photon confocal photomicrograph identifying a subset of Mrc2 interstitial myofibroblasts (PDGFR-β+) and macrophages (F4/80+) after UUO. A representative kidney section illustrates Mrc2 staining in green (detected using TSA amplified DyLight and Alexa Fluor 488-labeled secondary antibodies and the 488 laser) (A), PDGFR-β in orange (detected using TSA amplified Alexa Fluor 568-labeled secondary antibodies and the 559 laser) (B), and F4/80 in red (detected using Alexa Fluor 633-labeled secondary antibodies and the 635 laser) (C). Analysis of the merged images (D) using the Olympus FV1000MPE co-localization software program determined that 15% of PDGFR-β+ myofibroblasts and 16% of F4/80+ macrophages were Mrc2+. The photomicrographs are magnified ×400.
Renal Fibrosis Is More Severe in Mrc2−/− Mice with UUO
To assess the functional significance of Mrc2 in CKD, the degree of renal fibrosis was compared between Mrc2+/+ and Mrc2−/− mice 7, 14, and 21 days after UUO. Total kidney collagen measured using the hydroxyproline assay was significantly higher in the Mrc2−/− mice at 14 and 21 days (28% and 76%, respectively) (Figure 4). These differences were confirmed by quantitative computer-assisted image analysis of the interstitial area occupied by picrosirius red-positive collagen fibrils (Figure 4). Because of the presence of a normal contralateral kidney, measures of glomerular function cannot be used to assess the effect of fibrosis on renal function in the UUO model. As surrogate measures of parenchymal damage, the number of terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) positive apoptotic tubular cells were measured and found to be significantly higher, whereas the density of CD31+ interstitial capillaries was significantly lower in the Mrc2−/− kidneys (Figure 5). We next investigated the possibility that differences in TGF-β activity might contribute to greater tubular cell damage in the Mrc2−/− mice. By quantitative real-time PCR (qPCR), kidney mRNA levels for both TGF-β1 and its receptor TGF-βR1 were significantly higher on days 14 and 21 in the Mrc2−/− mice (Figure 6, A and B) and increased activity was suggested by higher phospho-Smad3 (pSmad) proteins levels measured by Western blotting (Figures 6, C and D). Although immunostaining identified several pSmad3 positive cell nuclei in the obstructed kidneys, the majority of the cells were tubular epithelia (Figure 6E).
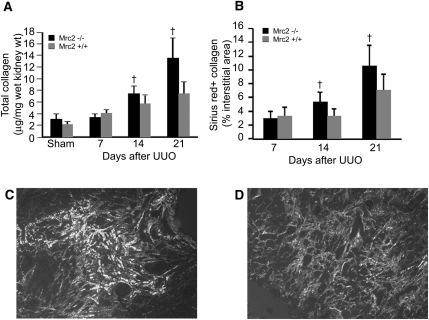
Figure 4.
Interstitial fibrosis induced by UUO is significantly more severe in Mrc2-deficient mice. Compared with wild-type mice (Mrc2+/+), mice genetically engineered to express a mutant Mrc2 gene missing exons 2–6, which includes the collagen-binding domain (Mrc2−/−), have significantly higher total kidney collagen levels 14 and 21 days after UUO (A). Worse fibrosis was confirmed by computer-assisted image analysis of picrosirius red+ interstitial areas (B). Representative polarized light photomicrographs illustrate more extensive interstitial collagen fibril accumulation in the Mrc2−/− mice (C) compared with the Mrc2+/+ mice (D). Bar graphs represent results expressed as mean ± SD, n=9–11 mice per group. †P<0.05 for Mrc2−/− versus Mrc2+/+ groups. The photomicrographs are magnified ×400.
Figure 5.
Worse fibrosis in Mrc2−/− mice is associated with more extensive tubular cell apoptosis and interstitial capillary loss. Apoptosis is a major pathway leading to tubular atrophy in CKD. (A, B) The number of TUNEL+ tubular cells was significantly higher on the Mrc2−/− mice, as illustrated by the representative day 14 photomicrographs. (C) The graph displays the semi-quantitative data at days 7, 14, and 21 after UUO (n=7 per group). Another pathologic feature of CKD, interstitial capillary rarefaction, was significantly worse in the Mrc2−/− kidneys as determined by computer-assisted image analysis of CD31+ interstitial capillaries. Day 14 data illustrate representative photomicrographs for Mrc2+/+ (D) and Mrc2−/− (E) mice and the graph shows the results of semi-quantitative analysis (n=8 per group) (F). The bar graphs are mean ± SD. †P<0.05 for Mrc2−/− versus Mrc2+/+ groups. The photomicrographs are magnified ×400.
Figure 6.
TGF-β expression is enhanced in Mrc2−/− mice after UUO. TGF-β is a central mediator of renal fibrosis. Measured by qPCR, total kidney TGF-β1 (A) and TGF-β receptor1 (B) mRNA levels were significantly higher in the Mrc2−/− kidneys at 14 and 21 days after UUO. Evidence of increased TGF-β signaling was suggested by significantly higher kidney phospho-Smad3 protein levels measured by Western blotting on days 14 (C) and 21 (D). Using phospho-Smad3 immunohistochemistry, most of the positive cells are tubular epithelium-like cells (two highlighted by arrows), as shown by the representative photomicrograph from a Mrc2+/+ animal 21 days after UUO (E). The bar graphs are mean ± SD. †P<0.05 for Mrc2−/− versus Mrc2+/+ groups. The photomicrographs are magnified ×400.
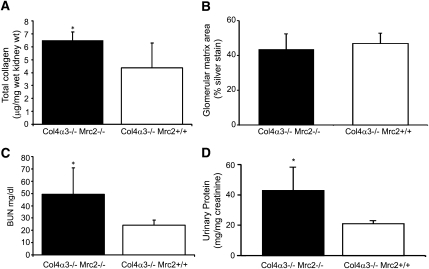
The fibrosis-attenuating effects of Mrc2 were confirmed in a mouse model of hereditary nephritis caused by a genetic mutation in procollagen IV (α1) (Col4α3−/−). Col4α3−/− × Mrc2−/− matings (all on a C57BL/6 background) were performed to generate Col4α3−/− Mrc2+/+ and Col4α3−/− Mrc2−/− offspring that were studied at 4 months of age. All mice underwent a unilateral nephrectomy at 2 months of age, on the basis of our preliminary findings that the pace of interstitial fibrosis was accelerated in single kidney hereditary nephritis mice (data not shown). Total kidney collagen levels, serum BUN levels, and urinary protein/creatinine ratios were significantly higher in the Col4α3−/− Mrc2−/− mice (Figure 7). Glomerular matrix areas, measured as the methenamine silver-positive glomerular tuft area, were similar in Mrc2+/+ and Mrc2−/− mice, suggesting that the antifibrotic effects of Mrc2 are primarily operative at the interstitial level.
Figure 7.
