Abstract
Maintenance of metabolic alkalosis generated by chloride depletion is often attributed to volume contraction. In balance and clearance studies in rats and humans, we showed that chloride repletion in the face of persisting alkali loading, volume contraction, and potassium and sodium depletion completely corrects alkalosis by a renal mechanism. Nephron segment studies strongly suggest the corrective response is orchestrated in the collecting duct, which has several transporters integral to acid-base regulation, the most important of which is pendrin, a luminal Cl/HCO3− exchanger. Chloride depletion alkalosis should replace the notion of contraction alkalosis.
Chloride depletion is the commonest of the three major causes of metabolic alkalosis; the others relate to potassium depletion/mineralocorticoid excess and to very low or absent glomerular filtration with base loading and are not further discussed in detail. Metabolic alkalosis has generation, maintenance, and recovery phases.1 This article focuses on the factors that affect the latter two phases.
In a seminal paper published in 1965, contraction alkalosis produced by ethacrynic acid was described in humans that gave rise to the hypothesis that extracellular fluid (ECF) volume contraction produces alkalosis.2 The authors concluded that abrupt change in ECF volume was the primary event, while acknowledging that chloride depletion might influence renal bicarbonate retention.
In an effort to separate the correction of volume depletion from that of chloride depletion, Cohen maintained alkalosis for 5 days in dogs treated with ethacrynic acid and a NaCl-deficient diet, and then expanded ECF volume with a fluid containing chloride and bicarbonate in concentrations identical to that in the plasma of the dogs with stable alkalemia.3 These isometric infusions completely corrected alkalosis by 24 hours without an increase in GFR, despite increasing potassium depletion; chloride repletion was acknowledged. On the basis of the known characteristics of fluid and electrolyte handling in the various nephron segments at that time, the volume hypothesis in which the intranephronal redistribution of fluid reabsorption plays the central role was expounded. ECF volume depletion accompanying alkalosis augments fluid reabsorption in the proximal tubule where bicarbonate is preferentially reabsorbed compared with chloride; the increased bicarbonate reabsorption in this segment thus maintains the alkalosis. During correction, volume expansion decreases proximal tubule fluid reabsorption, thereby delivering more bicarbonate to the distal nephron, which has a limited capacity to reabsorb bicarbonate; bicarbonaturia ensues and the alkalosis corrects.4
Schwartz et al. studied the pathogenesis of alkalosis produced by chloride depletion (CDA) in men and dogs.5 Gastric aspiration, chloruretic diuretics, NaNO3 infusion (an effect of un-reabsorbable anions), and prior hypercapnia (posthypercapnic CDA) were all used to generate CDA. These studies establish that Cl− repletion by NaCl or KCl—but not replacement of Na+ and K+ losses without Cl−—fully corrects CDA in the maintenance phase. The issue of the specific role of ECF volume depletion was not resolved at this time.
To separate chloride from volume repletion, we first studied rats with selective CDA produced by peritoneal dialysis against NaHCO3 and a normal serum potassium concentration. In rats given a 70 mEq/L Cl− drink with either Na+ or choline, CDA was completely corrected despite negative Na+ and K+ balances, decreased body weight, and obligatory sodium or choline bicarbonate loading.6 Acid-base status in choline-receiving controls was not altered. Rats treated in like manner with CDA maintained for 7–10 days responded in the same manner.7 The corrective response occurs by a renal mechanism.8
To more rigorously exclude a role for volume expansion, CDA rats were infused with 5% dextrose with either 6% albumin or an isometric Cl− solution. CDA persisted in rats infused with albumin despite 15% ECF volume expansion, but was corrected by the Cl--containing solution despite persistent volume depletion and decreased GFR.9 Delivery of chloride to the collecting duct was not statistically different between the groups but was absolutely greater in rats that corrected. Urinary bicarbonate excretion increased as chloride was infused, whereas it decreased further in the volume-expanded rats. The magnitude of bicarbonaturia approximated the estimated amount of chloride delivered to the collecting ducts. Renal chloride conservation persisted until plasma chloride concentration returned to normal.10
In normal human participants, we turned to diuretic-induced CDA. Furosemide, Na+, K+ citrate supplementation, and dietary Cl− restriction produced stably maintained CDA for 5 days that was completely corrected thereafter by oral KCl, despite the presence of maintained negative Na+ balance and plasma volume contraction (measured by the 131I albumin space and plasma albumin concentrations) and persistently lowered GFR and estimated renal plasma flow.11 During correction, net acid excretion decreased with HCO3− diuresis. In contrast, neutral sodium phosphate given in lieu of KCl was associated with increased serum HCO3− concentration despite increased plasma volume. In control participants, furosemide administration without Cl− restriction did not cause CDA and serum electrolyte concentrations and net acid excretion did not change with the same amount of KCl administration. Thus, in both rats and humans, chloride repletion in the face of persistent volume depletion corrects CDA, whereas volume expansion without chloride does not.
