Abstract
Serum levels of creatinine, cystatin C, or β trace protein allow estimation of GFR, but whether these markers contribute additional prognostic information beyond that reflected in GFR is unknown. Here, we analyzed data from the Modification of Diet in Renal Disease study, which provided baseline levels of these markers for 816 participants with a median follow-up of 16.6 years. We examined associations between the reciprocals of these filtration markers and 125I iothalamate GFR, expressed per SD, with kidney failure and mortality. In univariate analysis, lower GFR and higher levels of each filtration marker associated with a higher risk for all outcomes. After adjustment for GFR in a Cox proportional hazards model, higher creatinine associated with a higher risk for kidney failure but a lower risk for all-cause mortality. Higher cystatin C and β trace protein associated with a higher risk for both kidney failure and all-cause mortality. In models including either cystatin C or β trace protein, the association of GFR with all-cause mortality was no longer significant after the addition of the filtration marker, suggesting the possibility of multicollinearity. In summary, after adjustment for GFR, levels of creatinine, cystatin C, and β trace protein, each remained directly associated with kidney failure but differed with respect to their associations with mortality. These differences may be a result of non-GFR–related associations of filtration markers, residual confounding by GFR, or collinearity between the filtration markers and GFR. β trace protein and cystatin C seem to provide more consistent prognostic information than creatinine.
GFR is considered to be the best overall index of kidney function, and is critical for detection, evaluation, and management of kidney disease.1 The level of GFR provides important prognostic information about progression to kidney failure, cardiovascular disease (CVD), and mortality.2 GFR is not easily measured, and it is therefore most often estimated from the serum concentration of endogenous filtration markers.3 The serum concentration of these markers is determined by factors other than GFR leading to errors in estimating GFR as well as variation among markers in assessing prognosis.4–6 The prognostic effects of these markers adjusted for measured GFR has not been determined.
Creatinine is the most commonly used filtration marker; however, its non-GFR determinants, including muscle mass and protein intake, vary substantially by age, sex, race, ethnicity, and chronic illness.3,7,8 Cystatin C is advocated as an alternative to creatinine because it seems to be less affected by these factors and has been shown in several studies to be more strongly associated with CVD and mortality outcomes.9,10 β trace protein (BTP) has recently emerged as another alternative filtration marker.11–15 BTP is a low molecular weight glycoprotein that is freely filtered by the glomerulus, and has minimal nonkidney elimination.14 Some studies have suggested that BTP is more accurate than creatinine in estimating measured GFR11,13–21 and is more strongly associated with CVD.22 It is unclear whether the stronger associations of cystatin C and BTP with outcomes are due to more accurate GFR estimation or to confounding by non-GFR effects.
The Modification of Diet in Renal Disease (MDRD) study was a randomized trial of a dietary protein intake and strict BP control in patients with CKD.23 Long-term follow-up of the MDRD study has continued since the end of the randomized trial in 1993, with ascertainment of mortality and kidney failure from registries. We previously showed strong relationships of baseline serum creatinine, cystatin C, and measured GFR with 11 years of follow-up for the outcomes of kidney failure and all-cause mortality in the MDRD study.9 The current analysis adds to our previous analysis by evaluating a second novel filtration marker (BTP), adding 7 additional years of follow-up, and incorporating new analyses to examine associations of these markers after adjustment for measured GFR.
Results
Study Population
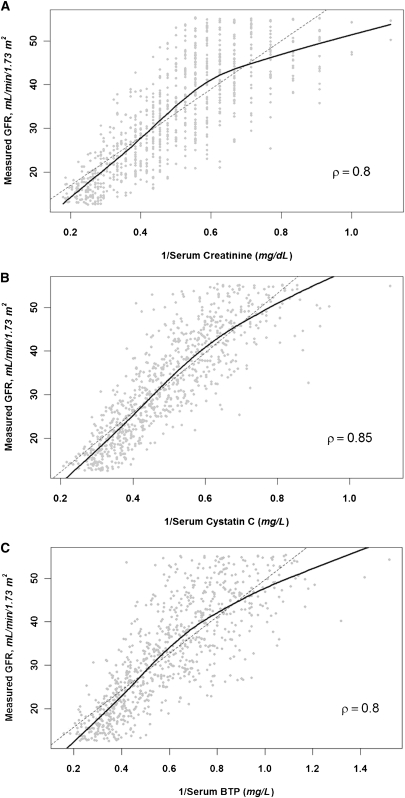
The mean age of the study population was 52 years; 60% of participants were male and 5% had diabetes. The mean (SD) GFR, serum creatinine, cystatin C, and BTP at the time of randomization were 33 (12) ml/min per 1.73 m2, 2.4 (0.9) mg/dl, 2.2 (0.7) mg/L, and 2.0 (0.8) mg/L, respectively. Age, sex, and cause of kidney disease were similar across quartiles of measured GFR. Urinary protein excretion was lower in patients with a higher GFR, but there was no association between cardiovascular risk factors, such as LDL and HDL cholesterol and C-reactive protein (CRP), and measured GFR. All of the filtration markers (creatinine, cystatin C, and BTP) were lower in patients with higher measured GFRs (Table 1). The filtration markers also correlated with each other (ρ=0.78 and ρ=0.85 for BTP with creatinine and cystatin C, respectively), and exhibited a strong correlation with measured GFR (ρ=0.85 for the inverse of serum cystatin C [1/Scys] and ρ=0.80 for the inverse of serum creatinine [1/Screat] and the inverse of BTP [1/SBTP]) (Figure 1).