Mrc2 reduces the severity of interstitial fibrosis in Col4α3−/− mice with hereditary nephritis. Col4α3−/− Mrc2+/+ and Col4α3−/− Mrc2−/− male mice on a C57BL/6 background underwent a unilateral nephrectomy at 2 months of age and were sacrificed at age 4 months (n=5 –7 per group). The graphs show significantly higher total kidney collagen levels (A) and serum BUN levels (C) and urinary protein/creatinine ratios (D) in the Col4α3−/− Mrc2−/− mice. (B) The mean glomerular matrix areas, measured as the percentage of glomerular tuft area stained with methenamine silver, were not significantly different. The bar graphs are mean ± SD. *P<0.05.
Collagen Turnover Is Reduced in Mrc2−/− Mice with UUO
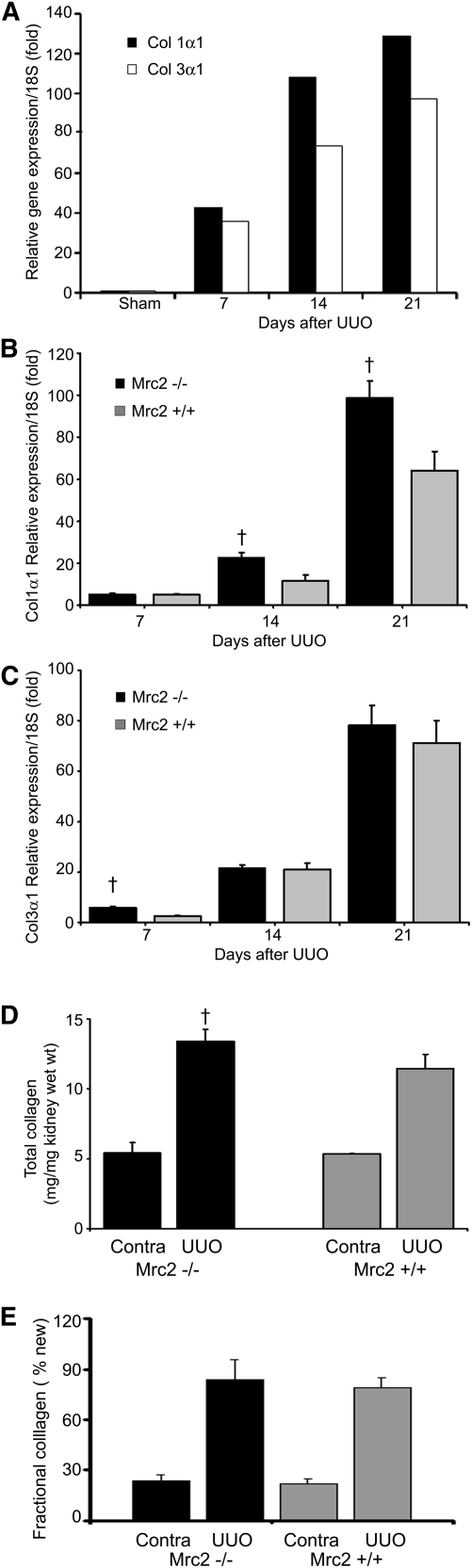
Kidney mRNA levels for procollagens I and III—the primary fibrillar collagens expressed in the kidney in response to chronic injury—are dramatically upregulated in the UUO model (Figure 8A). In a separate cohort of wild-type mice, we confirmed that kidney procollagen I (α1) and III (α1) mRNA levels were persistently upregulated 7–21 days after UUO. Because transcription is considered the rate-limiting step in collagen synthesis, these data suggest a remarkable increase in fibrillar collagen synthesis in response to UUO. Procollagen I (α1) but not procollagen III (α1) kidney mRNA levels were significantly higher in the Mrc2−/− mice at 14 and 21 days (n=10 mice per group) (Figure 8, B and C). To further investigate the dynamics of collagen turnover, collagen synthesis rates were measured by adding 8% deuterium to the drinking water (days 0–14) after an intraperitoneal loading dose, to measure the rate of deuterium incorporation into hydroxyproline in newly synthesized collagen.39 The amount of newly synthesized collagen 14 days after ureteral obstruction was similar between Mrc2+/+ and Mrc2−/− mice (Figure 8, D and E). Together, these data imply that decreased collagen breakdown contributes at least partially to the increase in collagen levels in the Mrc2−/− mice.
Figure 8.
Kidney collagen synthesis rates in Mrc2+/+ and Mrc2−/− mice after UUO. (A) Interstitial collagens I and III are dramatically upregulated in CKD as reflected by kidney procollagen mRNA levels measured by qPCR relative to 18S levels using samples that were pooled from five individual wild-type mice at each time-point after UUO surgery. Kidney procollagen I (α1) (B) but not procollagen III (α1) (C) levels were significantly higher in the Mrc2−/− mice 14 and 21 days after UUO. These data are shown as mean relative expression levels corrected to 18S ± SD, using groups of 10 individual mice at each time point. A separate cohort of mice was continuously labeled with deuterated water after the UUO procedure (n=3 per group) and the level of deuterium incorporation into stable C-H bonds of hydroxyproline was measured by gas chromatography/mass spectroscopy to calculate the accumulation of newly synthesized collagen in the obstructed and contralateral (contra) kidneys. Total kidney collagen was confirmed to be significantly higher in this new cohort of Mrc2−/− mice (D), but the fractional collagen synthesis was similar in Mrc2+/+ and Mrc2−/− mice when measured on day 14 after UUO (E). Results for the contralateral (contra) nonobstructed kidneys show no differences in total collagen and fractional new collagen between the genotypes. †P<0.05 for Mrc2−/− versus Mrc2+/+ Contra and UUO groups.
Mrc2 Expression by Interstitial Myofibroblasts and Macrophages
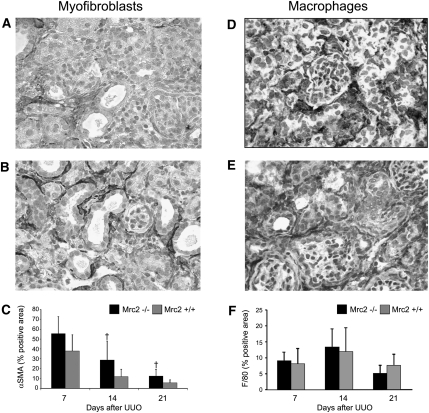
The cellular response to chronic kidney injury is characterized by the de novo appearance of a population of interstitial αSMA+ and PDGFR-β+ myofibroblasts and a significant increase in the number of interstitial macrophages. After UUO, the number of αSMA+ myofibroblasts was significantly higher in the Mrc2−/− mice on days 14 and 21 (Figure 9, A–C), whereas the number of F4/80+ macrophages were similar between the genotypes at 7, 14, and 21 days (Figures 9, D–F). A similar pattern was observed in the hereditary nephritis mice with no difference in the F4/80+ macrophage interstitial areas (15.1±2.3 versus 16.1±1.7% positive areas) and higher αSMA expression in the Col4α3−/− Mrc2−/− mice (2.1±1.4 versus 0.7±0.1 relative band density on Western blotting, after correction for β-actin protein loading, P<0.05).
Figure 9.