The contraction alkalosis viewpoint was consistent with then-current views of the mechanism of renal bicarbonate reabsorption by sodium-proton exchange. Aldosterone was regarded as necessary to increase the sodium avidity of ECF volume contraction. Chloride was regarded as the mendicant anion, allowing for sodium reabsorption without obligating proton secretion. Knowledge about specialized cells in the distal nephron capable of secreting protons or bicarbonate and of chloride bicarbonate exchange independent of sodium was yet to come.
The site at which this renal maintenance and correction of CDA occurs was addressed in micropuncture and microperfusion studies in our peritoneal dialysis rat model. First, GFR was inversely correlated with the degree of CDA by tubuloglomerular feedback.12 Although intact function of the proximal tubule and the loop of Henle are essential to the renal response, these segments have no identifiable adaptive role in the corrective response to chloride repletion in that glomerulotubular balance is maintained whether CDA is being corrected.8,9 Delivery of chloride and bicarbonate out of the loop of Henle was not different whether rats were maintaining or correcting CDA.9,13 In the distal convoluted tubule, alkalosis can induce HCO3 secretion, which is inhibited by the removal of luminal chloride; it is likely that such effects emanate from the connecting tubule.14
In perfused cortical collecting ducts, HCO3− is secreted in tubules from CDA rats and is dependent on luminal chloride, whereas it is reabsorbed in normal rats.15 The magnitude and direction of HCO3− transport is dependent upon the degree of alkalosis and chloride repletion in vivo at the time of tubule harvesting.16
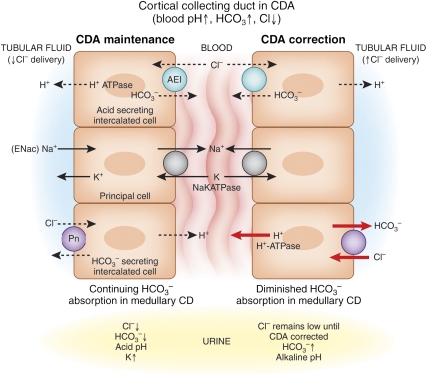
Thus, our studies in rats and humans suggest that the single and necessary correction effect for CDA is an increase in distal nephron chloride; the B-type intercalated cells along the cortical collecting duct were poised to secrete bicarbonate in exchange for administered chloride (Figure 1). H+-ATPase activity was increased at the basolateral membrane of B-type cells,17 but pendrin (the chloride bicarbonate exchanger) was not yet identified as the luminal anion exchanger.
Figure 1.
In the CCD during reduced Cl− delivery to that segment in CDA, pendrin activity is increased but secretion of HCO3− is inhibited by insufficient Cl- for anion exchange. HCO3− reabsorption may be partly maintained by Na+/H+ exchange by the electrical coupling between the principal cell and the H+-secreting A cell (although it is morphologically less active than normal17) and by the remainder of the collecting duct in the medulla. Coupled Na+/K+ exchange causes kaliuresis. After Cl− delivery to the cortical collecting ducts increases, HCO3− secretion occurs and medullary HCO3− reabsorption diminishes,32 allowing correction of the hypochloremic alkalosis. In our rat studies, bicarbonaturia occurred within minutes of Cl− administration intravenously and Cl− did not increase in the urine until correction of serum [Cl−] was nearly complete. (Both H+ and HCO3− are produced in the A- and B-type cells by carbonic anhydrase, and during acute acid-base changes, pendrin and H+ ATPase shuttle back and forth from cytosol to the luminal or basolateral membrane18).
Abundant evidence now supports pendrin as an important regulatory transporter in the cortical collecting ducts, which responds briskly18 in vivo and in vitro16 to alterations in chloride intake19 and to acid-base perturbations including metabolic alkalosis and acidosis20 and respiratory acidosis21 (Table 1). The main stimuli seem to be distal chloride delivery22 and intracellular pH.23 In CDA, pendrin is stimulated both by low chloride distal delivery22 and by metabolic alkalosis,20 including intracellular alkalosis. In potassium depletion metabolic alkalosis, a high serum bicarbonate is maintained by intracellular acidosis in the renal tubular cells with resulting increased bicarbonate reabsorption at several sites along the nephron. Pendrin is reduced in potassium depletion24; this suggests that the signal for increased activity for the luminal anion exchanger is related to changes in intracellular pH, rather than extracellular pH or urine pH. Pendrin has been studied in mice, rats, rabbits, and the gills of rays22 (where it responds to changes in acid-base status on moving from sea to fresh water).
Table 1.
