Abstract
A variety of chronic kidney diseases exhibit reactivation of Wnt/β-catenin signaling. In some tissues, β-catenin transcriptionally regulates matrix metalloproteinase-7 (MMP-7), but the association between MMP-7 and Wnt/β-catenin signaling in chronic kidney disease is unknown. Here, in mouse models of both obstructive nephropathy and focal segmental glomerulosclerosis (adriamycin nephropathy), we observed upregulation of MMP-7 mRNA and protein in a time-dependent manner. The pattern and extent of MMP-7 induction were positively associated with Wnt/β-catenin signaling in these models. Activation of β-catenin through ectopic expression of Wnt1 promoted MMP-7 expression in vivo, whereas delivery of the gene encoding the endogenous Wnt antagonist Dickkopf-1 abolished its induction. Levels of MMP-7 protein detected in the urine correlated with renal Wnt/β-catenin activity. Pharmacologic blockade of Wnt/β-catenin signaling by paricalcitol inhibited MMP-7 expression in diseased kidneys and reduced the levels detected in the urine. In vitro, β-catenin activation induced the expression and secretion of MMP-7 and promoted the binding of T cell factor to the MMP-7 promoter in kidney epithelial cells. We also observed higher levels of MMP-7 expression, which correlated with β-catenin, in kidney tissue from patients with various nephropathies. In summary, levels of renal MMP-7 correlate with Wnt/β-catenin activity, and urinary MMP-7 may be a noninvasive biomarker of this profibrotic signaling in the kidney.
Matrix metalloproteinase-7 (MMP-7), also known as matrilysin, is a secreted, zinc- and calcium-dependent endopeptidase that degrades a broad range of extracellular matrix substrates, such as type IV collagen, laminin, gelatin, fibronectin, and entactin.1 It also cleaves additional substrates, such as osteopontin and cell-associated Fas ligand; promotes the release of TNF-α; mediates E-cadherin ectodomain shedding; and activates other proteinases, such as urokinase plasminogen activator and pro–MMP-1, -2 and -9.2–5 Not surprisingly, the ability to act on such a broad spectrum of the substrates makes MMP-7 a critical player in regulating a diverse array of cellular processes, including extracellular matrix turnover and remodeling, cell proliferation and apoptosis, inflammation, and epithelial-mesenchymal transition.
MMP-7 is synthesized as an inactive enzymogen consisting of two structural domains: a pro-domain and a catalytic domain. Activation of the proenzyme involves the proteolytic removal of a 9-kD N-terminal pro-region. This results in the final 19-kD active enzyme, consisting of a catalytic domain with a zinc-binding motif. MMP-7 is preferentially expressed, at a low level, in the epithelial cells of various normal tissues. Its expression is upregulated in a variety of tumors, including gastric and colorectal cancers and lung carcinoma.6–8 Dysregulated expression of MMP-7 by malignant cells is thought to contribute to their neoplastic phenotype and the tissue degradation that accompanies tumor metastasis and invasion.9 MMP-7 expression is also induced and is clearly implicated in regulating tissue remodeling in many organs, such as liver, lung, and kidney, after chronic injury. In that regard, MMP-7 has been identified as a major proteinase upregulated in billary atresia–associated liver fibrosis, and its expression is thought to best discriminate between cirrhosis and precirrhotic stages.10,11 Induction of MMP-7 also occurs in human idiopathic pulmonary fibrosis, and it has been postulated as a potential peripheral blood biomarker for disease progression.12,13 As for the kidney, an earlier study documented an increased expression of MMP-7 in polycystic kidney disease, acute kidney injury induced by folic acid, and obstructive nephropathy.14 However, what regulates MMP-7 expression in diseased kidneys in vivo remains enigmatic. Furthermore, little is known about the relevance of MMP-7 regulation to the progression of renal fibrotic lesions in CKDs.
MMP-7 is transcriptionally regulated by β-catenin, the principal downstream mediator of the canonical Wnt signaling, in various malignant tissues, including colorectal cancer, intestinal adenoma, and ovarian endometrial carcinoma.6,7,15 Wnt/β-catenin signaling, an evolutionarily conserved signal cascade that plays an important role in regulating nephron formation and kidney development,16 is reactivated in a wide variety of CKDs, such as diabetic nephropathy, adriamycin nephropathy, polycystic kidney disease, obstructive nephropathy, and chronic allograft nephropathy.17–20 Activation of canonical Wnt signaling leads to β-catenin stabilization and nuclear translocation, wherein it binds to T cell factor (TCF)/lymphoid enhancer–binding factor to stimulate the transcription of Wnt target genes.21 We hypothesized that MMP-7 expression in the diseased kidneys is controlled by the canonical Wnt/β-catenin and that its expression level could serve as a surrogate marker for predicting renal Wnt/β-catenin activity.
In this study, we investigated MMP-7 regulation in diseased kidneys and delineated its relation to renal Wnt/β-catenin signaling. Our results suggest that urinary MMP-7 level, as a noninvasive surrogate biomarker, closely correlates with and predicts the activity of the canonical Wnt/β-catenin signaling in diseased kidneys.