Mrc2 deficiency increases the number of interstitial myofibroblasts but does not change interstitial macrophage recruitment after UUO. Compared with Mrc2+/+ mice (A), there were significantly more αSMA+ interstitial myofibroblasts in the kidneys of Mrc2−/− mice 14 and 21 days after UUO (B), as illustrated by the representative immunohistochemical photomicrographs taken 14 days after UU0. (C) The graph represents the results of the computer-assisted image analysis of the αSMA+ interstitial area (n=7–8 mice per group). Representative immunohistochemical photomicrographs illustrate similar numbers of F4/80+ interstitial macrophages in Mrc2+/+ (D) and Mrc2−/− (E) kidneys 14 days after UUO. (F) The graph summarizing the results of the computer-assisted image analysis of the F4/80+ interstitial area (n=8–10 mice per group) shows no significant differences between Mrc2+/+ and Mrc2−/− at 7, 14, and 21 days (F). The bar graphs are mean ± SD. †P<0.05 for Mrc2−/− versus Mrc2+/+ groups. The photomicrographs are magnified ×400.
Renal Cathepsins Are Upregulated and Promote Kidney Fibrosis
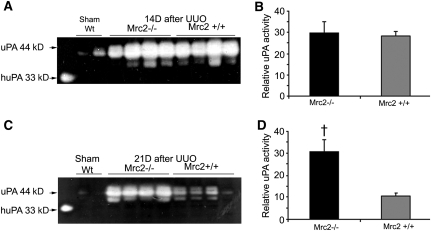
Because the identity of the specific collagenolytic enzymes that regulate interstitial collagen turnover rates in the kidney is unknown, we considered the possibility that the presence of Mrc2 might enhance uPA activity via a mechanism that involves its known interactions with uPAR (Mrc2 is alternatively known as uPAR-associated protein). Measurement of renal urokinase (uPA) activity via plasminogen gel zymography confirmed increased uPA activity in response to UUO (as previously reported).40,41 Levels were similar in Mrc2+/+ and Mrc2−/− mice at 14 days but significantly higher in the Mrc2−/− mice with worse fibrosis at 21 days (Figure 10). Because previous studies on the protease-dependent effects of uPA have found either no effect on fibrosis severity or an antifibrotic effect, mediated by proteolytic activation of hepatocyte growth factor,42–44 the fibrosis-attenuating effects of Mrc2 cannot be explained by differences in uPA activity. An alternative possibility is that collagenolytic lysosomal enzymes—primarily members of the cathepsin family—degrade collagen after its import into endolysosomes by Mrc2. Measured by qPCR, whole kidney mRNA levels for the collagenolytic cathepsins L, K, S, and C were markedly increased in response to UUO, whereas cathepsin B mRNA levels decreased (Figure 11A). Levels were similar in Mrc2+/+ and Mrc2−/− kidneys. By dual staining confocal microscopy, cathepsin K (the most potent collagenase)45 was shown to be expressed by both tubular cells (which lack Mrc2) and Mrc2+ interstitial cells (Figure 11B). To determine the collagenolytic cathepsin expression profile within fibroblasts and macrophages and to determine if cathepsin expression is dependent on the Mrc2 genotype, mRNA levels were measured by qPCR in four mouse cell lines: Mrc2+/+ fibroblasts, Mrc2−/− fibroblasts, Mrc2high macrophages (expression induced by IFN-γ), and Mrc2low macrophages (Figure 11, C and D). Expressed relative to CtsS, which was the least abundant collagenolytic cathepsin in all four cell types, differential expression was similar in Mrc2+ fibroblasts (Figure 11) and macrophages (Figure 11D): CtsL>CtsK>CtsB>CtsC. Similarly to the kidney data, Mrc2 genotype had little effect on the relative mRNA levels (data not shown), suggesting that factors other than Mrc2 regulate the expression of the collagenolytic cathepsins. Treatment of a group of mice with the cathepsin inhibitor E64d significantly increased kidney collagen levels after UUO, supporting a role for renal cathepsins in collagen turnover in vivo (Figure 11E). By electron microscopy, most of the interstitial cells within the widened interstitial space of a day 14 UUO kidney that express prominent lysosomes seem to be macrophages (Figure 11F).
Figure 10.
Renal uPA activity is increased after UUO and not influenced by Mrc2 expression until day 21. Renal uPA activity measured by plasminogen gel zymography showed similar levels on day 14 post UUO (A and B) and significantly higher levels in the Mrc2−/− mice with worse fibrosis on day 21 (C and D). The uPA standard in the first lane on the left (Std) is purified low molecular mass human uPA (33 kD). The graphs (B and D) represent mean combined size of the two major uPA lytic bands (40–44 kD) measured using the Image Quant software program. The bars are mean ± SD. †P<0.05 for Mrc2−/− versus Mrc2+/+ groups.
Figure 11.
Renal collagenolytic cathepsins are upregulated after UUO and promote collagen turnover. (A) Whole kidney mRNA levels for the five known collagenolytic cathepsins (Cts), measured 14 days after UUO by qPCR relative to 18S, showed abundant expression of four of them—Cts L, K, S, and C. (B) Expression levels were similar between Mrc2+/+ and Mrc2−/− kidneys (n=6 mice per group). Representative confocal photomicrograph of a 14-day UUO kidney co-stained for the potent collagenolytic cathepsin K (green) and Mrc2 (red) localized cathepsin K expression to Mrc2-negative tubular epithelium-like cells and a subset of Mrc2+ interstitial cells (yellow). The inset is an enlargement of dual-stained interstitial cells. Cathepsin mRNA profiling results in mouse Mrc2+ fibroblasts (C) and Mrc2+ RAW 264.7 macrophages (D) are shown graphically. Each bar represents the mean of three independent experiments, each run in triplicate. The data represent expression levels relative to 18S and are expressed in arbitrary units using 1.0 as the level for the least abundant, cathepsin S. (E) Treatment with the cathepsin inhibitor E64d for 14 days after UUO resulted in significantly higher collagen levels in the kidneys of wild-type mice compared with vehicle-treated mice (n=4 per group). (F) Using electron microscopy, a macrophage with prominent lysosomes is visible within the widened interstitial space of a kidney 14 days after UUO. The photomicrograph is magnified ×3150.
Discussion
Mrc2 Expression Is Minimal in Normal Kidneys
To our knowledge, this study is the first to report Mrc2 expression by subsets of interstitial macrophages and myofibroblasts that invade the renal interstitium in chronically damaged kidneys. Expression was demonstrated in three experimental models (a genetic model of GN, an immune-mediated model of chronic GN, and a model of progressive obstructive nephropathy), suggesting that the presence of Mrc2-positive interstitial cells is a common response to chronic renal parenchymal injury in which matrix remodeling is in progress. Mrc2 was shown to serve an important fibrosis-attenuating role in wild-type mice compared with Mrc2 knockout mice. What is particularly remarkable is that the relative differences between the genotypes become progressively greater as the duration of UUO-induced chronic injury increased such that total kidney collagen was 36%–46% lower in the Mrc2+/+ mice on day 21, depending upon the measurement method. Renal fibrosis attenuation and functional preservation were confirmed in the Col4α3−/− mice that expressed Mrc2 compared with those that were Mrc2 deficient.
