Abstract
Activation of the sphingosine 1-phosphate receptor 1 (S1P1R) protects against renal ischemia-reperfusion (IR) injury and inflammation, but the role of other members of this receptor family in modulating renal IR injury is unknown. We found that a selective S1P2R antagonist protected against renal IR injury in a dose-dependent manner. Consistent with this observation, both S1P2R-deficient mice and wild-type mice treated with S1P2R small interfering RNA had reduced renal injury after IR. In contrast, a selective S1P2R agonist exacerbated renal IR injury. The S1P2R antagonist increased sphingosine kinase-1 (SK1) expression via Rho kinase signaling in renal proximal tubules; the S1P2R agonist decreased SK1. S1P2R antagonism failed to protect the kidneys of SK1-deficient mice or wild-type mice pretreated with an SK1 inhibitor or an S1P1R antagonist, suggesting that the renoprotection conferred by S1P2R antagonism results from pathways involving activation of S1P1R by SK1. In cultured human proximal tubule (HK-2) cells, the S1P2R antagonist selectively upregulated SK1 and attenuated both H2O2-induced necrosis and TNF-α/cycloheximide-induced apoptosis; the S1P2R agonist had the opposite effects. In addition, increased nuclear hypoxia inducible factor-1α was critical in mediating the renoprotective effects of S1P2R inhibition. Finally, induction of SK1 and S1P2R in response to renal IR and S1P2R antagonism occurred selectively in renal proximal tubule cells but not in renal endothelial cells. Taken together, these data suggest that S1P2R may be a therapeutic target to attenuate the effects of renal IR injury.
AKI is a major clinical complication with high mortality, morbidity, and cost.1,2 Renal ischemia and reperfusion (IR) injury is a major cause of perioperative AKI for patients undergoing surgery involving the kidney, liver, or aorta.3,4 Unfortunately, the severity and incidence of AKI have been increasing, without any improvements in therapy or patient survival over the past 50 years.5 The incidence of renal dysfunction in high-risk patients after major cardiovascular, hepatobiliary, or aortic surgery approaches 70%–80%.3,4,6 Despite continued research searching for renal protective agents, there are no proven therapies to reduce AKI in the perioperative setting1,7
Sphingolipids are pleiotropic regulators of kidney physiology that modulate diverse pathways of cell death, including necrosis, apoptosis, inflammation, and immunity.8,9 In particular, phosphorylation of sphingosine by sphingosine kinases (SK1 and SK2) leads to the formation of sphingosine 1-phosphate (S1P), a lysophospholipid targeting G-protein–coupled receptor that has diverse extracellular as well as intracellular effects.9 Of five G-protein–coupled receptors for S1P, activation of endothelial S1P1R receptor (S1P1R) reduces permeability and maintains the integrity of the vascular endothelial cell barrier.10 S1P1R activation also protects against cardiac,11,12 renal,13,14 and hepatic15 IR injury and inflammation. In contrast, S1P2R activation may have the opposite effects, with potentially adverse vascular signaling events.16 These previous studies suggest that a balance of S1P1R and S1P2R activation may modulate the tissue response to endogenous and exogenous S1P.17,18 However, unlike the better-characterized role of the S1P1R, the role of the S1P2R in tissue injury secondary to IR remains unclear. Moreover, the direct renal tubular effects of S1P2R activation have never been described. In this study, we aimed to test the role of S1P2R in modulating renal injury after IR.