Responses of CCD luminal B cell pendrin to changes in dietary chloride and acid-base perturbations
| Condition | B Cell Pendrin Expression/Activity |
|---|---|
| Low chloride diet | ↑ |
| High chloride diet | ↓ |
| Chloride depletion alkalosis | ↑ |
| Metabolic acidosis | ↓ |
| Respiratory acidosis | ↓ |
| Potassium depletion alkalosisa | ↓ |
aAssociated with intracellular acidosis.
In renal pendrin null mice, serum HCO3− concentration is higher and urine is less acidic than in controls.25 Humans with Pendred syndrome (hypothyroid goiter and deafness) seem to have normal acid-base status. However, two recent descriptions of a severe metabolic alkalosis in a child, given thiazide for excess endolymph26 and severe alkalosis and potassium depletion in response to vomiting,27 suggest that an intact functioning of B-type intercalated cells are important for defense against chloride depletion.
The role of aldosterone, if any, in regulating pendrin’s response to changes in dietary chloride and to acid-base changes is not clear. We showed that angiotensin II was not regulatory in maintenance or correction of CDA.28 High aldosterone levels are also not necessary for maintenance of CDA29 and correction by chloride occurs despite rising levels.10 Aldosterone, within its physiologic range of levels, is not important for direct pendrin regulation.20,21 Adrenalectomized rats ingesting a low chloride diet and drinking isotonic neutral sodium phosphate excrete extremely low concentrations of chloride and maintain chloride balance.30 Again, this suggests that chloride conservation in the distal nephron is not aldosterone dependent.
Recently, a new mechanism of electroneutral sodium chloride absorption, which is not inhibited by amiloride but is by thiazides, has been shown in the mouse cortical collecting duct.31 It is dependent on pendrin and another sodium-dependent anion exchanger. Thus, reduced chloride delivery to the cortical collecting ducts causes sodium conservation to be more dependent on ENaC and may explain the high losses of urinary potassium in CDA.
Our conceptual model of maintenance and correction of CDA is shown (Figure 1). In the rat model, bicarbonate secretion in exchange for administered chloride in the cortical collecting duct can account for correction of CDA. Clearly there must be a coordinated response in more distal sites to allow secreted bicarbonate to pass into the final urine. We have shown in medullary collecting duct segments that bicarbonate reabsorption is decreased in tubules obtained from rats with correcting CDA32 and chloride is intensely conserved along the collecting ducts on a low chloride diet.33
In summary, CDA can be corrected by selective chloride repletion despite maintained or increasingly negative sodium or potassium balance, continued bicarbonate loading, and continuing high levels of angiotensin II or aldosterone, and is not corrected by sodium or potassium repletion without chloride repletion, by chloride repletion in the absence of renal function, by plasma volume expansion by as much as 15%, or by restoring baseline GFR without chloride repletion. In addition, we have demonstrated in vitro and in vivo correction mechanisms by anion exchange along the collecting duct, which are consistent with current physiology. The more proximal nephron segments do not contribute to the adaptive response for the correction of CDA by administered chloride.
Chloride administration without volume expansion is necessary and sufficient to correct CDA. Volume deficits, whether associated with metabolic acidosis or alkalosis, should be corrected. We suggest that the term chloride depletion alkalosis is clinically valuable because it indicates the correct pathophysiology and focuses on the ion in the blood and urine that is fundamental to that mechanism.
DISCLOSURES
None.