RESULTS
MMP-7 Expression Is Induced in Obstructive Nephropathy
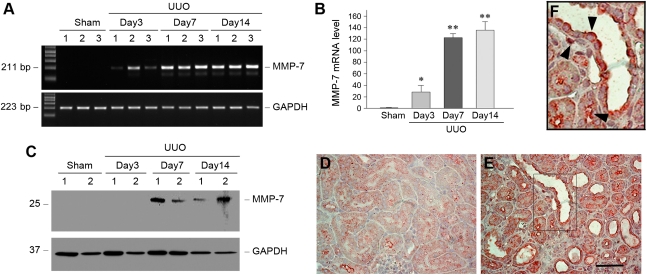
We investigated the expression of MMP-7 in obstructive nephropathy, a well characterized and widely used model of renal interstitial fibrosis. As shown in Figure 1A, the steady-state level of MMP-7 mRNA was markedly increased in the obstructed kidneys in a time-dependent fashion. Quantitative determination revealed a >120-fold induction of MMP-7 mRNA abundance in the obstructed kidneys 7 days after unilateral ureter obstruction, compared with sham controls. Increased MMP-7 protein expression was also evident in the obstructed kidney by Western blot analyses of whole kidney lysates. Immunohistochemical staining illustrated that MMP-7 protein was primarily localized in the tubular epithelial cells, as well as interstitial cells (Figure 1, D–F). Interestingly, increased MMP-7 protein was preferentially distributed in the apical region of renal tubular epithelium (Figure 1F, arrowheads), implying the possibility for its inclined secretion into tubular lumens. Of note, the pattern and extent of this MMP-7 induction appear to be closely associated with the activation of Wnt/β-catenin signaling in this model, as documented elsewhere.20
Figure 1.
MMP-7 expression is induced in a mouse model of obstructive nephropathy. (A and B) Representative RT-PCR results (A) and graphic presentation (B) show MMP-7 mRNA expression in different groups of mice as indicated. Numbers (1, 2, and 3) in A denote each individual animal in a given group. Data in B are presented as mean ± SEM of five animals (n=5). *P<0.05 and **P<0.01 versus sham controls. (C) Western blot analyses show MMP-7 protein induction in the obstructed kidney at different time points after unilateral ureter obstruction (UUO). Kidney lysates were immunoblotted with antibodies against MMP-7 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively. (D and E) Immunohistochemical staining shows the localization of MMP-7 protein in obstructive nephropathy. Kidney sections from the sham (D) and unilateral ureter obstruction (E) groups 7 days after surgery were immunostained with antibody against MMP-7. Scale bar, 50 μm. (F) Enlarged image from the boxed area in E. Arrowheads indicate MMP-7–positive cells.
MMP-7 Expression Is Correlated with Renal β-Catenin Induction after Injury
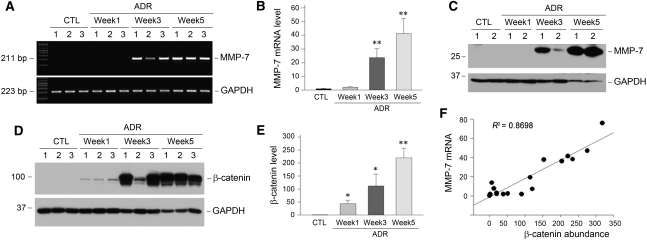
To further study MMP-7 expression in other CKDs, we used adriamycin nephropathy, a model of FSGS that is characterized by initial podocyte injury and proteinuria and subsequent interstitial inflammation and fibrosis.22,23 As shown in Figure 2A, renal MMP-7 mRNA was markedly induced at 3 weeks after adriamycin injection. At 5 weeks, when significant renal fibrotic lesions developed in this model,24 MMP-7 mRNA level was increased by approximately 40-fold compared with levels in the controls (Figure 2B). Western blot analyses also showed a time-dependent induction of MMP-7 protein in the kidneys after adriamycin injection. Of note, Wnt/β-catenin signaling was dramatically activated in this model, as illustrated by an increased expression and accumulation of renal β-catenin protein in a time-dependent manner (Figure 2, D and E). Linear regression showed a close correlation between renal MMP-7 mRNA levels and β-catenin abundance (Figure 2F), suggesting that renal MMP-7 may be a transcriptional target of the canonical Wnt/β-catenin signaling in this model.
Figure 2.
MMP-7 expression is closely correlated with renal β-catenin induction in a mouse model of adriamycin (ADR) nephropathy. (A) Representative RT-PCR results demonstrate a time-dependent induction of MMP-7 mRNA in a mouse model of adriamycin nephropathy. Numbers (1, 2, and 3) denote each individual animal in a given group. (B) Quantitative data on renal MMP-7 mRNA abundance at different time points after adriamycin injection. **P<0.01 versus controls (CTL) (n=5). (C) Representative Western blot analyses show MMP-7 protein induction in the kidneys at different time points after adriamycin injection. (D and E) Representative Western blot analyses (D) and quantitative data (E) show the induction of renal β-catenin protein at different time points after adriamycin injection. Numbers (1, 2, and 3) in D denote each individual animal in a given group. *P<0.05 and **P<0.01 versus sham controls (n=5). (F) Linear regression shows a close correlation between renal MMP-7 mRNA levels and β-catenin abundance (arbitrary units). The correlation coefficient (R2) is shown.
Wnt/β-Catenin Signaling Promotes MMP-7 Expression In Vivo
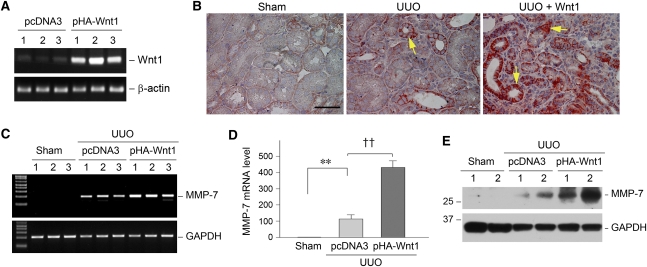
To establish a direct relationship between MMP-7 expression and Wnt/β-catenin signaling in vivo, we sought to activate canonical Wnt/β-catenin signaling by ectopic expression of Wnt1, the prototypic Wnt that transmits its signal via the canonical pathway, through a hydrodynamics-based gene delivery strategy.25–27 As shown in Figure 3A, a single intravenous injection of Wnt1 expression vector (pHA-Wnt1) induced significant Wnt1 mRNA expression in the kidneys. Quantitative analyses revealed a >15-fold induction of renal Wnt1 mRNA 24 hours after plasmid injection (15.6±2.4 in the pHA-Wnt1 group versus 1.0±0.15 in the pcDNA3 group; P<0.01; n=3). As reported elsewhere,27 ectopic expression of Wnt1 in the obstructed kidney significantly induced β-catenin expression at 7 days after unilateral ureter obstruction. Immunohistochemical staining revealed that increased β-catenin was primarily localized in the cytoplasm and nuclei of renal tubular epithelia (Figure 3B, arrows), indicating an activation of this signaling protein. Interestingly, activation of Wnt/β-catenin signaling via ectopic expression of Wnt1 resulted in increased MMP-7 mRNA expression in the obstructed kidney (Figure 3, C and D). Similarly, MMP-7 protein was also induced in the obstructed kidneys after ectopic expression of Wnt1, as shown by Western blot analyses (Figure 3E).