Mrc2 is a 180-kD cellular receptor that is characterized structurally by a cysteine-rich N-terminal domain, a fibronectin type II domain (the collagen binding site), eight C-type lectin-like domains, and a short transmembrane domain. Current evidence indicates that Mrc2 serves primarily as an endocytic receptor that shuttles extracellular cargo to endolysosomes for degradation via a process that involves clathrin-coated pits.18 Once this process is complete, Mrc2 recycles back to the cell membrane—a cycle that may occur as often as 10 times per hour.31 Extensive in vitro evidence, based primarily on studies in fibroblasts, suggests that collagens (I, III, IV, and likely others) are the major Mrc2 ligands. Remarkably, Mrc2 can internalize native as well as denatured collagens, although collagenase-mediated cleavage increases Mrc2 binding.18,23 Mrc2 expression by either stromal cells or malignant cells has been associated with enhanced tumor invasion and metastasis.25,46–48 This study reveals that it also serves an important role in solid organ fibrosis by reducing the rate of interstitial collagen accumulation, resulting in protection of the surrounding parenchyma from fibrosis-associated destruction.
Our findings in the UUO model of CKD are consistent with in vitro studies, which have established an important role for Mrc2 in collagen turnover. Despite approximately 40% lower total kidney collagen levels in the wild-type mice 21 days after UUO compared with Mrc2−/− mice, kidney collagen III mRNA levels were similar in Mrc2+/+ and Mrc2−/−, whereas collagen I mRNA levels were only 1.5- to 1.8-fold higher in the Mrc2−/− mice on days 14 and 21, respectively. Collagen synthesis rates measured as the rate of formation of new collagen labeled with deuterium were similar in mice of both genotypes. Together these data are consistent with the view that degradation is faster in the Mrc2+/+ mice. Although the concept that collagen turnover may occur as an intracellular process is not new, it has received very little attention until recently. In fact, most studies on the pathogenetic mechanisms of renal fibrosis still assume that this is an extracellular event mediated by MMPs despite the lack of any compelling evidence.
Since 2000, a rapidly growing body of evidence, primarily based on in vitro observations, has clearly established Mrc2 as a high-capacity collagen turnover pathway. Although the specific lysosomal proteases that degrade collagen molecules after they are imported by Mrc2 are unknown, in vitro data suggest that cathepsins play a major role. When Mrc2+/+ fibroblasts were allowed to internalize fluorochrome-labeled collagens, collagen accumulated within the lysosomes without degradation if cells were pretreated with the cell-permeable cathepsin inhibitor E64d.26 We found a 70% increase in kidney collagen 14 days after UUO in E64d-treated mice, supporting an important fibrosis-attenuating role for the endogenous cathepsins, although this finding alone does not establish Mrc2 dependency. Five members of the cathepsin family have known collagenolytic activity and they represent the candidate mediators of collagen turnover during renal fibrogenesis: K, B, L, S, and C/dipeptidyl peptidase 1.49–51 Cathepsin K is of particular interest because it has been identified as one of the most potent mammalian collagenases; impaired activity has been associated with other fibrotic disorders.45,52–55 Other than limited descriptive expression studies, very little is currently known about the role of cathepsins in renal fibrogenesis. In this study, relative to sham-operated kidneys, mRNA levels 14 days after UUO were increased to similar levels in the Mrc2+/+ and Mrc2−/− mice: cathepsin L (1.8–1.9×), cathepsin K (3.3–4.8×), cathepsin S (14–27×), and cathepsin C (10–22×). Because Mrc2-negative tubules are a rich source of cathepsins, in vitro studies were performed to gain further insight into which collagenolytic cathepsins might degrade collagen after its delivery to endolysosomes by Mrc2. We found similar mRNA expression profiles in macrophages and fibroblasts (CtsL>CtsK>CtsB>CtsC>CtsS) with no significant differences between Mrc2+/+ and Mrc2−/− cells. These data suggest that Mrc2 expression and collagen delivery, rather than lysosomal cathepsin levels, are likely to be the rate-limiting step in this turnover pathway. However, it is acknowledged that mRNA levels do not determine proteolytic activity, that these data need to be confirmed in kidney-derived cell populations, and that functional studies are needed to establish whether one or more of the lysosomal cathepsins degrade the collagen molecules that are imported by Mrc2.
Interstitial myofibroblasts are considered the primary source of the extracellular matrix proteins that accumulate in the interstitium in CKD and mechanisms to prevent the appearance of these cells or to enhance their clearance are considered promising therapeutic approaches.56,57 The remarkable finding in this study is that Mrc2 expression may enable interstitial myofibroblasts to potentially serve an antifibrotic role by degrading rather than synthesizing collagen. It has long been known that fibroblasts isolated from fibrotic organs such as the lung are functionally distinct from fibroblasts isolated from normal tissues.58–62 Less attention has been paid to the possibility that within diseased tissues such as the kidney, (myo)fibroblasts may be phenotypically and functionally distinct and that mechanisms that skew these cells to an antifibrotic phenotype may mediate adaptive wound healing responses that help minimize permanent tissue damage. On the basis of the results of dual staining confocal microscopy, only a subset of these cells (approximately 15%) express Mrc2. In addition to their ability to degrade collagen, it is possible that Mrc2+ fibroblasts manifest other functions that differ from Mrc2− fibroblasts and help to reduce parenchymal injury when they are recruited to heal wounds. These findings suggest an important functional polarization of renal interstitial myofibroblasts, including some that propagate and others that attenuate renal scarring.