Table 1.
Baseline characteristics of the study population by quartiles of GFR
| Variable | Quartile 1 (n=204) | Quartile 2 (n=204) | Quartile 3 (n=204) | Quartile 4 (n=204) | Total (n=816) | P Value |
|---|---|---|---|---|---|---|
| Age (yr) | 51 (13) | 51 (13) | 54 (12) | 52 (11) | 52 (12) | 0.51 |
| Male | 56 | 60 | 61 | 64 | 60 | 0.12 |
| White | 86 | 87 | 82 | 84 | 85 | 0.33 |
| Current smoker (%) | 7 | 13 | 9 | 9 | 10 | 0.72 |
| Diabetes (%) | 4 | 5 | 6 | 5 | 5 | 0.62 |
| Cause of kidney failure | 0.93 | |||||
| glomerular diseases | 35 | 30 | 29 | 35 | 32 | |
| PKD | 26 | 22 | 23 | 25 | 24 | |
| Other | 39 | 48 | 48 | 41 | 44 | |
| Systolic BP (mmHg) | 133 (17) | 132 (19) | 132 (16) | 130 (18) | 132 (18) | 0.07 |
| Diastolic BP (mmHg) | 81 (10) | 81 (10) | 81 (10) | 81 (10) | 81 (10) | 0.98 |
| HDL cholesterol (mg/dl) | 39 (15) | 40 (14) | 40 (15) | 40 (13) | 40 (14) | 0.14 |
| LDL cholesterol (mg/dl) | 146 (38) | 146 (41) | 147 (43) | 150 (43) | 148 (41) | 0.50 |
| BMI (kg/m2) | 26 (4) | 27 (5) | 28 (4) | 28 (4) | 27 (4) | <0.01 |
| Serum albumin (g/L) | 4.0 (0.3) | 4.0 (0.3) | 4.1 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 0.01 |
| CRP (mg/l) | 0.2 (0.1–0.5) | 0.2 (0.1–0.7) | 0.3 (0.1–0.7) | 0.2 (0.1–0.6) | 0.2 (0.1–0.6) | 0.09 |
| Proteinuria (g/d) | 0.7 (0.2–2.0) | 0.5 (0.1–2.0) | 0.1 (0.1–0.9) | 0.1 (0.0–0.8) | 0.3 (0.1–1.5) | < 0.01 |
| GFR (ml/min per 1.73 m2)a | 18 (3) | 28 (3) | 37 (3) | 49 (4) | 33 (12) | NA |
| Serum creatinine (mg/dl) | 3.5 (0.9) | 2.5 (0.6) | 1.9 (0.4) | 1.6 (0.3) | 2.4 (0.9) | <0.01 |
| Serum cystatin C (mg/L) | 3.1 (0.5) | 2.3 (0.4) | 1.9 (0.3) | 1.5 (0.3) | 2.2 (0.7) | <0.01 |
| Serum BTP (mg/L) | 3.0 (0.8) | 2.1 (0.5) | 1.5 (0.3) | 1.3 (0.3) | 2.0 (0.8) | <0.01 |
Data are reported as percentages, mean (SD), or median (interquartile range), P value is for trend analysis. Quartile 1: 13–23 ml/min per 1.73 m2. Quartile 2: 24–32 ml/min per 1.73 m2. Quartile 3: 33–42 ml/min per 1.73 m2, Quartile 4: 43–59 ml/min per 1.73 m2. PKD, polycystic kidney disease BMI, body mass index; BTP, β trace protein; NA, not appropriate.
GFR is the mean of two values 3 months apart. P value is for trend.
Figure 1.
Correlation of filtration markers (inverted) and measured GFR. Scatter plot of filtration markers and measured GFR for (A) creatinine, (B) cystatin C, and (C) BTP. ρ denotes the Pearson correlation coefficient. Solid line is the lowess smoother fit and the dashed line is the least-squares regression fit.