Figure 3.
Canonical Wnt/β-catenin signaling promotes MMP-7 expression in vivo. (A) Ectopic expression of Wnt1 in the kidney. Mice were injected intravenously with Wnt1 expression vector (pHA-Wnt1) or empty vector (pcDNA3) as indicated. Renal expression of Wnt1 mRNA was assessed by RT-PCR. Numbers (1, 2, and 3) denote each individual animal in a given group. (B) Ectopic expression of Wnt1 in vivo induces renal β-catenin accumulation in obstructive nephropathy. Representative micrographs demonstrate renal β-catenin expression and localization in the sham group (left), group with unilateral ureter obstruction (UUO) injected with pcDNA3 (center), and group with unilateral ureter obstruction injected with pHA-Wnt1 plasmid (right). Bar = 50 μm. (C and D) Representative RT-PCR results (C) and quantitative determination (D) show renal MMP-7 mRNA expression in different groups as indicated. Numbers (1, 2, and 3) in C denote each individual animal in a given group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Data in D are presented as mean ± SEM of five animals (n=5). **P<0.01 versus sham controls; ††P<0.01 versus pcDNA3. (E) Western blot analyses show renal MMP-7 protein abundance in the obstructed kidney in different groups as indicated.
Inhibition of Wnt/β-Catenin Signaling Represses MMP-7 Expression In Vivo
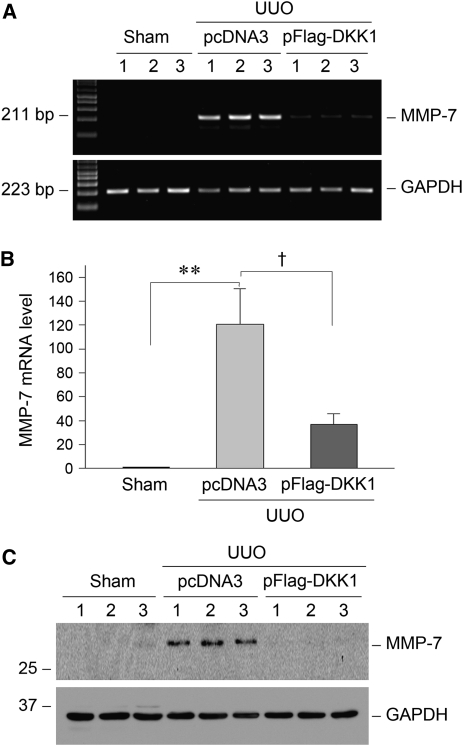
We next investigated the role of Wnt/β-catenin signaling in regulating MMP-7 expression in vivo by blocking Wnt signaling. To this end, the Dickkopf-1 (DKK1) gene, a natural, secreted Wnt antagonist that specifically blocks the canonical Wnt/β-catenin signaling by disrupting the engagement of Wnts to their LDL-related protein-5/6 co-receptors,28,29 was administrated to mice by the hydrodynamics-based gene delivery approach. As reported elsewhere,20 delivery of the DKK1 gene resulted in substantial elevation of the circulating DKK1 protein levels, as well as kidney expression of transgene. We found that blockade of Wnt/β-catenin signaling by the DKK1 gene substantially inhibited MMP-7 expression in the obstructed kidneys 7 days after unilateral ureter obstruction. As shown in Figure 4, A and B, renal MMP-7 mRNA was suppressed by more than 65% in the obstructed kidney after ectopic expression of exogenous DKK1. Accordingly, renal MMP-7 protein expression was also suppressed by DKK1 in the obstructed kidneys after injury (Figure 4C).
Figure 4.
Inhibition of Wnt/β-catenin signaling by its antagonist represses MMP-7 expression in obstructive nephropathy. (A) Representative RT-PCR results demonstrate that ectopic expression of DKK1 gene inhibited MMP-7 mRNA expression in the obstructed kidney after unilateral ureter obstruction (UUO). Numbers (1, 2, and 3) denote each individual animal in a given group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Quantitative data of renal MMP-7 mRNA abundance in different groups as indicated. *P<0.05 and **P<0.01 versus sham controls and †P<0.05 versus pcDNA3 (n=4–5). (C) Representative Western blot analyses show MMP-7 protein expression in the obstructed kidneys in different groups as indicated.
Urinary MMP-7 Correlates with Its Renal Expression and Wnt/β-Catenin Activity
Because MMP-7 is a secreted protein, we next tested the hypothesis that MMP-7 might be detectable in urine and that the urinary MMP-7 level could reflect its renal expression in CKDs. To this end, we examined urinary MMP-7 using a specific ELISA in a mouse model of adriamycin nephropathy. As shown in Figure 5A, a substantial increase in urinary MMP-7 was detected 5 weeks after adriamycin injection compared with that in the controls. Paricalcitol, a synthetic vitamin D analogue, was recently shown to block Wnt/β-catenin signaling in this model in vivo.24 We consistently found that paricalcitol also significantly reduced urinary MMP-7 excretion (Figure 5A). To investigate whether urinary MMP-7 levels reflect renal expression of MMP-7, we further examined MMP-7 expression in the diseased kidneys after adriamycin injection. As shown in Figure 5B, renal expression of MMP-7 protein as detected by Western blot analyses was closely correlated with its urinary levels. Similar results were obtained when MMP-7 mRNA expression was examined in the kidneys 5 weeks after adriamycin injection (Figure 5, C and D). Therefore, it appears that urinary MMP-7 levels faithfully reflect renal expression of MMP-7, thereby predicting renal Wnt/β-catenin activity.