Results
Pharmacologic Blockade, Genetic Deletion, or In Vivo Knockdown of S1P2R Protects against Renal IR Injury in Mice
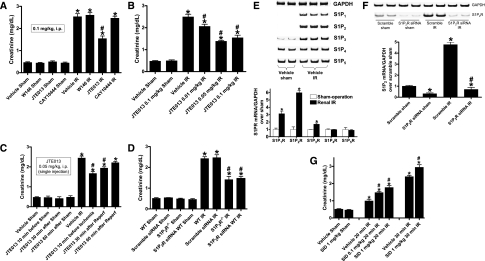
We initially tested the effects of selective S1P1R (W146), S1P2R (JTE-013), or S1P3R (CAY10444) blockade on renal IR injury in mice (Figure 1A); all drugs were given at a dose of 0.1 mg/kg body wt intraperitoneally 10 minutes before and 30 minutes after renal ischemia. Renal IR caused statistically significant increases in plasma creatinine in all groups. However, blockade of the S1P2R produced significant renal protection against IR injury compared with vehicle-treated mice. Neither S1P1R nor S1P3R antagonist pretreatment affected renal IR injury. We subsequently showed dose-dependent renal protection with JTE-013, 0.05–0.1 mg/kg injected intraperitoneally 10 minutes before and 30 minutes after renal ischemia, which produced maximal renal protection in mice after IR injury (Figure 1B). We also tested whether blockade of S1P2R after renal ischemia protected against renal IR injury. Figure 1C shows that JTE-013, 0.1 mg/kg, injected intraperitoneally 10 minutes before ischemia or 30 minutes after reperfusion protected against renal IR injury. However, JTE-013 administered 60 minutes after reperfusion did not produce renal protection after IR.
Figure 1.
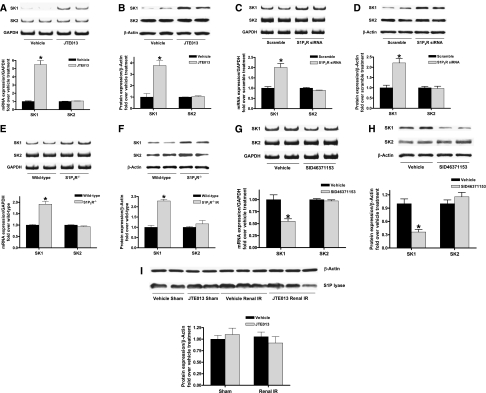
S1P2R activation modulates renal injury after IR. (A) Treatment with a selective S1P2R inhibitor (JTE-013; 0.1 mg/kg interperitoneally 10 minutes before and 30 minutes after renal ischemia) significantly reduced acute kidney injury after renal IR. Selective S1P1R (W146; 0.1 mg/kg intraperitoneally) or S1P3R (CAY10444; 0.1 mg/kg interperitoneally (i.p.) antagonists did not decrease renal injury after IR (n=5–6 in each group). (B) JTE-013 protected the kidney against renal IR in a dose-dependent manner (0.01–0.1 mg/kg interperitoneally given twice at 10 minutes before ischemia and at 30 minutes after reperfusion; n=6). (C) JTE-013 (0.1 mg/kg, single intraperitoneal injection) administered 10 minutes before ischemia or 30 minutes after reperfusion (Reperf) protected against renal IR injury. However, JTE-013 (0.1 mg/kg interperitoneally) administered 60 minutes after reperfusion did not produce renal protection after IR (n=6 per group). (D) Mice deficient in the S1P2R (S1P2R−/−) or wild-type (WT) mice treated with S1P2R siRNA (50 μg intravenously 48 hours before IR for in vivo gene knockdown of the S1P2R) were protected against renal IR compared with wild-type mice or mice treated with scrambled control siRNA (n=4–6 per group). (E) Renal S1P1-5 mRNA expression after sham operation or 30 minutes of renal ischemia followed by 24 hours of reperfusion (IR). Representative reverse transcriptase PCR bands (top) and densitometric quantifications of relative band intensities normalized to GAPDH (bottom, n=4) are shown. S1P2R induction was most robust after renal IR. (F) Evidence for in vivo knockdown of the S1P2R with siRNA treatment. Representative bands and densitometric quantification of relative S1P2R mRNA (normalized to GAPDH) in mouse kidney. Mice were injected with scrambled control siRNA or siRNA targeting the S1P2R 48 hours before renal IR (n=4). (G) Treatment with a selective S1P2R agonist (SID46371153, SID) exacerbated mild (20-minute) and severe (30-minute) renal injury after IR. Mice were treated with vehicle or SID46371153 (0.1 or 1 mg/kg, injected intraperitoneally 10 minutes before ischemia) and subjected to sham operation (n=4) or to 20 or 30 minutes of renal ischemia followed by 24 hours of reperfusion (IR, n=6). *P<0.05 versus vehicle-treated sham operated mice; #P<0.05 versus vehicle-treated mice subjected to renal IR. Error bars represent 1 SEM.