Association with Clinical Outcomes
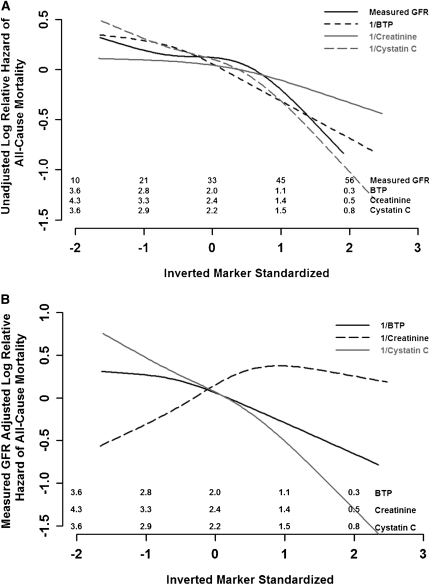
The median follow-up time to death was 16.6 years. Table 2 shows the number of events and event rates. The event rates for kidney failure, all-cause mortality, and CVD mortality for quartiles 1–4 were 9.5, 3.3, and 1.5 per 100 person-years, respectively. Event rates were higher in patients with lower measured GFR, corresponding to higher levels of the serum filtration markers (lower levels of reciprocal serum levels). In unadjusted analyses, all measures of kidney function were directly and significantly associated with each outcome (a lower value for the reciprocal of the serum level was associated with a higher risk) (Table 3, Figure 2). Cystatin C and BTP had slightly higher hazard ratios (HRs) for mortality than GFR. For each measure, HRs were higher for kidney failure than either CVD or all-cause mortality.
Table 2.
Incidence rates overall and by quartile of markers of kidney function
| Outcome, Marker of Kidney Function | Quartile 1 (n=204) | Quartile 2 (n=204) | Quartile 3 (n=204) | Quartile 4 (n=204) | Total (n=816) |
|---|---|---|---|---|---|
| Kidney failure | |||||
| 1/Screat | 24.32 (180) | 13.38 (178) | 7.46 (149) | 4.25 (95) | 9.55 (602) |
| 1/Scyc | 21.92 (184) | 12.77 (167) | 8.07 (144) | 4.51 (107) | – |
| 1/SBTP | 22.62 (183) | 14.04 (174) | 6.98 (134) | 4.75 (111) | – |
| measured GFR | 21.99 (182) | 12.48 (171) | 7.50 (136) | 4.93 (113) | – |
| All-cause mortality | |||||
| 1/Screat | 3.60 (98) | 3.52 (100) | 3.36 (104) | 2.54 (71) | 3.26 (373) |
| 1/Scyc | 4.34 (115) | 4.05 (111) | 2.87 (84) | 2.01 (63) | – |
| 1/SBTP | 4.36 (115) | 3.43 (97) | 3.40 (96) | 2.06 (65) | – |
| measured GFR | 4.11 (111) | 3.49 (98) | 3.44 (97) | 2.14 (67) | – |
| Cardiovascular mortality | |||||
| 1/Screat | 1.91 (52) | 1.51 (43) | 1.36 (42) | 1.29 (36) | 1.51 (173) |
| 1/Scyc | 2.04 (54) | 1.82 (50) | 1.43 (42) | 0.86 (27) | – |
| 1/SBTP | 2.16 (57) | 1.41 (40) | 1.59 (45) | 0.98 (31) | – |
| measured GFR | 2.00 (54) | 1.57 (44) | 1.59 (45) | 0.96 (30) | – |
Incidence rates are calculated as the ratio of the total number of events to the total follow-up time within each quartile and expressed per 100 person-years.
Table 3.
Association between baseline filtration markers and adverse outcomes
| Outcome | Marker of Kidney Function | |||
|---|---|---|---|---|
| Measured GFR | 1/Screat | 1/Scyc | 1/SBTP | |
| Kidney failure | ||||
| unadjusted | 2.04 (1.86–2.23) | 2.26 (2.05–2.49) | 2.12 (1.92–2.33) | 2.19(1.99–2.41) |
| adjusted for measured GFRa | – | 1.79 (1.54–2.08) | 1.62 (1.37–1.91) | 1.73 (1.48–2.01) |
| additional adjustment for potential confounders | 1.88 (1.71–2.08) | 2.07 (1.67–2.56) | 1.46 (1.20–1.77) | 1.38 (1.15–1.67) |
| All-cause mortality | ||||
| unadjusted | 1.27 (1.14–1.40) | 1.12 (1.01–1.24) | 1.41 (1.26–1.57) | 1.34 (1.20–1.49) |
| adjusted for measured GFRb | – | 0.82 (0.69–0.96) | 1.72 (1.38–2.15) | 1.33 (1.10–1.61) |
| additional adjustment for potential confounders | 1.39 (1.23–1.56) | 1.07 (0.85–1.36) | 1.82 (1.41–2.36) | 1.43 (1.14–1.80) |
| Cardiovascular mortality | ||||
| unadjusted | 1.29 (1.10–1.50) | 1.18 (1.01–1.38) | 1.46 (1.24–1.72) | 1.33 (1.13–1.55) |
| adjusted for measured GFRc | – | 0.90 (0.70–1.15) | 1.90 (1.37–2.63) | 1.24 (0.94–1.63) |
| additional adjustment for potential confounders | 1.43 (1.20–1.70) | 1.21 (0.85–1.73) | 2.14 (1.46–3.14) | 1.43 (1.02–2.00) |
Cells contain the point estimate and 95% confidence intervals (95% CIs) for HRs for the outcome per 1-SD lower value of the variable. Models adjusted for potential confounders are nested upon models adjusted for GFR. Potential confounders include age, race, sex, smoking, history of diabetes and CVD, body mass index, LDL cholesterol, HDL cholesterol, systolic BP, log-transformed CRP, serum albumin level, and log-transformed proteinuria.