Figure 5.
Urinary MMP-7 correlates with its renal expression in adriamycin (ADR) nephropathy. (A) Urinary MMP-7 levels in mice. Mouse urinary MMP-7 protein was quantitatively determined by a specific ELISA and expressed as ng/mg creatinine. *P<0.05 versus sham controls and §P<0.05 versus vehicle (n=5). CTL, controls. (B) Representative Western blot analyses show renal MMP-7 protein expression in different groups as indicated. Numbers (1, 2, and 3) denote each individual animal in a given group. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (C) Representative RT-PCR results show renal MMP-7 mRNA expression 5 weeks after adriamycin injection. Numbers (1, 2, and 3) denote each individual animal in a given group. (D) Quantitative data on renal MMP-7 mRNA abundance in different groups as indicated. *P<0.05 and **P<0.01 versus sham controls and §P<0.05 versus vehicle (n=5).
Activation of β-Catenin Induces MMP-7 Expression In Vitro
To further confirm a direct role of β-catenin activation in mediating MMP-7 expression, we investigated MMP-7 gene regulation in cultured kidney tubular epithelial (HKC-8) cells. To this end, HKC-8 cells were transfected with N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat), as reported elsewhere.30 As shown in Figure 6A, ectopic expression of the constitutively active β-catenin in HKC-8 cells significantly induced MMP-7 mRNA expression, suggesting that β-catenin activation is sufficient for inducing MMP-7 expression. However, incubation of the cells with ICG-001, a small-molecule inhibitor that disrupts β-catenin–mediated gene transcription,30,31 was able to completely block MMP-7 mRNA induction (Figure 6, A and B). Similarly, MMP-7 protein expression in HKC-8 cells was also controlled by β-catenin, as shown by Western blot analyses (Figure 6C). Of note, human MMP-7 protein in cultured tubular epithelial cells in vitro displayed two predominant bands corresponding to pro–MMP-7 (28 kD) and active MMP-7 (19 kD), respectively. It appeared that the majority of MMP-7 in HKC-8 cells was the active form (Figure 6C). Notably, MMP-7 protein produced by HKC-8 cells was readily secreted into the extracellular space; it was detectable in the supernatants of cell cultures by a specific ELISA. As shown in Figure 6D, ectopic expression of β-catenin increased MMP-7 secretion by HKC-8 cells, whereas ICG-001 completely abolished the elevation of MMP-7 level in the supernatant. Of note, ICG-001 displayed the tendency to suppress MMP-7 secretion without overexpression of constitutively active β-catenin, suggesting a role for β-catenin signaling in mediating MMP-7 secretion in the basal conditions as well.
Figure 6.
Active β-catenin directly induces MMP-7 expression in kidney tubular epithelial cells in vitro. (A) Representative RT-PCR results show that ectopic expression of constitutively active β-catenin induced MMP-7 expression. Human kidney proximal tubular epithelial (HKC-8) cells were transiently transfected with N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat) or empty vector (pcDNA3), followed by incubation without or with small-molecule inhibitor ICG-001 (10 μM) for 24 hours, respectively. (B) Quantitative data on MMP-7 mRNA abundance in HKC-8 cells after various treatments as indicated. **P<0.01 versus sham controls and §§P<0.01 versus pDel-β-cat alone (n=3). (C) Western blot analyses show MMP-7 expression in HKC-8 cells after various treatments as indicated. Both pro–MMP-7 (28 kD) and active MMP-7 (19 kD) are indicated. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (D) MMP-7 secretion detected by a specific ELISA. The supernatants of HKC-8 cells after various treatments as indicated were collected and assayed for MMP-7 protein abundance by ELISA. **P<0.01 versus sham controls and §§P<0.01 versus pDel-β-cat alone (n=3).
Activation of β-Catenin Promotes TCF Binding to MMP-7 Promoter
To gain molecular insights into how β-catenin regulates MMP-7 expression, we investigated the interaction between TCF and MMP-7 gene promoter in the kidney cells using an in situ chromatin immunoprecipitation (ChIP) assay. Bioinformatics analysis revealed that human MMP-7 gene promoter contained two putative TCF-binding elements (TBEs) (Figure 7A), a proximal TBE1 and a distal TBE2, that could mediate the transcriptional regulation of MMP-7 by β-catenin. As shown in Figure 7, B and C, ectopic expression of the N-terminally truncated, stabilized β-catenin in HKC-8 cells clearly promoted TCF binding to the proximal TBE1 in the MMP-7 promoter. In addition, TCF binding to distal TBE2 appeared to increase after β-catenin expression in HKC-8 cells as well (Figure 7B), although it did not reach statistical significance (Figure 7C). These results suggest that β-catenin activation can facilitate TCF binding to the proximal TBE1 and, to less extent, the distal TBE2 in tubular epithelial cellls, leading to MMP-7 gene trans-activation.
Figure 7.
Active β-catenin promotes T cell factor (TCF) binding to the TCF-binding elements (TBE) in the MMP-7 promoter. (A) Partial sequence of human MMP-7 gene promoter region. Bold letters indicate the proximal TBE1 and distal TBE2. P1 and P2 indicate the primer pair encompassing the proximal TBE1, and P3 and P4 are the primer pair for the distal TBE2. (B and C) Chromatin immunoprecipitation (ChIP) assay revealed that ectopic expression of constitutively active, stabilized β-catenin promoted TCF binding to the proximal TBE1 and, to a lesser extent, to distal TBE2 in human kidney proximal tubular epithelial cells. Representative ChIP assay (B) and quantitative ChIP data (C) are presented. ‡P<0.05 versus pcDNA3 (n=3).