Most studies to date have focused on the functional role of Mrc2 as a fibroblast receptor although other cell types may express it, including macrophages, osteoblasts/osteocytes, and chondrocytes. In this study, interstitial macrophages were identified as a significant subpopulation of the Mrc2+ cells and, like the myofibroblasts, only a subset of F4/80+ macrophages were positive (4%–16% depending upon the phase of injury). In mice, two distinct populations of macrophages have been identified and shown to have unique functions: the classically activated M1 group typically associated with tissue injury and the alternatively activated M2 subset.63 Fibrosis- or scar-associated macrophages are assumed to belong to the M2 population, although what determines their ability to repair tissues without excessive matrix deposition and parenchymal destruction remains unclear. Mannose receptor 1 (Mrc1), widely used as a marker of M2 macrophages, is also known to bind and degrade collagen, although its specific role in the wound healing has not been determined.20 This study suggests that the presence of macrophages expressing Mrc2 directs tissue repair toward functional preservation. What remains to be determined is whether Mrc2+ macrophages and Mrc2+ fibroblasts both contribute to collagen during renal fibrogenesis in vivo. A recent in vitro study using human cell lines concluded that fibroblasts were the primary site of Mrc2-mediated collagen processing.64
The possibility remains that Mrc2 regulates additional cellular responses that lead to fibrosis attenuation in CKD. Although its short cytoplasmic tail is not known to activate classic extracellular signaling cascades, endosomes containing Mrc2 have been shown to facilitate cellular contraction and migration in vitro via a mechanism that involves Rho GTPase-Rho Rho kinase-myosin light chain signaling.65 However, in this study, the number of interstitial myofibroblasts was actually significantly lower in the Mrc2+/+ kidneys, whereas the number of macrophages was similar in Mrc2+/+ and Mrc2−/− mice, which does not support a pro-migratory role for cells recruited to the site of chronic kidney injury. TGF-β activity seemed to be higher in the Mrc2−/− mice and may have contributed to worse tubular damage but this effect is likely secondary as tubules themselves are Mrc2 negative. It remains possible that future studies using isolated populations of interstitial kidney cells will also detect differences in TGF-β expression between isolated kidney Mrc2+/+ and Mrc2−/− (myo)fibroblasts and/or macrophages. Like other members of the mannose receptor family, Mrc2 expresses several calcium-dependent lectin-like domains. Mrc2 expresses only one functional lectin domain, which recognizes N-acetylglucosamine. This carbohydrate is not a common terminal sugar on mammalian oligosaccharides and thus the relevance of this potential interaction to Mrc2’s function in vivo is unclear.18 Finally, it remains possible that Mrc2 elicits pericellular effects relevant to fibrogenesis that have not yet been elucidated. Mrc2 is known to bind to uPAR, a nonsignaling receptor that has also been shown to have antifibrotic effects in the kidney.32,35,66,67 Whether uPAR and Mrc2 function in an independent, complementary, or competitive manner is unclear. Collagen binding induces a significant conformational change in the Mrc2 domain that recognizes pro-uPA/uPAR such that Mrc2 and uPAR cross-linking can be blocked by collagens I, IV, and V in vitro.66,68,69 uPAR has both uPA-dependent proteolytic effects and uPA-independent/integrin-mediated effects that are relevant to renal fibrosis.44 Kidney uPA activity was upregulated to similar levels at day 14 after UUO, whereas by day 21, it was significantly higher in the Mrc2−/− mice, yet fibrosis was worse. Thus, the antifibrotic effects of Mrc2 cannot be explained via enhanced uPAR-uPA–mediated pericellular proteolysis and is consistent with our previous finding that genetic uPA deficiency does not exacerbate renal fibrosis after UUO.42
In summary, this study reports striking expression of the collagen endocytic receptor Mrc2 by subsets of interstitial myofibroblasts and macrophages that invade the interstitium in response to chronic kidney injury. Expression of this receptor is associated with features suggesting that greater collagen turnover at least partially contributes to the attenuation in renal scarring and the extent of renal parenchymal damage—that is, promoting an adaptive tissue repair response. However, it is acknowledged that a gold standard in vivo measure of collagen degradation rates has yet to be developed that could provide unequivocal evidence of this difference. Nevertheless, the lysosomal cathepsins L, K, B, and C seem to be the leading candidates to function as endogenous mediators of collagen turnover by Mrc2+ fibroblasts and macrophages. Identifying mechanisms to enhance the activity of Mrc2-lysosomal cathepsin collagenolytic pathway represents a promising new therapeutic strategy to reduce kidney fibrosis and preserve kidney function.
Concise Methods
Animal Models
Endo180−/− mice (Mrc2−/− mice in this article) were generated by Dr. T. Bugge as previously described.23 Breeding colonies of the Mrc2−/− mice were maintained in our vivarium. The genotype of all study mice was confirmed by PCR using genomic DNA isolated from tails and the following primers: Mrc2+/+, 5′-TCCTACAAATACACGCTGGTGGCGATA-3′ and 5′-GCAGTTCCCTTTTAAATGCAAATCA-3′ (band size, approximately 400 bp); and Mrc2−/−, 5′-TTAAACTGGTAACAGCTGTCAGTC-3′ and 5′-TCTACACCTSCCAGGGAAACTCAC-3′ (band size, approximately 200 bp). Genotype was further confirmed by Western blotting. Using the anti-human Endo180 antibody generated by Dr. Isacke, no protein product was identified in the Mrc2−/− kidneys after UUO. Using the anti-Mrc2 antibody from R&D Systems, a 140-kD protein band was detected, consistent with the targeting strategy that deleted exons 2–6 to yield a truncated Mrc2 protein that lacks the cysteine-rich domain, collagen-binding fibronectin type II domain, and C-type lectin-like domain 1, as reported by East et al.68 All studies that compared outcomes between Mrc2+/+ and Mrc2−/− mice were performed in age-matched male mice on the FVB/NJ background, except for the hereditary nephritis mice, which were on the C57BL/6 background.
UUO, the primary experimental model for these studies, was induced as previously described.7 Groups of Mrc2+/+ and Mrc2−/− male mice were sacrificed after sham or UUO surgery on days 7, 14, or 21. The chronic NTS nephritis model70 was induced in C57BL/6 male mice by two intraperitoneal injections of IgG purified NTS (15 mg/20g body wt) on day 0 and 7.5 mg/20g body wt on day 7. Groups of two mice were sacrificed after 2, 4, 6, 8, and 12 weeks. Mice with hereditary nephritis due to a null mutation in the Col4α3 gene were investigated at 4 months of age.38 The genotype of each study mouse was confirmed by PCR using the following primers: WT forward, AAC ACC AGC TCT GAT GCC AAT G and WT reverse, AAT GAA AGA AAA ACC TTT CCA GAG (300-bp product); and mutant forward, ACG ACC TTT GTT AAA CTA GAA GAA GTC and mutant reverse, TGC TAA AGC GCA TGC TCC AGA CTG C (900-bp product). C57BL/6 Col4α3−/−Mrc2+/+ (n=5) and Col4α3−/−Mrc2−/− (n=7) male mice were generated for this study. A unilateral nephrectomy was performed at 2 months of age based on our earlier findings that the pace of CKD is accelerated in these mice by uninephrectomy. Renal outcomes were investigated at 4 months of age.
A separate cohort of wild-type mice (n=5 per group) was used to generate a pool of RNA to measure kidney procollagen I and III levels on days 0, 7, 14, and 21. In a final study, a group of wild-type C57BL/6 mice was treated with daily intraperitoneal injections of the cell-permeable cathepsin inhibitor E64d (Sigma-Aldrich, St. Louis, MO) dissolved in 1% DMSO or vehicle alone for 14 days. At the time of sacrifice the UUO or sham kidneys were harvested, the capsule was removed, and each kidney was divided in an identical fashion into sections that were processed for total collagen, zymography, RNA, protein, and histology (frozen and paraffin-embedded). Frozen tissue samples were stored at −80°C. All animal procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were approved by the Seattle Children’s Research Institute Institutional Animal Care and Use Committee.
Cell Lines
The mouse monocytic macrophage RAW 264.7 cell line was purchased from the American Type Culture Collection (Manassas, VA) and cultured in DMEM high-glucose media supplemented with 10% FBS. Mrc2 expression was induced by adding 2.5 ng/ml recombinant IFN-γ (PeproTech, Rocky Hill, NJ) for 48 hours as described by Ye et al.71; in these studies, Mrc2 mRNA levels were increased >10-fold. Cells were then harvested for RNA isolation. Immortalized newborn skin fibroblasts that were originally derived from Mrc2+/+ and Mrc2−/− mice23 were obtained from Dr. J. Sottile, University of Rochester School of Medicine and Dentistry,72 and grown in DMEM/F2 media supplemented with 10% FCS. When 90% confluent, the cells were harvested for RNA isolation. Mrc2 expression was confirmed by qPCR.