For the kidney failure models: HRs (95% CIs) for mGFR are 1.32 (1.14–1.52) in the 1/Screat model, 1.37 (1.16–1.61) in the 1/Scyc model, and 1.33 (1.15–1.54) in the 1/SBTP model.
For the all-cause mortality models: HRs (95% CIs) for mGFR are 1.50 (1.26–1.79) in the 1/Screat model, 0.80 (0.65–0.99) in the 1/Scyc model, and 1.01 (0.84–1.20) in the 1/SBTP model.
For the CVD mortality models: HRs (95% CIs) for mGFR are 1.41 (1.09–1.82) in the 1/Screat model, 0.76 (0.56–1.02) in the 1/Scyc model, and 1.08 (0.83–1.41) in the 1/SBTP model.
Figure 2.
Unadjusted and GFR-adjusted hazards of filtration markers with all-cause mortality. (A) Unadjusted hazards for GFR and filtration markers with all-cause mortality. (B) GFR-adjusted hazards for filtration markers with all-cause mortality. Restricted cubic splines for unadjusted (A) and GFR-adjusted (B) log relative hazard for the standardized inverted filtration markers (subtracting the mean and dividing by the SD) and all-cause mortality. Numbers shown on top of x-axis for each marker are the corresponding values on the untransformed scale at the specific ticks.
Creatinine
In unadjusted and GFR-adjusted analyses, 1/Screat remained strongly associated with kidney failure. After adjustment for additional potential confounders including age, sex, race, and body mass index, the statistical association between creatinine and kidney failure remained strong, and it was stronger than any of the other filtration markers and slightly stronger than measured GFR. In contrast, after adjustment for measured GFR, 1/Screat was negatively associated with all-cause mortality (Figure 2B), with a null association after additional adjustment for other potential confounding variables. Similar findings were observed for CVD mortality, although they were not significant in GFR-adjusted analyses.
Cystatin C
1/Scyc was associated with all adverse outcomes, including kidney failure, CVD death, and all-cause mortality in unadjusted and adjusted analyses (Table 3). In contrast to 1/Screat, the adjusted HRs for 1/Scyc were strongest for CVD and all-cause mortality and were weaker for the outcome of kidney failure. Adjustment for GFR attenuated the association between 1/Scyc and kidney failure. In contrast, adjustment for GFR strengthened the association with mortality outcomes (Table 3, Figure 2). To note, the HRs for GFR with all-cause mortality and CVD mortality were <1.0 in these models (Table 3). Further adjustment for demographic variables and cardiovascular risk factors did not diminish the associations of 1/Scyc with mortality.
BTP
In unadjusted analyses and after adjusting for GFR and other potential confounding variables, 1/SBTP was associated with kidney failure, all-cause mortality, and CVD mortality. The statistical association of 1/SBTP with kidney failure was not as strong as 1/Screat, and for CVD and all-cause mortality, it was not as strong as the association of 1/Scyc. Adjustment for GFR and other potential confounders attenuated the strength of the association between 1/SBTP and kidney failure, but had minimal effect on the association between 1/SBTP and mortality outcomes (Table 3 and Figure 2).
Sensitivity Analyses
Findings for the composite of kidney failure and mortality were similar to the kidney failure outcome alone, whereas findings for mortality prior to kidney failure were similar to the all-cause mortality data presented. Findings were also consistent in a comparison of the 80th and 20th percentiles for each biomarker versus comparisons per SD, or when GFR was modeled as a nonlinear variable.
Results in study A were similar to those when individuals in study A and B were combined. Finally, when only patients with screening GFR values within 25% of baseline GFR were included, the results were essentially unchanged (data not shown).
Discussion
In this long-term follow-up of the MDRD study, higher serum concentrations of creatinine, cystatin C, and BTP and lower measured GFR were all associated with a higher risk of kidney failure, all-cause mortality, and CVD mortality. After adjustment for GFR, all markers remained directly associated with kidney failure. In contrast, after adjustment for measured GFR, the relationships with all-cause and CVD mortality differed among the markers: serum creatinine became inversely associated with both outcomes, serum cystatin C became more strongly directly associated with both outcomes, and serum BTP was relatively unaffected.
The serum concentration of endogenous filtration markers is determined by the level of GFR and factors other than GFR, such as generation, renal tubular reabsorption or secretion, and extrarenal elimination of the marker (non-GFR determinants). In principle, non-GFR associations of filtration markers with prognosis could be due either to direct effects of the markers or to associations of their non-GFR determinants with outcomes. Because none of these markers is known to have direct effects on the kidney or cardiovascular system, our results may reflect differences among markers in the association of their non-GFR determinants with these outcomes. There are, however, two alternate explanations for our results. First, it is possible that the association of cystatin C and BTP independent of GFR reflects residual confounding by GFR despite adjustment. Second, because the HRs for GFR were not >1.0 in mortality models, and in fact were significantly <1.0 in the model relating cystatin C to all-cause mortality, it is possible that models with filtration markers and GFR are unstable because of high collinearity. The latter may be true despite relatively low variance inflation factors and little change in the magnitude of standard errors.