MMP-7 Mediates E-Cadherin Degradation
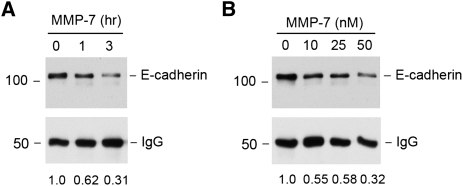
To explore the potential implication of MMP-7 in the pathogenesis of renal fibrosis, we examined its effect on E-cadherin in tubular epithelial cells because previous studies have indicated that MMP-7 mediates E-cadherin ectodomain shedding.2 To this end, HKC-8 cell lysates were immunoprecipitated with anti–E-cadherin antibody, and the resultant immunocomplexes were incubated with recombinant MMP-7 in vitro. As shown in Figure 8, MMP-7 clearly induced E-cadherin degradation in a time- and dose-dependent manner. Furthermore, this MMP-7–mediated E-cadherin degradation was prevented by MMP inhibitor II (data not shown). Therefore, it is conceivable that MMP-7 can induce proteolytic degradation of E-cadherin and thereby impair the structural integrity of renal tubular epithelia.32
Figure 8.
Recombinant MMP-7 degrades E-cadherin in vitro. Human kidney proximal tubular epithelial cell lysates were immunoprecipitated with anti–E-cadherin antibody, followed by incubating with recombinant MMP-7 (50 nM) for various periods of time (A) or different concentrations of MMP-7 for 3 hours (B). E-cadherin protein abundance after MMP-7 incubation was assessed by Western blot and was normalized with the input protein (IgG heavy chain). The relative levels of E-cadherin after MMP-7 treatment were quantitated and are presented in the bottom of pictures.
Increased MMP-7 Expression in Human Diseased Kidneys
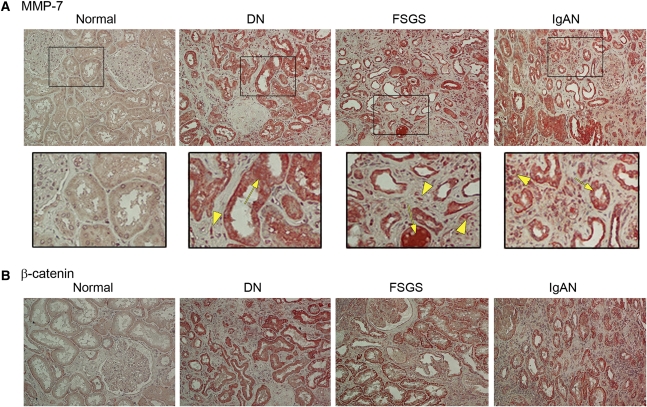
To establish the relevance of MMP-7 regulation to the pathogenesis and progression of human CKDs, we investigated its expression in kidney biopsy specimens from patients with various CKDs. As shown in Figure 9A, MMP-7 was expressed at extremely low levels in three specimens from normal human kidneys, if it was expressed at all. However, MMP-7 protein was readily detectable in all six biopsy specimens from patients with CKD, according to immunohistochemical staining (albeit with varying staining intensity). Figure 9A shows the representative micrographs of MMP-7 staining in the kidney sections from patients with diabetic nephropathy, FSGS, and IgA nephropathy (IgAN). MMP-7 protein was predominantly localized in renal tubular epithelia in the diseased kidneys (Figure 9A, arrows). Occasionally, fluids in the lumens of renal tubules also stained positively for MMP-7, suggesting its secretion by tubular cells. Besides tubular cells, some interstitial fibroblast-like cells with spindle shape, glomerular podocytes, and infiltrated inflammatory cells were apparently positive for MMP-7 staining as well (Figure 9A, arrowheads). Immunohistochemical staining for β-catenin in these human kidney specimens produced similar results (Figure 9B). Thus, MMP-7 expression is also closely correlated with renal canonical Wnt/β-catenin signaling in humans.
Figure 9.
MMP-7 expression is increased in human kidney biopsy specimens from patients with various CKDs. (A) Representative immunohistochemical staining shows an increased expression of MMP-7 protein in various human CKDs. Normal, nontumor kidney tissue from the patients who had renal cell carcinoma was used for normal controls. DN, diabetic nephropathy; IgAN, IgA nephropathy. Arrows indicate the predominant tubular localization of MMP-7. Arrowheads denote increased MMP-7 expression in other cell types, including interstitial cells, glomerular podocytes, and infiltrated inflammatory cells. (B) Representative immunohistochemical staining shows increased β-catenin expression in various human CKDs. Kidney tissues from normal controls and from patients with diabetic nephropathy, FSGS, or IgAN, respectively, were immunostained with antibody against β-catenin.
DISCUSSION
Using animal models, cultured kidney cells, and human biopsy specimens, we have carried out a comprehensive investigation on the expression and regulation of MMP-7 in normal and diseased kidneys. Our results indicate that MMP-7 is markedly induced primarily in renal tubular epithelial cells in a variety of fibrotic kidney diseases. The induction of MMP-7 is temporally and spatially correlated with the activation of Wnt/β-catenin signaling, a developmental signal pathway that is reactivated in the pathogenesis of chronic renal fibrosis. Furthermore, MMP-7 expression in diseased kidneys is dictated by the manipulations of the canonical Wnt/β-catenin signaling through ectopic expression of Wnt1 or its antagonist DKK1 genes. These findings provide significant insights into understanding the regulation of MMP-7 and its underlying mechanism in diseased kidneys, and further raise the possibility that MMP-7 level could predict the activity of renal Wnt/β-catenin signaling, thereby forecasting the progression of renal fibrotic lesions.