Antibodies
Primary antibodies used in these studies for immunostaining and Western blotting were: rabbit anti-human Endo180,30 sheep anti-mouse anti-Mrc2 (R&D Systems Inc, Minneapolis, MN), mouse anti-mouse αSMA-Cy3 and mouse monoclonal anti-β-actin (Sigma-Aldrich, St. Louis, MO), peroxidase-conjugated mouse anti-human αSMA (Dako Corp.), rabbit anti-PDGFR β-PE (Santa Cruz Biotechnology, Santa Cruz, CA), rat anti-mouse F4/80 (AbD Serotec, Raleigh, NC), biotinylated rat anti-mouseCD31/PECAM (BD Pharmingen, San Diego, CA), rabbit anti-cathepsin K (BioVision Inc., Mountain View, CA), rabbit anti-laminin DyLight 549 (Novus Biologicals, Littleton, CO), and rabbit anti-phospho-Smad3 (Rockland Immunochemicals, Gilbertsville, PA). The secondary antibodies were: biotinylated goat anti-rabbit IgG and biotinylated rabbit anti-rat IgG (Vector Labs., Burlingame, CA); donkey anti-sheep DyLight 488, horseradish peroxidase (HRP) conjugated anti-rat IgG and HRP-conjugated donkey anti-sheep IgG (Jackson ImmunoResearch Lab. Inc., West Grove, PA); goat anti-rabbit Alexa Flour 568, HRP-conjugated goat anti-rabbit IgG, and goat anti-rat Alexa Flour 633 (Invitrogen, Carlsbad, CA). Tyramide signal amplification (TSA) kits were from Invitrogen.
Fibrosis Severity Assessment
Total kidney collagen was measured by acid hydrolysis using the hydroxyproline assay, as previously described.41 Results were expressed as micrograms of collagen per milligrams of kidney wet weight. The interstitial area containing collagen fibrils was determined by computer-assisted image analysis of paraffin-embedded kidney sections after staining with picrosirius red. The mean positive interstitial area was calculated by an observer blinded to the animal group using the ImagePro software program from Media Cybernetics. Intraglomerular matrix areas were measured in the hereditary nephritis mice using periodic acid methenamine silver staining methods as previously described.73 Serum BUN levels were measured in samples from the hereditary nephritis mice using a kit from Teco Diagnostics (Anaheim, CA). Using aliquots from 24-hour urine samples collected from fasting mice housed individually in metabolic cages, urinary protein was measured with a Pierce total protein assay kit and urinary creatinine using the picric acid assay.
Immunohistochemical and TUNEL Staining and Electron Microscopy
Immunostaining was performed on sections of paraffin-embedded tissue or cryosections of snap-frozen tissue using standard laboratory procedures previously described.41 VECTASTAIN Elite ABC Kits (Vectors Labs) and AEC Chromagen K3464 (Dako Corp., Carpinteria, CA) were used for immunohistochemistry. For confocal microscopy, fluorescence signals were enhanced by tyramide amplification using kits (Vector Laboratories or Invitrogen) and nuclei were stained with TO-PRO-3 iodide. After excluding glomeruli and large vessels, the mean positive tubulointerstitial area was measured for each experimental animal using a computerized image analysis system with a Leica DM4000 microscope and ImagePro software program (Media Cybernetics, Bethesda, MD). All immunostaining procedures included confirmation that staining was negative when the secondary antibody was used alone. All slides were coded to ensure that the observer is blinded to the animal group at the time of quantitative analyses. Confocal microscopy was performed using an Olympus FluoView 1000MPE multi-photon laser scanning confocal microscope. The percentage of dual-positive cells (PDGFR-β or αSMA+ cells expressing Mrc2 and F4/80+ cells expressing Mrc2) were determined using the co-localization analysis tool.
Apoptotic cells were detected in kidney sections using the TUNEL assay on formalin-fixed tissue sections as previously described, using proteinase K pretreatment (6.7 μg/ml; Boehringer-Mannheim, Indianapolis, IN), followed by incubation with TdT (280 units/ml; Pharmacia Biotech) and Bio-14-dATP (7.2 μM; Invitrogen) in One-Phor-All buffer.7 Biotinylated ATP was detected using the using the VECTASTAIN Elite ABC kit (Vector Labs.). Cells were regarded as TUNEL-positive if their nuclei were both stained and had an apoptotic morphology characterized by typical nuclear pyknosis and chromatin condensation. The number of TUNEL-positive cells in each specimen was calculated in a blinded fashion by counting the number of TUNEL-positive tubular cells in an average of eight sequentially selected nonoverlapping fields of renal cortex at ×400 magnification. Results were expressed as the mean number of apoptotic cells per ×400 field.
Samples from two sham and two day-14 UUO kidneys were processed for routine electron microscopy after 3.0% glutaraldehyde fixation.
Western Blotting
Protein was isolated from homogenized frozen kidney in the presence of Complete Protease Inhibitor Cocktail without EDTA (tablets from F. Hoffmann-La Roche Ltd, USA), equally loaded into 9% polyacrylamide gels according to protein concentration, separated by electrophoresis, and transferred to polyvinylidene fluoride membranes and stained with specific antibodies according to methods routinely performed in our laboratory. Specific bands were visualized and quantified using the Li-Cor Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE). Band densities were corrected for protein loading based on β-actin band densities.
mRNA Levels
Total kidney RNA was isolated from kidney tissue homogenates and isolated kidney cells using the automated Maxwell 16 System and Total RNA Purification kit from Promega (Madison, WI). RNA integrity and concentration were determined using Agilent RNA 6000 Nano Chips and the Agilent 2100 Bioanalyzer system (Agilent Technologies, Foster City, CA); samples (1 μg) with a RNA integrity number >8 were reverse transcribed with MMLV reverse transcription using a combination of random hexamers and oligo-dTs and the cDNA synthesis kit from Bioline (Taunton, MA). qPCR was performed according to the IQ SYBR Green Superkit (Bio-Rad) instructions using gene-specific primers shown in Supplemental Table 1 for Mrc2; procollagen I (α1) and III (α1); cathepsins B, L, K, S, and C; and TGF-β1 receptor. Real-time qPCR reactions containing 1.5 μl (6 ng) of cDNA, 0.2 μM primers, and the 2× SYBR Green Supermix were run in the Bio-Rad iQ thermal cycler with programs specific for the respective gene. Reactions were run in triplicate and genes of interest were normalized to the 18S housekeeping gene. All primers were optimized for efficiency and absence of primer-dimers confirmed primer specificity. Single amplicons of the right size were confirmed by gel electrophoresis. Data analysis was performed using the Pfaffl algorithm with the REST analysis software (version 1.9.9; Corbett Research Pty Ltd). Final results were expressed as fold-increase relative to wild-type or control samples as appropriate.