The main non-GFR determinants of serum creatinine are generation by muscle or meat intake. Our results confirm suggestions by other studies that non-GFR determinants of creatinine may confound the relationship of kidney function with all-cause and CVD mortality. For example, several studies have documented a U-shaped relationship between estimated GFR (eGFR) based on serum creatinine and outcomes in elderly patients, in which individuals with a very high eGFR (>90 ml/min per 1.73 m2) and corresponding low serum creatinine tend to have higher mortality rates than those with eGFRs between 60 and 90 ml/min per 1.73 m2.24 More recently, a study examining urinary creatinine excretion noted an independent association with all-cause mortality, independent of eGFR based on serum cystatin C.25 These findings are consistent with observations in dialysis patients, in whom higher levels of serum creatinine are associated with increased survival. In dialysis patients, variation in GFR likely contributes less to variation in serum creatinine levels than to variation in its non-GFR determinants (muscle mass and nutritional status).26,27 In our study, adjustment for variables in addition to GFR removed the inverse association between creatinine and mortality, suggesting that these other variables may be associated with the non-GFR determinants of serum creatinine. The inverse relationship was not observed for kidney failure, even after accounting for GFR and potential non-GFR determinants, likely reflecting bias arising from the clinicians’ use of serum creatinine in the decision of when to start dialysis, despite guidelines that recommend a symptom-guided approach to initiation of renal replacement therapy.
The main non-GFR determinants of serum cystatin C are less well known than for creatinine. Multiple studies have now demonstrated that serum cystatin C has a stronger and more linear relationship to adverse outcomes than serum creatinine or eGFR based on creatinine.10,28,29 These studies have not been able to adjust for GFR and therefore were unable to identify whether the unique contribution of cystatin C is due to more accurate GFR estimation or confounding by its non-GFR determinants. We found that cystatin C remained independently associated with kidney failure, all-cause mortality, and CVD mortality after adjusting for GFR. These findings may be consistent with the hypothesis that non-GFR determinants of cystatin C are associated with these outcomes. In a recent study in a general population cohort, CVD risk factors were associated with higher cystatin C independent of measured GFR, suggesting that they are associated with non-GFR determinants of cystatin C and could possibly affect its association with prognosis.30 In our additional analyses, further adjustment for CVD risk factors and other variables did not attenuate the risk estimates for cystatin C, but this does not eliminate the possibility that the observed results could be due, at least in part, to residual confounding by GFR, or due to collinearity between cystatin C and GFR such that the models are unstable.
Recently, BTP has been identified as a prostaglandin D synthase, an enzyme with actions ranging from platelet aggregation to smooth muscle relaxation.31,32 Higher serum BTP values have been associated with lower serum albumin values and more severe sleep apnea, suggesting a possible link with vascular or endothelial dysfunction.33–36 The Mild and Moderate Kidney Disease study examined the association of BTP adjusted for measured GFR with CKD progression in 177 patients (29 kidney failure outcomes).37 The results showed a HR of 2.80 for BTP after adjustment for age, sex, measured GFR, and proteinuria, consistent with the hypothesis that non-GFR determinants of BTP may be associated with kidney disease progression. Because of the small sample size, the authors were not able to examine all-cause or CVD mortality or the effect of adjustment for other variables. Our study suggests that BTP is associated with kidney failure independent of measured GFR. As with cystatin C, measured GFR was not significantly associated with mortality in these models, again possibly due to collinearity of BTP and GFR.
There are several clinical and research implications to our results. First, filtration markers are strongly associated with prognosis. However, one needs to be cautious in ascribing the prognostic importance of filtration markers to measured GFR, because the associations with adverse outcomes may reflect associations of non-GFR determinants as well. Second, like cystatin C, BTP, is more strongly associated with mortality than creatinine. Although the non-GFR determinants of serum creatinine have been well characterized, additional studies are required to understand the non-GFR determinants of cystatin C and BTP and the potential mechanisms that may account for their associations with kidney disease progression and potentially mortality. Third, because measurement of GFR is not practical in most clinical practices, GFR estimates based on serum levels of endogenous filtration markers will continue to be used to estimate GFR and to predict prognosis. We previously showed that a combination of creatinine and cystatin C provides a more accurate estimate of measured GFR than either marker alone, presumably due to smaller errors of their non-GFR determinants.38,39 A panel of filtration markers may possibly provide a better prediction of prognosis than any marker alone, and this hypothesis requires explicit testing. Finally, although several studies have evaluated determinants and prognostic potential of filtration markers independent of GFR, we add caution to the interpretation of these studies given both the possibility of residual confounding due to GFR and collinearity between the variables which may affect interpretation.4,6,30,37 Future studies should report associations with measured GFR as well as endogenous filtration markers of interest in multivariable models with outcomes of chronic kidney disease and mortality.