We also demonstrated that mice genetically deficient in S1P2R (S1P2R−/−) or wild-type mice treated with small interfering RNA (siRNA) targeting the S1P2R were also protected against renal IR injury compared with wild-type or scrambled control siRNA–treated mice (Figure 1D). In addition, we determined that renal cortical mRNA expression of S1P1R, S1P2R, and S1P3R were significantly increased after renal IR. Of these three S1P receptor subtypes, we observed the greatest induction in S1P2R (approximately sixfold) after renal IR (Figure 1E). In contrast, the expression of S1P4R and S1P5R did not change after renal IR. Figure 1F demonstrates significantly reduced S1P2R mRNA expression in the kidneys of mice treated with siRNA targeting S1P2R compared with scrambled control siRNA–treated mice. The S1P2R siRNA also prevented the increase in S1P2R mRNA expression after renal IR. Other S1P receptors (S1P1R, S1P3R, S1P4R, and S1P5R) were not affected by S1P2R siRNA treatment (not shown).
Selective Activation of the S1P2R Exacerbates Renal IR Injury in Mice
In contrast to the renal protective effects of S1P2R blockade, a selective S1P2R agonist, SID46371153, produced dose-dependent exacerbation of renal injury in mice subjected to mild (20-minute) or severe (30-minute) renal IR (Figure 1G). Plasma creatinine levels were similar in sham-operated mice treated with vehicle or those treated with SID46371153 alone.
S1P2R Blockade or Deletion Reduces Renal Tubular Necrosis after Renal IR Injury
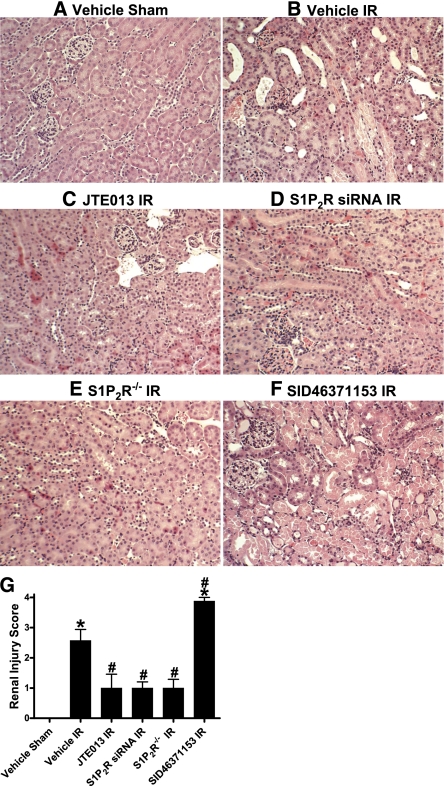
Compared with vehicle-treated sham-operated mice (Figure 2A, representative of four experiments), significant renal injury was evident in vehicle-treated mice subjected to renal IR (Figure 2B), with severe tubular necrosis, corticomedullary congestion, and hemorrhage, and development of proteinaceous casts (30-minute renal ischemia and 24-hour reperfusion). However, mice treated with a selective S1P2R antagonist (JTE-013; 0.1 mg/kg) displayed markedly reduced renal tubular damage and necrosis after renal IR compared with vehicle-treated mice (Figure 2C). Consistent with this, wild-type mice treated with siRNA for S1P2R (Figure 2D) or S1P2R−/− mice (Figure 2E) had improved renal histologic findings after IR. In contrast, mice treated with a selective S1P2R agonist (SID46371153) showed increased renal injury after IR (Figure 2F).
Figure 2.
S1P2R blockade reduces renal tubular necrosis after renal IR injury. Representative photomicrographs (magnification ×200) of hematoxylin and eosin–stained kidney sections. Mice were subjected to sham-operation (A) or to 30 minutes of renal ischemia followed by 24 hours of reperfusion (IR). Wild-type mice were treated with vehicle (B), JTE-013 (a selective S1P2R antagonist; 0.1 mg/kg intraperitoneally 10 minutes before ischemia and again 30 minutes after reperfusion) (C), S1P2R siRNA (50 μg intravenously 48 hours before ischemia) (D), or SID46371153 (a selective S1P2R agonist; 1 mg/kg intraperitoneally 10 minutes before ischemia) (F). We also subjected S1P2R−/− mice to renal IR (E). Photographs are representative of five independent experiments. (G) Summary of Jablonski scale Renal Injury Scores (scale, 0–4) for mice subjected to sham operation or renal IR. *P<0.05 versus vehicle-treated sham operation; #P<0.05 versus vehicle-treated mice subjected to renal IR. Error bars represent 1 SEM.