In normal kidneys, MMP-7 is expressed at extremely low basal levels, if at all. Using different approaches, including reverse transcriptase (RT)–PCR, Western blot, and immunostaining, no or little MMP-7 was detected in normal kidneys in mice and humans, suggesting that it is dispensable in the maintenance of kidney structure and function in normal physiologic conditions. This finding is in line with earlier observations that mice with genetic ablation of MMP-7 are normal in size and do not display any gross physical or behavior abnormalities.33 However, both MMP-7 mRNA and protein are dramatically induced in the injured kidneys in response to diverse types of insults. The magnitude of MMP-7 induction over the controls is remarkable in mouse model of obstructive nephropathy (Figure 1). In fact, among many MMPs examined in this model (data not shown), MMP-7 is the most upregulated metalloproteinase, suggesting a predominant response of this enzyme in the kidneys to injury. It should be noted that significant MMP-7 induction in adriamycin nephropathy occurs only 3 weeks after adriamycin injection, despite early proteinuria and podocyte damage observed at 7 days in this model.24,34 These results suggest that MMP-7 induction is temporally associated with the onset of tubulointerstitial fibrotic lesions, but not glomerular lesions, in diseased kidney. Consistent with this notion, increased MMP-7 was primarily localized in renal tubular epithelia in kidney biopsy specimens from patients with various kidney disorders (Figure 9). Of interest, MMP-7 induction occurs in a broad spectrum of CKDs, such as obstructive nephropathy, adriamycin nephropathy (and human FSGS), diabetic nephropathy, and IgA nephropathy. This finding suggests that regardless of the initial causes, MMP-7 induction could represent a general and converging response of the kidneys to various toxic, metabolic, immune, and hemodynamic insults.
One of the interesting findings in this study is that MMP-7 expression in the diseased kidneys is primarily controlled by canonical Wnt/β-catenin signaling. This conclusion is substantiated by several lines of evidence. First, MMP-7 expression in animal models of kidney disease and in human kidney biopsy specimens is closely correlated with β-catenin, the principal transcriptional regulator downstream of Wnt signaling. Second, activation of endogenous β-catenin by ectopic expression of the Wnt1 gene in vivo promotes MMP-7 expression in the obstructed kidney. Conversely, delivery of the Wnt natural antagonist DKK1 gene inhibits renal MMP-7 induction in vivo. In addition, pharmacologic blockade of Wnt/β-catenin signaling by the vitamin D analogue paricalcitol also represses renal MMP-7 expression in adriamycin nephropathy, although we cannot exclude the possibility that paricalcitol may regulate MMP-7 by other mechanisms as well. Third, β-catenin is sufficient to induce MMP-7 expression in proximal tubular epithelial cells in vitro, and it facilitates TCF binding to its cognate TBE in the MMP-7 gene promoter region. These results are also compatible with earlier reports on the importance of Wnt/β-catenin in controlling MMP-7 expression in cancer cells.6,7 In this context, it becomes clear that MMP-7 is a transcriptional target of the canonical Wnt/β-catenin signaling in diseased kidneys. Given increased Wnt/β-catenin signaling in various fibrotic kidney diseases, as recently reported,19,20,35 the present study has defined an important pathogenic mechanism by which Wnt/β-catenin exerts its actions through inducing MMP-7 expression.
The magnitude of MMP-7 induction is closely correlated with the extent of renal fibrotic lesions in both models of obstructive and adriamycin nephropathy (Figures 1 and 2), and alterations in MMP-7 expression via manipulating Wnt/β-catenin signaling are also associated with the changes in the degree of renal fibrosis in vivo.20,24 At this stage, however, the exact role of MMP-7 induction in renal fibrogenesis remains to be determined. We have shown herein that MMP-7 induces proteolytic degradation of E-cadherin (Figure 8), an epithelial cell adhesion receptor that is essential for the maintenance of tubular epithelial integrity. This is in line with previous reports that MMP-7 mediates the ectodomain shedding of E-cadherin in injured lung epithelium.2,36 Of note, proteolytic shedding of E-cadherin also causes β-catenin nuclear translocation and Slug induction,37 which further induces MMP-7 expression and represses E-cadherin transcription in tubular epithelial cells. Because destruction of E-cadherin is the initial step during the entire course of epithelial-mesenchymal transition,38 such a MMP-7–mediated E-cadherin ectodomain shedding undoubtedly contributes to the tubular epithelial injury and promotes epithelial-mesenchymal transition in diseased kidneys. Furthermore, because MMP-7 degrades collagen IV and laminin, the major components of tubular basement membrane, it is conceivable that increased MMP-7 would aggravate TBM damage, which facilitates tubular epithelial-mesenchymal transition in the fibrotic kidneys.39 In addition, MMP-7 stimulates the activation of pro–MMP-2 and -9, which promote renal fibrotic lesions.40,41 MMP-7 also promotes the release of TNF-α,5 thereby deteriorating renal inflammation and leading to fibrotic demolition. Therefore, it is conceivable that MMP-7 may promote renal fibrosis by a multitude of mechanisms.
By illustrating a close correlation between renal MMP-7 level and β-catenin, our study further raises the possibility that this protein could serve as a surrogate biomarker for predicting the activity of the canonical Wnt/β-catenin signaling in diseased kidneys. The very nature of this enzyme as a secreted protein that is readily detectable in urine makes it particularly attractive for developing it as a noninvasive biomarker for monitoring renal Wnt/β-catenin, a critical fibrogenic signaling implicated in renal fibrosis. In that regard, a recent study using a urinary peptidomics approach has identified MMP-7 as one of the biomarkers for acute rejection in renal transplantation.42 Similarly, plasma MMP-7 is proposed as a potential biomarker in human idiopathic pulmonary fibrosis and as a metastatic marker and survival predictor in patients with renal cell carcinomas.13,43 Whether plasma MMP-7 level is also elevated in the fibrotic kidney diseases remains to be determined. Clearly, more clinical studies and validations in large cohorts are needed in this area.