There are some limitations to our analysis. First, we assumed that measured GFR is the gold standard for assessment of kidney function. However, measured GFR has a much higher coefficient of variation than measurements of endogenous filtration markers, likely reflecting biologic variation and measurement error.40 Steady state serum levels of cystatin C and BTP may possibly more accurately reflect average true GFR than measured GFR at any one time. We attempted to reduce the influence of biologic variability and measurement error in GFR by averaging two measurements. Sensitivity analysis using a more restrictive definition of GFR did not change our findings. Nonetheless, interpretation of our findings is limited by imprecision in GFR ascertainment. Second, the MDRD study included patients with primarily nondiabetic CKD stages III–IV, with a restricted range of GFR, who had a high risk of kidney failure. As a result, the findings may not be applicable to the larger population of patients with earlier stages of kidney disease in whom the range of kidney function is broader and the risk of kidney failure is lower. Sensitivity analysis confirmed that the results were similar in study A (i.e., those higher levels of GFR). Third, we used baseline measurements of filtration markers, cardiovascular risk factors, and measured GFR in this study with 18 years of follow-up. The lack of repeated measurements during follow-up limits our ability to examine the effect of changes in filtration markers or measured GFR. Finally, we used data from the US Renal Data System and the National Death Index (NDI) for outcome ascertainment. As a result, intermediate outcomes such as nonfatal cardiovascular events and doubling of serum creatinine were not included in our analysis, and the definition of CVD mortality could be subject to misclassification bias.
The strengths of our analyses are the comparison of the associations of serum levels of the novel and established filtration markers, using state of the art assays, with the outcomes of kidney failure, all-cause mortality, and death from CVD. We performed these analyses in a well established cohort of patients with CKD, who had accurate risk factor ascertainment at baseline and a long follow-up to allow sufficient events. We were able to examine these associations in unadjusted analyses and analyses adjusted for measured GFR, allowing us to examine potential non-GFR effects of these filtration markers on adverse outcomes. Finally, we also performed analyses adjusting for important cardiovascular risk factors, further delineating the independent contribution of the filtration markers to kidney failure, all-cause mortality, and CVD mortality.
In conclusion, we find that higher levels of serum creatinine, BTP, and cystatin C are associated with kidney failure, independent of GFR. In contrast, higher levels of creatinine are associated with lower mortality, independent of GFR, whereas higher levels of cystatin C and BTP are associated with higher mortality, independent of GFR. Although these results may imply non-GFR associations of each of these filtration markers, they also may reflect residual confounding from GFR itself, or collinearity between the filtration markers and GFR. Taken together, these findings suggest that BTP, like cystatin C, is a promising filtration marker; however, these results also highlight the difficulty with evaluating non-GFR associations in statistical analyses.
Concise Methods
Study Sample
Briefly, key inclusion criteria for the MDRD study were age 18–70 years and a serum creatinine concentration of 1.2–7.0 mg/dl in women and 1.4–7.0 mg/dl in men. Patients with diabetes requiring insulin were excluded. GFR was measured at screening and again after a 3-month baseline period, during which patients were instructed about the study procedures, dietary protein intake, and BP control. Patients were eligible for study A if their 3-month baseline GFR was 25–55 ml/min per 1.73 m2 and for study B if it was 13–24 ml/min per 1.73 m2. A total of 840 patients with predominantly nondiabetic kidney disease were included in the randomized cohort.23 Patients in studies A and B were combined for the current analyses. Patients with a cystatin C and BTP measurement available (n=816) at baseline were included in the present analysis.
Baseline Measurement of Kidney Function
GFR was measured by urinary clearance of 125I iothalamate as previously described.23,40 GFR is expected to be stable over the baseline period, and we therefore averaged the measured GFR at the beginning and end of the baseline period to reduce the effects of biologic variability and measurement error, as we previously described.41 The mean, median, 25th percentile, 75th percentile, and SD difference in GFR between the two measurements were −1.0 ml/min per 1.73 m2, −1.1 ml/min per 1.73 m2, −3.3 ml/min per 1.73 m2, 1.4 ml/min per 1.73 m2, and 4.9, respectively. Serum creatinine was measured at the 3-month visit at the Cleveland Clinic Foundation, Cleveland, Ohio, using a kinetic alkaline picrate assay on a Beckman Astra CX3 instrument (Beckman, Fullerton, CA). Cystatin C and BTP were measured in frozen samples collected at the third baseline visit and stored at −70°C until assay. Samples assayed for cystatin C and BTP were measured at the Cleveland Clinic in 2005 and 2008, respectively, using a particle enhanced immunonephelometric assay on a BN-II nephelometer (Siemens Healthcare Diagnostics, Deerfield, IL). Measurement of a quality control specimen was included in each analytic run. Calculation of the coefficient of variation showed an inter-run precision of 5.6% for cystatin C and 6.0% for BTP.
Outcomes
We evaluated the following three outcomes: kidney failure (defined as the need for dialysis or transplantation), all-cause mortality, and CVD mortality. The decisions to initiate dialysis or transplantation were made by the patients’ treating physicians who had access to follow-up serum creatinine measurements during and after the study. We obtained kidney failure outcomes from the US Renal Data System. The criteria used included either certification of ESRD status from the Medical Evidence form or at least 22 claims for dialysis treatments over a 60-day period.