The Jablonski scale19 Renal Injury Score for histology grading was used to grade renal tubular necrosis 24 hours after renal IR (Figure 2G). Thirty minutes of renal ischemia and 24 hours of reperfusion resulted in severe acute tubular necrosis in vehicle-treated mice (score, 2.6±0.4; n=5). In contrast, wild-type mice treated with JTE-013, S1P2R−/− mice, and wild-type mice treated with siRNA targeting the S1P2R had significantly lower Renal Injury Scores than vehicle-treated mice subjected to renal IR (Figure 2G). We also show that mice treated with SID46371153 showed even greater renal tubular necrosis after IR, consistent with significantly increased plasma creatinine.
S1P2R Blockade or Deletion Reduces Renal Apoptosis after Renal IR Injury
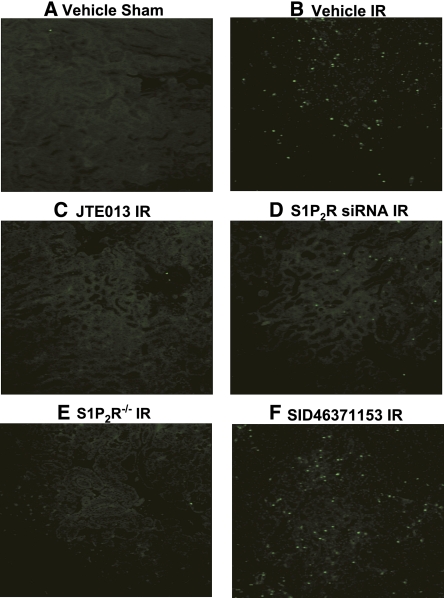
We failed to detect significant apoptotic cells positive for terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) in kidney sections from sham-operated mice (Figure 3A, representative of four experiments). Thirty minutes of renal ischemia and 24 hours of reperfusion resulted in increased apoptotic cells in the kidney (Figure 3B). In contrast, wild-type mice treated with JTE-013 (Figure 3C), wild-type mice treated with siRNA for S1P2R (Figure 3D), and S1P2R−/− mice (Figure 3E) had fewer apoptotic positive cells in the kidney scores compared with vehicle-treated mice subjected to renal IR. We also show that mice treated with SID46371153 showed even more apoptotic cells in the kidney after IR, consistent with significantly increased plasma creatinine (Figure 3F).
Figure 3.
S1P2R blockade reduces renal apoptosis after renal IR injury. Representative fluorescence photomicrographs (four experiments) of kidney sections illustrating apoptotic nuclei TUNEL fluorescence staining; original magnification ×100) in the outer medulla of the kidneys of mice subjected to sham operation (A) or to 30 minutes of renal ischemia followed by 24 hours of reperfusion (IR). Wild-type mice were treated with vehicle (B), JTE-013 (a selective S1P2R antagonist; 0.1 mg/kg intraperitoneally 10 minutes before ischemia and again 30 minutes after reperfusion) (C), S1P2R siRNA (50 μg intravenously 48 hours before ischemia) (D) or SID46371153 (a selective S1P2R agonist; 1 mg/kg intraperitoneally 10 minutes before ischemia) (F). We also subjected S1P2R−/− mice to renal IR (E).