CONCISE METHODS
Animal Model
Male CD-1 and BALB/c mice weighing about 20–23 g were obtained from Harlan Sprague–Dawley (Indianapolis, IN). All animal studies were performed by use of the procedures approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh, Pittsburgh, PA. Unilateral ureter obstruction was performed in CD-1 mice using an established protocol, as described elsewhere.44 Sham-operated mice were used as normal controls. Mice were killed at 3, 7, and 14 days after unilateral ureter obstruction. For delivery of the Wnt1 or DKK1 gene, naked Wnt1 expression plasmid (pHA-Wnt1; Upstate Biotechnology) or DKK1 expression plasmid (pFlag-DKK1; provided by Dr. Xi He, Harvard Medical School, Boston, MA) was injected intravenously at 1 mg/kg body wt 1 day before (day −1) unilateral ureter obstruction by use of a hydrodynamics-based in vivo gene transfer approach, as described elsewhere.25,26 For empty vector controls, pcDNA3 plasmid was injected into the mice that had undergone unilateral ureter obstruction, in an identical manner. Groups of mice (n=4–5) were killed at 7 days after unilateral ureter obstruction, and the kidney tissues were removed for various analyses.
BALB/c mice were used for establishing adriamycin nephropathy, as described elsewhere.34 Briefly, adriamycin (doxorubicin hydrochloride; Sigma, St. Louis, MO) was administrated by a single intravenous injection at 10 mg/kg body wt. Paricalcitol (provided by Abbott Laboratories, Abbott Park, IL) was given by daily subcutaneous injection at 50 ng/kg body wt, starting at 1 day before adriamycin was administrated. Five weeks after adriamycin injection, urine was collected and all mice (n=5 per group) were sacrificed. Kidney tissues were collected for various analyses.
Cell Culture and DNA Transfection
HKC-8 cells were cultured in DMEM-Ham F12 medium supplemented with 5% calf serum, as described elsewhere.44 HKC-8 cells were transiently transfected with Flag-tagged N-terminally truncated, stabilized β-catenin expression vector (pDel-β-cat) or empty vector pcDNA3 using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), as described elsewhere.17 After transfection, cells were treated with the small-molecule β-catenin–signaling inhibitor ICG-001 (10 μM) for 24 hours. ICG-001 compound was described elsewhere.27,31 Cells were then collected for various analyses.
RT-PCR and Real-Time RT-PCR
Total RNA was extracted from kidney tissues or cultured cells using TRIzol RNA isolation system (Invitrogen). The first strand of cDNA was synthesized using 2 µg of RNA in 20 µl of reaction buffer by RT using AMV-RT (Promega, Madison, WI) and random primers at 42°C for 30 min. PCR was carried out using a standard PCR kit and a 1-µl aliquot of cDNA, HotStarTaq polymerase (Qiagen Inc., Valencia, CA), with specific primer pairs. Quantitative, real-time RT-PCR was also performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA), as described elsewhere.24 The PCR reaction mixture in a 25-μl volume contained 12.5 μl 2× SYBR Green PCR Master Mix (Applied Biosystems), 5 μl diluted RT product (1:10), and 0.5 μM sense and antisense primer sets. The sequences of the primer pair for Wnt1 were described elsewhere.20 The sequences of other primer pairs are given in Supplementary Table 1. PCR reaction was run using standard conditions. The mRNA levels of various genes were calculated after normalizing with glyceraldehyde 3-phosphate dehydrogenase or β-actin.
Western Blot Analysis
Western blot analysis for specific protein expression was performed essentially according to an established procedure.27 The primary antibodies used were as follows: anti–MMP-7 (#11603; GeneTex, Irvine, CA); anti–β-catenin (#610154; BD Transduction Laboratories, San Jose, CA); anti-Flag (clone M2; #F3165; Sigma); and anti–glyceraldehyde 3-phosphate dehydrogenase (#4300; Ambion, Austin, TX). For human cell lysates, anti–MMP-7 antibody obtained from Abcam (ab5706; Cambridge, MA) was used.
Immunohistochemical Staining
Human kidney specimens were obtained from diagnostic renal biopsies performed at the University of Pittsburgh Medical Center. Nontumor kidney tissue from the patients who had renal cell carcinoma and underwent nephrectomy was used for normal controls. All studies involving human tissues were approved by the Institutional Review Board at the University of Pittsburgh. Paraffin-embedded mouse kidney sections (3- to 4-μm thickness) were prepared by a routine procedure. Immunohistochemical staining of kidney sections for MMP-7 and β-catenin was performed using the Vector M.O.M. immunodetection kit, according to the protocol specified by the manufacturer (Vector Laboratories, Burlingame, CA). The following primary antibodies were used: anti–MMP-7 (Genetex) and anti–β-catenin (ab15180; Abcam). As a negative control, the primary antibody was replaced with nonimmune IgG, and no staining occurred. Stained sections were viewed with a Nikon Eclipse E600 microscope equipped with a digital camera (Melville, NY).
MMP-7 Secretion Assay by ELISA
Human MMP-7 secretion was quantitatively assessed by measuring total MMP-7 protein in the supernatant of cultured HKC-8 cells using an ELISA kit (Quantikine Human MMP-7 Immunoassay; R&D Systems, Minneapolis, MN). This assay uses the quantitative sandwich enzyme immunoassay technique. Briefly, the supernatants of HKC-8 cells after various treatments as well as standard MMP-7 were added onto a microplate precoated with monoclonal anti–MMP-7 antibody and incubated for 2 hours. After extensive washing, an enzyme-linked polyclonal anti–MMP-7 antibody was added to the wells and incubated for additional 2 hours. After substrate solution was added and color-developed, optical density was measured at 450 nm using a microplate reader. For determining urinary MMP-7 levels, a specific ELISA kit for mouse MMP-7 was used, and the assay was carried out according to the protocol specified by the manufacturer (Mouse MMP-7 ELISA kit; Wuhan EIAab Science Co., Wuhan, China).