We ascertained survival status and cause of death from the NDI and ascribed deaths to CVD if the primary cause of death was coded according to the International Classification of Diseases 390–459 or I00–I99 codes (ninth revision and tenth revision, respectively) or if kidney disease or diabetes was listed as the primary cause of death and CVD was the secondary cause. A combination of first and last names, sex, date of birth, and social security number were used for the NDI match. We defined survival time as the time from randomization to death or end of follow-up (December 31, 2007). The institutional review board of Tufts Medical Center approved the data collection procedures.
Statistical Analyses
We compared baseline characteristics of the study sample across quartiles of GFR values and calculated P values for linear trend. Because serum BTP, cystatin C, and creatinine vary as the inverse of GFR, we used 1/SBTP, 1/Scyc, and 1/Screat to facilitate direct comparison with GFR. Pearson correlations were used to examine linear associations among the three measures of kidney function and with measured GFR. We calculated incidence rates for kidney failure, all-cause mortality, and CVD mortality by quartiles of each baseline measure of kidney function.
We examined the association of the baseline measures of kidney function with outcomes by using unadjusted and adjusted Cox proportional hazards models. We entered the measures of kidney function as continuous variables and calculated the HRs and 95% confidence intervals per 1-SD lower value of each measure to allow a standardized comparison across measures with the same scale. The models for kidney failure were censored at death or the end of follow-up. The models for the mortality outcomes included patients with kidney failure and were censored only at the end of follow-up.
To examine the unique contribution of each marker to prognosis separate from measured GFR, we constructed Cox models for each outcome, using the filtration markers as the variable of interest and adjusting for the mean measured GFR. In principle, the HR for each marker adjusted for measured GFR represents the contribution of the non-GFR effects to outcomes. In subsequent models we adjusted for age, race, sex, smoking, history of diabetes and CVD, body mass index, LDL cholesterol, HDL cholesterol, systolic BP, CRP, serum albumin level, and proteinuria. CRP and proteinuria were log transformed due to their skewed distribution. Models for kidney failure were also adjusted for cause of kidney disease (glomerular diseases, polycystic kidney disease, or other). We tested for nonlinearity by examining the functional form of the relationship of the outcomes versus each marker as a continuous variable by fitting a cubic smoothing spline controlling for the covariates. We examined the effect of collinearity in these models by evaluating the variance inflation factors and the change in magnitude of standard errors. No significant changes in variance inflation factors (>5) or magnitude of standard errors were found. We checked the proportional hazards assumption by testing the significance of the correlation coefficient between survival time and the scaled Schoenfeld residuals. A violation of the proportional hazards assumption was noted for serum creatinine and the outcome of kidney failure. The HR for serum creatinine and kidney failure outcome when modeled at different blocks of follow-up was higher than the HR of the other markers but showed a significant decline as follow-up time increased. For ease of reporting, the HR for serum creatinine and kidney failure outcome reported in this analysis ignores its significant interaction with follow-up time. We used SAS software (version 9.2; SAS Institute Inc, Cary, NC) to perform the statistical analyses.
Sensitivity Analyses
We examined the association of the filtration markers with the composite outcome of kidney failure or death, and with the outcome of death before kidney failure. To determine if the results were driven by patients with lower levels of GFR, we repeated analyses exclusively in study A. To evaluate the potential bias in GFR measurement, we adjusted for GFR using a more restrictive definition, in which only patients with screening GFR values within 25% of baseline GFR were included.41 We also considered a nonlinear relationship with measured GFR and outcomes, and created models in which GFR was treated as a restricted cubic spline with 5 knots.
Finally, because the distribution of the kidney markers differs, a 1-SD change in each marker may not be comparable across the markers. To address this issue, we repeated the Cox models with HRs comparing the 80th with the 20th percentile of decreasing kidney function.
Disclosures
None.