Acknowledgments
This study was supported by grants from the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Seldin DW, Rector FC, Jr: Symposium on acid-base homeostasis. The generation and maintenance of metabolic alkalosis. Kidney Int 1: 306–321, 1972 [DOI] [PubMed] [Google Scholar]
- 2.Cannon PJ, Heinemann HO, Albert MS, Laragh JH, Winters RW: “Contraction” alkalosis after diuresis of edematous patients with ethacrynic acid. Ann Intern Med 62: 979–990, 1965 [DOI] [PubMed] [Google Scholar]
- 3.Cohen JJ: Correction of metabolic alkalosis by the kidney after isometric expansion of extracellular fluid. J Clin Invest 47: 1181–1192, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz WB, Cohen JJ: The nature of the renal response to chronic disorders of acid-base equilibrium. Am J Med 64: 417–428, 1978 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz WB, Kassirer JP. Van Ypersele de Strihou: Role of anions in metabolic alkalosis and potassium deficiency. N Engl J Med 279: 630–639, 1968 [DOI] [PubMed] [Google Scholar]
- 6.Galla JH, Bonduris DN, Luke RG: Correction of acute chloride-depletion alkalosis in the rat without volume expansion. Am J Physiol 244: F217–F221, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Wall BM, Byrum GV, Galla JH, Luke RG: Importance of chloride for the correction of chronic chloride depletion metabolic alkalosis in the rat. Am J Physiol Renal Physiol 253: F1031–F1039, 1987 [DOI] [PubMed] [Google Scholar]
- 8.Craig DM, Galla JH, Bonduris DN, Luke RG: Importance of the kidney in the correction of chloride-depletion alkalosis in the rat. Am J Physiol 250: F54–F57, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Galla JH, Bonduris DN, Luke RG: Effects of chloride and extracellular fluid volume on bicarbonate reabsorption along the nephron in metabolic alkalosis in the rat. Reassessment of the classical hypothesis of the pathogenesis of metabolic alkalosis. J Clin Invest 80: 41–50, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galla JH, Bonduris DN, Dumbauld SL, Luke RG: Segmental chloride and fluid handling during correction of chloride-depletion alkalosis without volume expansion in the rat. J Clin Invest 73: 96–106, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen RA, Julian BA, Dubovsky EV, Galla JH, Luke RG: On the mechanism by which chloride corrects metabolic alkalosis in man. Am J Med 84: 449–458, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Galla JH, Bonduris DN, Sanders PW, Luke RG: Volume-independent reductions in glomerular filtration rate in acute chloride-depletion alkalosis in the rat. Evidence for mediation by tubuloglomerular feedback. J Clin Invest 74: 2002–2008, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galla JH, Bonduris DN, Luke RG: Superficial distal and deep nephrons in correction of metabolic alkalosis. Am J Physiol 257: F107–F113, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Levine DZ, Vandorpe D, Iacovitti M: Luminal chloride modulates rat distal tubule bidirectional bicarbonate flux in vivo. J Clin Invest 85: 1793–1798, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gifford JD, Sharkins K, Work J, Luke RG, Galla JH: Total CO2 transport in rat cortical collecting duct in chloride-depletion alkalosis. Am J Physiol 258: F848–F853, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Gifford JD, Ware MW, Luke RG, Galla JH: HCO3- transport in rat CCD: Rapid adaptation by in vivo but not in vitro alkalosis. Am J Physiol 264: F435–F440, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Verlander JW, Madsen KM, Galla JH, Luke RG, Tisher CC: Response of intercalated cells to chloride depletion metabolic alkalosis. Am J Physiol 262: F309–F319, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Purkerson JM, Tsuruoka S, Suter DZ, Nakamori A, Schwartz GJ: Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney Int 78: 993–1005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM: Dietary Cl(-) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Frische S, Kwon TH, Frøkiaer J, Madsen KM, Nielsen S: Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284: F584–F593, 2003 [DOI] [PubMed] [Google Scholar]
- 21.de Seigneux S, Malte H, Dimke H, Frøkiaer J, Nielsen S, Frische S: Renal compensation to chronic hypoxic hypercapnia: Downregulation of pendrin and adaptation of the proximal tubule. Am J Physiol Renal Physiol 292: F1256–F1266, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Vallet M, Picard N, Loffing-Cueni D, Fysekidis M, Bloch-Faure M, Deschênes G, Breton S, Meneton P, Loffing J, Aronson PS, Chambrey R, Eladari D: Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol 17: 2153–2163, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Adler L, Efrati E, Zelikovic I: Molecular mechanisms of epithelial cell-specific expression and regulation of the human anion exchanger (pendrin) gene. Am J Physiol Cell Physiol 294: C1261–C1276, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP: Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Amlal H, Petrovic S, Xu J, Wang Z, Sun X, Barone S, Soleimani M: Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl(-)/HCO3(-) exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol 299: C33–C41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pela I, Bigozzi M, Bianchi B: Profound hypokalemia and hypochloremic metabolic alkalosis during thiazide therapy in a child with Pendred syndrome. Clin Nephrol 69: 450–453, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Kandasamy N, Fugazzola L, Evans M, Chatterjee K, Karet F: Life-threatening metabolic alkalosis in Pendred syndrome. Eur J Endocrinol 165: 167–170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters EA, Rome L, Luke RG, Galla JH: Absence of a regulatory role of angiotensin II in acute chloride-depletion alkalosis in the rat. Am J Physiol 261: F741–F745, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Kassirer JP, Appleton FM, Chazan JA, Schwartz WB: Aldosterone in metabolic alkalosis. J Clin Invest 46: 1558–1571, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luke RG: Effect of adrenalectomy on the renal response to chloride depletion in the rat. J Clin Invest 54: 1329–1336, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galla JH, Rome L, Luke RG: Bicarbonate transport in collecting duct segments during chloride-depletion alkalosis. Kidney Int 48: 52–55, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Kirchner KA, Galla JH, Luke RG: Factors influencing chloride reabsorption in the collecting duct segment of the rat. Am J Physiol 239: F552–F559, 1980 [DOI] [PubMed] [Google Scholar]