Collagen Turnover and Synthesis
In a group of Mrc2+/+ and Mrc2−/− male mice (n=3 per group), kidney collagen synthesis was determined by quantifying incorporation of deuterium (2H) into hydroxyproline during the 14 days after UUO. After the surgery, each mouse received an intraperitoneal loading dose of 100% deuterated water (2H2O) (35 μl/g body wt) that is calculated to achieve an initial 5% enrichment of the total body water with 2H. Beginning immediately after the surgery, the drinking water was replaced with 8% 2H2O until the mice were sacrificed 14 days later. The incorporation of deuterium into the stable C-H bonds of hydroxyproline in newly synthesized collagen by gas chromatography/mass spectroscopy as previously described.39,74
Protease Activity
Kidney uPA activity was measured by plasminogen-casein gel zymography as previously described.40,41 In brief, pieces of frozen kidney were homogenized in buffer (1% SDS in 50 mM Tris pH 7.6). Protein samples (10 μg) were separated by electrophoresis in 10% SDS-polyacrylamide gels containing plasminogen (10 μg/ml) and casein (2 mg/ml). The gels were washed with 2.5% TritonX-100 to remove SDS, and incubated for 16 hours at 37°C in a 0.1 mol/L glycine solution, and stained with Coomassie brilliant blue. The gel was photographed, and the size of each lytic band was measured using the Image Quant software program (Molecular Dynamics, Sunnyvale, CA). A human low molecular mass (33 kD) uPA standard (Calbiochem-Novabiochem Corp, La Jolla, CA) was loaded into the outer lane. uPA activity was confirmed in a separate gel by the disappearance of the lytic band in the presence of amiloride.40
Statistical Analyses
All data are presented as the mean ± SD. A nested ANOVA was used for data generated by computer-assisted image analysis. For image analysis data, the arithmetic mean of all randomly selected images from each slide (6–9) for each individual animal was used to calculate the reported mean for each group. Comparisons between two experimental groups used an unpaired t test for parametric data and the Mann–Whitney U test for nonparametric data. P<0.05 was considered statistically significant.
Disclosures
C.L.E. and S.M.T. received income and research support as employees of KineMed Inc.
Acknowledgments
The authors acknowledge the excellent technical assistance of Nadia M. Bahrami, Mercades Diaz, and Angela Tchao; Dr. Jacques Garrigues for his technical expertise with the confocal microscope; Dr. Kathy Patterson and Dr. Laura Finn for performing the electron microscopy; Dr. Jeffrey Miner and the Washington University Center for Kidney Disease Research (P30DK079333) for supplying the Alport mouse line; and Dr. Jane Sottile, Aab Cardiovascular Research Institute, University of Rochester School of Medicine and Dentistry, for providing the immortalized fibroblast lines.
The authors gratefully acknowledge research support from the National Institutes of Health Grants DK54500 (A.A.E.), DK44757 (A.A.E.), DK080926 (I.Y.), and DK073497 (D.O.); Seattle Children’s Research Institute; and the NIDCR Intramural Research Program (T.H.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011030310/-/DCSupplemental.
References
- 1.Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Gill SE, Kassim SY, Birkland TP, Parks WC: Mouse models of MMP and TIMP function. Methods Mol Biol 622: 31–52, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Gill SE, Parks WC: Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol 40: 1334–1347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redondo-Muñoz J, Ugarte-Berzal E, Terol MJ, Van den Steen PE, Hernández del Cerro M, Roderfeld M, Roeb E, Opdenakker G, García-Marco JA, García-Pardo A: Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell 17: 160–172, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Rupp PA, Visconti RP, Czirók A, Cheresh DA, Little CD: Matrix metalloproteinase 2-integrin alpha(v)beta3 binding is required for mesenchymal cell invasive activity but not epithelial locomotion: A computational time-lapse study. Mol Biol Cell 19: 5529–5540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sbai O, Ould-Yahoui A, Ferhat L, Gueye Y, Bernard A, Charrat E, Mehanna A, Risso JJ, Chauvin JP, Fenouillet E, Rivera S, Khrestchatisky M: Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia 58: 344–366, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Oda T, López-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA: TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 12: 736–748, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH: Matrix metalloproteinase 2 and basement membrane integrity: A unifying mechanism for progressive renal injury. FASEB J 20: 1898–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, Wen P, Jiang L, Yang J: Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 299: F973–F982, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Surendran K, Simon TC, Liapis H, McGuire JK: Matrilysin (MMP-7) expression in renal tubular damage: Association with Wnt4. Kidney Int 65: 2212–2222, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Kernan KA, Collins SJ, Cai X, López-Guisa JM, Degen JL, Shvil Y, Eddy AA: Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: Role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Wu C, Mars WM, Liu Y: Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest 117: 3821–3832, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochrane AL, Kett MM, Samuel CS, Campanale NV, Anderson WP, Hume DA, Little MH, Bertram JF, Ricardo SD: Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol 16: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Eddy AA: Can renal fibrosis be reversed? Pediatr Nephrol 20: 1369–1375, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Garant PR: Collagen resorption by fibroblasts. A theory of fibroblastic maintenance of the periodontal ligament. J Periodontol 47: 380–390, 1976 [DOI] [PubMed] [Google Scholar]
- 17.Arora PD, Glogauer M, Kapus A, Kwiatkowski DJ, McCulloch CA: Gelsolin mediates collagen phagocytosis through a rac-dependent step. Mol Biol Cell 15: 588–599, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelholm LH, Ingvarsen S, Jürgensen HJ, Hillig T, Madsen DH, Nielsen BS, Behrendt N: The collagen receptor uPARAP/Endo180. Front Biosci 14: 2103–2114, 2009 [DOI] [PubMed] [Google Scholar]
- 19.East L, Isacke CM: The mannose receptor family. Biochim Biophys Acta 1572: 364–386, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC: Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295: 1898–1901, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hanasaki K, Arita H: Biological and pathological functions of phospholipase A(2) receptor. Arch Biochem Biophys 372: 215–223, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, Nussenzweig MC, Steinman RM: A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol 61: 729–738, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Engelholm LH, List K, Netzel-Arnett S, Cukierman E, Mitola DJ, Aaronson H, Kjøller L, Larsen JK, Yamada KM, Strickland DK, Holmbeck K, Danø K, Birkedal-Hansen H, Behrendt N, Bugge TH: uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J Cell Biol 160: 1009–1015, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wienke D, MacFadyen JR, Isacke CM: Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol Biol Cell 14: 3592–3604, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH: Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol 169: 977–985, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kjøller L, Engelholm LH, Høyer-Hansen M, Danø K, Bugge TH, Behrendt N: uPARAP/endo180 directs lysosomal delivery and degradation of collagen IV. Exp Cell Res 293: 106–116, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjøller L, Gårdsvoll H, Høyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N: Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem 282: 27037–27045, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K: Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol 27: 6309–6322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasquelle C, Sartelet A, Li W, Dive M, Tamma N, Michaux C, Druet T, Huijbers IJ, Isacke CM, Coppieters W, Georges M, Charlier C: Balancing selection of a frame-shift mutation in the MRC2 gene accounts for the outbreak of the Crooked Tail Syndrome in Belgian Blue Cattle. PLoS Genet 5: e1000666, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isacke CM, van der Geer P, Hunter