Acknowledgments
This work was supported by Grants K24 DK078204 (M.J.S.), CKD-EPI UO1 DK 053869, UO1 DK 067651, and UO1 DK 35073 (A.S.L. and L.A.I.), as well as K23DK081017 (L.A.I.). N.T. is supported by a KRESCENT postdoctoral fellowship, a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research, and the Canadian Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE: Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 65: 1416–1421, 2004 [DOI] [PubMed] [Google Scholar]
- 5.White CA, Akbari A, Doucette S, Fergusson D, Ramsay T, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA: Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis 54: 922–930, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS: Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG. Modification of Diet in Renal Disease (MDRD) Study Group: Creatinine filtration, secretion and excretion during progressive renal disease. Kidney Int Suppl 27: S73–S80, 1989 [PubMed] [Google Scholar]
- 8.Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J, Levey AS: Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int 9: 471–477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ: Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med 147: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Donadio C, Lucchesi A, Ardini M, Donadio E, Giordani R: Serum levels of beta-trace protein and glomerular filtration rate—preliminary results. J Pharm Biomed Anal 32: 1099–1104, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gerhardt T, Pöge U, Stoffel-Wagner B, Klein B, Klehr HU, Sauerbruch T, Woitas RP: Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant 23: 309–314, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Giessing M: Beta-trace protein as indicator of glomerular filtration rate. Urology 54: 940–941, 1999 [PubMed] [Google Scholar]
- 14.White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA: A novel equation to estimate glomerular filtration rate using beta-trace protein. Clin Chem 53: 1965–1968, 2007 [DOI] [PubMed] [Google Scholar]
- 15.White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA: Estimating GFR using serum beta trace protein: Accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76: 784–791, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Priem F, Althaus H, Birnbaum M, Sinha P, Conradt HS, Jung K: Beta-trace protein in serum: A new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem 45: 567–568, 1999 [PubMed] [Google Scholar]
- 17.Woitas RP, Stoffel-Wagner B, Poege U, Schiedermaier P, Spengler U, Sauerbruch T: Low-molecular weight proteins as markers for glomerular filtration rate. Clin Chem 47: 2179–2180, 2001 [PubMed] [Google Scholar]
- 18.Filler G, Priem F, Lepage N, Sinha P, Vollmer I, Clark H, Keely E, Matzinger M, Akbari A, Althaus H, Jung K: Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 48: 729–736, 2002 [PubMed] [Google Scholar]
- 19.Pham-Huy A, Leonard M, Lepage N, Halton J, Filler G: Measuring glomerular filtration rate with cystatin C and beta-trace protein in children with spina bifida. J Urol 169: 2312–2315, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pöge U, Gerhardt TM, Stoffel-Wagner B, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP: beta-Trace protein is an alternative marker for glomerular filtration rate in renal transplantation patients. Clin Chem 51: 1531–1533, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Pöge U, Gerhardt T, Woitas RP: Estimation of glomerular filtration rate by use of beta-trace protein. Clin Chem 54: 1403–1405, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Manzano-Fernández S, Januzzi JL, Jr, Boronat-Garcia M, Bonaque-González JC, Truong QA, Pastor-Pérez FJ, Muñoz-Esparza C, Pastor P, Albaladejo-Otón MD, Casas T, Valdés M, Pascual-Figal DA: β-trace protein and cystatin C as predictors of long-term outcomes in patients with acute heart failure. J Am Coll Cardiol 57: 849–858, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G. Modification of Diet in Renal Disease Study Group: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ: Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 63: 1121–1129, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avram MM, Bonomini LV, Sreedhara R, Mittman N: Predictive value of nutritional markers (albumin, creatinine, cholesterol, and hematocrit) for patients on dialysis for up to 30 years. Am J Kidney Dis 28: 910–917, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Avram MM, Mittman N, Bonomini L, Chattopadhyay J, Fein P: Markers for survival in dialysis: A seven-year prospective study. Am J Kidney Dis 26: 209–219, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cardiovascular Health Study: Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med 142: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF: Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 17: 254–261, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, Toft I, Eriksen BO: Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eguchi Y, Eguchi N, Oda H, Seiki K, Kijima Y, Matsu-ura Y, Urade Y, Hayaishi O: Expression of lipocalin-type prostaglandin D synthase (beta-trace) in human heart and its accumulation in the coronary circulation of angina patients. Proc Natl Acad Sci USA 94: 14689–14694, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreland RB, Nehra A, Kim NN, Min KS, Albadawi H, Watkins MT, Goldstein I, Traish AM: Expression of functional prostaglandin D (DP) receptors in human corpus cavernosum smooth muscle. Int J Impot Res 14: 446–452, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Bachmann G, Petereit H, Djenabi U, Michel O: Predictive values of beta-trace protein (prostaglandin D synthase) by use of laser-nephelometry assay for the identification of cerebrospinal fluid. Neurosurgery 50: 571–576, discussion 576–577, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Michel O, Bamborschke S, Nekic M, Bachmann G: Beta-trace protein (prostaglandin D synthase)–a stable and reliable protein in perilymph. Ger Med Sci 3: Doc04, 2005 [PMC free article] [PubMed]
- 35.Jordan W, Hagedohm J, Wiltfang J, Laier-Groeneveld G, Tumani H, Rodenbeck A, Rüther E, Hajak G: Biochemical markers of cerebrovascular injury in sleep apnoea syndrome. Eur Respir J 20: 158–164, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Jordan W, Tumani H, Cohrs S, Eggert S, Rodenbeck A, Brunner E, Rüther E, Hajak G: Prostaglandin D synthase (beta-trace) in healthy human sleep. Sleep 27: 867–874, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von Eckardstein A. Mild and Moderate Kidney Disease (MMKD) Study Group: Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem 56: 740–749, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, Waheed S, Coresh J: Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis 58: 682–684, 2011 [DOI] [PMC free article] [PubMed]
- 40.Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, Steffes MWModification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group: Glomerular filtration rate measurements in clinical trials. J Am Soc Nephrol 4: 1159–1171, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwong YT, Stevens LA, Selvin E, Zhang YL, Greene T, Van Lente F, Levey AS, Coresh J: Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 56: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]