ChIP Assay
A ChIP assay was performed to analyze in vivo interactions of TCF and putative TBE in human MMP-7 promoter. This assay was carried out essentially according to the protocols specified by the manufacturer (Upstate Biotechnology).27 Briefly, after various treatments as indicated, HKC-8 cells were cross-linked with 1% formaldehyde and then resuspended in SDS lysis buffer containing protease inhibitors. The chromatin solution was sonicated, and the supernatant was diluted 10-fold. An aliquot of total diluted lysate was used for total genomic DNA as input DNA control. The anti–TCF-1 antibody (clone 7H13; #05-510; Upstate) was added and incubated at 4°C overnight, followed by incubation with protein A-agarose for 1 hour. The precipitates were washed and chromatin complexes were eluted. After reversal of the cross-linking at 65°C for 4 hours, the DNA was purified, and ChIP samples were used as a template for PCR. Primer sets P1/P2 and P3/P4 encompass different regions of the MMP-7 promoter containing proximal and distal putative TBE, respectively. The sequences of primers used for ChIP assay are given in Figure 7A. PCR samples were analyzed by electrophoresis on a 2.0% agarose gel.
Assessment of E-Cadherin Degradation by MMP-7
For enriching E-cadherin protein, HKC-8 cell lysates were immunoprecipitated with anti–E-cadherin antibody (#610182; BD Transduction, San Jose, CA) according to the established procedure.45 The immunocomplexes were then incubated with different concentrations of recombinant MMP-7 (#444270; EMD Chemicals, Gibbstown, NJ) for various periods of time as indicated. In some experiments, MMP inhibitor II (#444247; EMD Chemicals) was added to the incubation mixture. E-cadherin protein abundance after MMP-7 incubation was assessed by Western blot analyses.
Statistical Analyses
All data examined were expressed as mean ± SEM. Statistical analyses of the data were performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparisons between groups were made using one-way ANOVA followed by the Student-Newman-Keuls test. A P value less than 0.05 was considered to indicate a statistically significant difference.
Disclosures
None.
Acknowledgments
This work was supported by National Institutes of Health Grant DK064005, National Natural Science Foundation of China Grant 81130011, and 973 Program Grant 2012CB517700.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011050490/-/DCSupplemental.
REFERENCES
- 1.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T: Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269–285, 2003 [DOI] [PubMed] [Google Scholar]
- 2.McGuire JK, Li Q, Parks WC: Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162: 1831–1843, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargo-Gogola T, Crawford HC, Fingleton B, Matrisian LM: Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys 408: 155–161, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I: Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 61: 577–581, 2001 [PubMed] [Google Scholar]
- 5.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM: Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 105: 143–150, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T: Beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155: 1033–1038, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford HC, Fingleton B, Gustavson MD, Kurpios N, Wagenaar RA, Hassell JA, Matrisian LM: The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumors. Mol Cell Biol 21: 1370–1383, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leinonen T, Pirinen R, Böhm J, Johansson R, Ropponen K, Kosma VM: Expression of matrix metalloproteinases 7 and 9 in non-small cell lung cancer. Relation to clinicopathological factors, beta-catenin and prognosis. Lung Cancer 51: 313–321, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Masaki T, Matsuoka H, Sugiyama M, Abe N, Goto A, Sakamoto A, Atomi Y: Matrilysin (MMP-7) as a significant determinant of malignant potential of early invasive colorectal carcinomas. Br J Cancer 84: 1317–1321, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, Chen YS, Chen CL, Tai MH: Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol 18: 941–950, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, Bahr M, Flemming P, Manns MP, Boeker KH: Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol 34: 239–247, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, Nakamura M, Tasaka S, Hasegawa N, Aikawa N, Ishizaka A, Okada Y: Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med 134: 1136–1142, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, Sciurba F, Dauber J, Selman M, Gochuico BR, Kaminski N: MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5: e93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surendran K, Simon TC, Liapis H, McGuire JK: Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 65: 2212–2222, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Machin P, Catasus L, Pons C, Muñoz J, Matias-Guiu X, Prat J: CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol 33: 206–212, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP: Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9: 283–292, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surendran K, Schiavi S, Hruska KA: Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 19.von Toerne C, Schmidt C, Adams J, Kiss E, Bedke J, Porubsky S, Gretz N, Lindenmeyer MT, Cohen CD, Gröne HJ, Nelson PJ: Wnt pathway regulation in chronic renal allograft damage. Am J Transplant 9: 2223–2239, 2009 [DOI] [PubMed] [Google Scholar]
- 20.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Wang YP, Tay YC, Harris DC: Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 24.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai C, Yang J, Liu Y: Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol 13: 411–422, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y: Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology 33: 848–859, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niehrs C: Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25: 7469–7481, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X: Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11: 951–961, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eguchi M, Nguyen C, Lee SC, Kahn M: ICG-001, a novel small molecule regulator of TCF/beta-catenin transcription. Med Chem 1: 467–472, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Liu Y: Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159: 1465–1475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Park PW, Wilson CL, Parks WC: Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111: 635–646, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Dai C, Saleem MA, Holzman LB, Mathieson P, Liu Y: Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int 77: 962–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H: β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant 25: 2437–2446, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M: Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu TT, Min D, Succar L, Rangan GK, Hu M, Henderson BR, Alexander SI, Harris DC: Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol 175: 580–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y: Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y: Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Invest 110: 1525–1538, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, Wen P, Jiang L, Yang J: Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 299: F973–F982, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH: Matrix metalloproteinase 2 and basement membrane integrity: A unifying mechanism for progressive renal injury. FASEB J 20: 1898–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Ling XB, Sigdel TK, Lau K, Ying L, Lau I, Schilling J, Sarwal MM: Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol 21: 646–653, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K: Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinomas. Cancer Sci 99: 1188–1194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan R, Zhang X, Yang J, Li Y, Liu Y: Molecular basis for the cell type specific induction of SnoN expression by hepatocyte growth factor. J Am Soc Nephrol 18: 2340–2349, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Dai C, Wen X, He W, Liu Y: Inhibition of proinflammatory RANTES expression by TGF-β1 is mediated by glycogen synthase kinase-3β-dependent β-catenin signaling. J Biol Chem 286: 7052–7059, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]