T, Trowbridge IS: p180, a novel recycling transmembrane glycoprotein with restricted cell type expression. Mol Cell Biol 10: 2606–2618, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheikh H, Yarwood H, Ashworth A, Isacke CM: Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J Cell Sci 113: 1021–1032, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Behrendt N, Rønne E, Danø K: A novel, specific pro-urokinase complex on monocyte-like cells, detected by transglutaminase-catalyzed cross-linking. FEBS Lett 336: 394–396, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Wu K, Yuan J, Lasky LA: Characterization of a novel member of the macrophage mannose receptor type C lectin family. J Biol Chem 271: 21323–21330, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Kim H, Cai X, Lopez-Guisa JM, Carmeliet P, Eddy AA: Urokinase receptor modulates cellular and angiogenic responses in obstructive nephropathy. J Am Soc Nephrol 14: 1234–1253, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Zhang G, Kim H, Cai X, López-Guisa JM, Alpers CE, Liu Y, Carmeliet P, Eddy AA: Urokinase receptor deficiency accelerates renal fibrosis in obstructive nephropathy. J Am Soc Nephrol 14: 1254–1271, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, Cai X, López-Guisa JM, Collins SJ, Eddy AA: Mitogenic signaling of urokinase receptor-deficient kidney fibroblasts: Actions of an alternative urokinase receptor and LDL receptor-related protein. J Am Soc Nephrol 15: 2090–2102, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Miner JH, Sanes JR: Molecular and functional defects in kidneys of mice lacking collagen alpha 3(IV): Implications for Alport syndrome. J Cell Biol 135: 1403–1413, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D: Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest 119: 3713–3722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oda T, Jung YO, Kim HS, Cai X, López-Guisa JM, Ikeda Y, Eddy AA: PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Matsuo S, López-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA: Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi I, Lopez-Guisa JM, Cai X, Collins SJ, Okamura DM, Eddy AA: Endogenous urokinase lacks antifibrotic activity during progressive renal injury. Am J Physiol Renal Physiol 293: F12–F19, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Hattori N, Mizuno S, Yoshida Y, Chin K, Mishima M, Sisson TH, Simon RH, Nakamura T, Miyake M: The plasminogen activation system reduces fibrosis in the lung by a hepatocyte growth factor-dependent mechanism. Am J Pathol 164: 1091–1098, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G, Eddy AA: Urokinase and its receptors in chronic kidney disease. Front Biosci 13: 5462–5478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaissé JM: The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem 273: 32347–32352, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Wienke D, Davies GC, Johnson DA, Sturge J, Lambros MB, Savage K, Elsheikh SE, Green AR, Ellis IO, Robertson D, Reis-Filho JS, Isacke CM: The collagen receptor Endo180 (CD280) is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res 67: 10230–10240, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Sulek J, Wagenaar-Miller RA, Shireman J, Molinolo A, Madsen DH, Engelholm LH, Behrendt N, Bugge TH: Increased expression of the collagen internalization receptor uPARAP/Endo180 in the stroma of head and neck cancer. J Histochem Cytochem 55: 347–353, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM: A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One 5: e9808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP: Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 24: 1359–1366, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Wolters PJ, Chapman HA: Importance of lysosomal cysteine proteases in lung disease. Respir Res 1: 170–177, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohamed MM, Sloane BF: Cysteine cathepsins: Multifunctional enzymes in cancer. Nat Rev Cancer 6: 764–775, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Bühling F, Röcken C, Brasch F, Hartig R, Yasuda Y, Saftig P, Brömme D, Welte T: Pivotal role of cathepsin K in lung fibrosis. Am J Pathol 164: 2203–2216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Srivastava M, Steinwede K, Kiviranta R, Morko J, Hoymann HG, Länger F, Buhling F, Welte T, Maus UA: Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis. Respir Res 9: 54, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rünger TM, Quintanilla-Dieck MJ, Bhawan J: Role of cathepsin K in the turnover of the dermal extracellular matrix during scar formation. J Invest Dermatol 127: 293–297, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Guo J, Bot I, de Nooijer R, Hoffman SJ, Stroup GB, Biessen EA, Benson GM, Groot PH, Van Eck M, Van Berkel TJ: Leucocyte cathepsin K affects atherosclerotic lesion composition and bone mineral density in low-density lipoprotein receptor deficient mice. Cardiovasc Res 81: 278–285, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G, Idell S: Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol 15: 78–87, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Jordana M, Schulman J, McSharry C, Irving LB, Newhouse MT, Jordana G, Gauldie J: Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis 137: 579–584, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Müller GA, Strutz FM: Renal fibroblast heterogeneity. Kidney Int Suppl 50: S33–S36, 1995 [PubMed] [Google Scholar]
- 61.Jelaska A, Strehlow D, Korn JH: Fibroblast heterogeneity in physiological conditions and fibrotic disease. Springer Semin Immunopathol 21: 385–395, 1999 [PubMed] [Google Scholar]
- 62.Moodley YP, Caterina P, Scaffidi AK, Misso NL, Papadimitriou JM, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA: Comparison of the morphological and biochemical changes in normal human lung fibroblasts and fibroblasts derived from lungs of patients with idiopathic pulmonary fibrosis during FasL-induced apoptosis. J Pathol 202: 486–495, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madsen DH, Ingvarsen S, Jürgensen HJ, Melander MC, Kjøller L, Moyer A, Honoré C, Madsen CA, Garred P, Burgdorf S, Bugge TH, Behrendt N, Engelholm LH: The non-phagocytic route of collagen uptake: A distinct degradation pathway. J Biol Chem 286: 26996–27010, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sturge J, Wienke D, Isacke CM: Endosomes generate localized Rho-ROCK-MLC2-based contractile signals via Endo180 to promote adhesion disassembly. J Cell Biol 175: 337–347, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Behrendt N, Jensen ON, Engelholm LH, Mørtz E, Mann M, Danø K: A urokinase receptor-associated protein with specific collagen binding properties. J Biol Chem 275: 1993–2002, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Behrendt N: The urokinase receptor (uPAR) and the uPAR-associated protein (uPARAP/Endo180): Membrane proteins engaged in matrix turnover during tissue remodeling. Biol Chem 385: 103–136, 2004 [DOI] [PubMed] [Google Scholar]
- 68.East L, McCarthy A, Wienke D, Sturge J, Ashworth A, Isacke CM: A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep 4: 710–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boskovic J, Arnold JN, Stilion R, Gordon S, Sim RB, Rivera-Calzada A, Wienke D, Isacke CM, Martinez-Pomares L, Llorca O: Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J Biol Chem 281: 8780–8787, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye Q, Xing Q, Ren Y, Harmsen MC, Bank RA: Endo180 and MT1-MMP are involved in the phagocytosis of collagen scaffolds by macrophages and is regulated by interferon-gamma. Eur Cell Mater 20: 197–209, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Shi F, Harman J, Fujiwara K, Sottile J: Collagen I matrix turnover is regulated by fibronectin polymerization. Am J Physiol Cell Physiol 298: C1265–C1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collins SJ, Alexander SL, López-Guisa JM, Cai X, Maruvada R, Chua SC, Zhang G, Okamura DM, Eddy AA: Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron Exp Nephrol 104: e23–34, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Gardner JL, Turner SM, Bautista A, Lindwall G, Awada M, Hellerstein MK: Measurement of liver collagen synthesis by heavy water labeling: Effects of profibrotic toxicants and antifibrotic interventions. Am J Physiol Gastrointest Liver Physiol 292: G1695–G1705, 2007 [DOI] [PubMed] [Google Scholar]