S1P2R Blockade Reduces Renal Pro-Inflammatory Gene Expression after Renal IR Injury
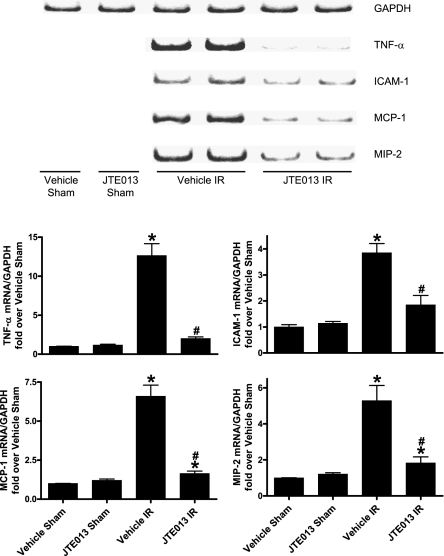
We found significantly increased mRNA expression of pro-inflammatory genes (TNF-α, intracellular adhesion molecule-1, monocyte chemotactic protein-1, or macrophage inflammatory protein-2) in vehicle-treated mice subjected to renal IR 5 hours earlier (collected 5 hours after surgery; Figure 4), and JTE-013 treatment significantly reduced expression of these pro-inflammatory mRNAs.
Figure 4.
S1P2R blockade reduces renal pro-inflammatory gene expression after renal IR injury. Renal pro-inflammatory mRNA expression after sham operation or 30 minutes of renal ischemia followed by 5 hours of reperfusion (IR; n=4 per group). Representative reverse transcriptase PCR bands and densitometric quantifications of relative mRNA band intensities normalized to GAPDH are shown. Mice were injected with vehicle or JTE-013 (a selective S1P2R antagonist; 0.1 mg/kg intraperitoneally) 10 minutes before sham surgery or 10 minutes before ischemia and 30 minutes after reperfusion. Treatment with JTE-013 significantly inhibited the increases in pro-inflammatory TNF-α, ICAM-1, MCP-1 and MIP-2 expression after renal IR. *P<0.05 versus vehicle-treated sham operation; #P<0.05 versus vehicle-treated mice subjected to renal IR. Error bars represent 1 SEM.
Inhibition of Rho or Rho-Associated Protein Kinase Prevents S1P2R-Mediated Exacerbation of Renal IR Injury
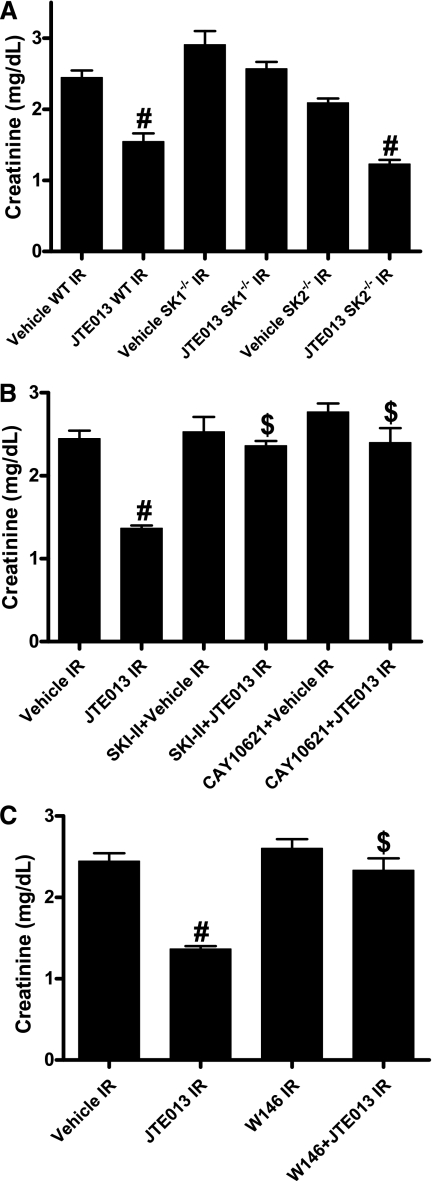
Because S1P2R can activate Rho guanosine triphosphatase as well as Rho-associated protein kinase (ROCK),16 we tested whether a selective S1P2R agonist, SID46371153, targets Rho, as well as ROCK to exacerbate renal dysfunction after IR injury. We pretreated mice with a specific Rho inhibitor C3-exotoxin20 (0.2 mg/kg administered intraperitoneally 1 hour before renal ischemia) or with a selective inhibitor of ROCK21 (Y27632; 10 mg/kg given intraperitoneally 1 hour before renal ischemia). We determined that both C3-exotoxin (creatinine, 1.8±0.2 mg/dl; n=4) and Y27632 (creatinine, 1.9±0.2 mg/dl; n=4) attenuated the renal dysfunction induced with 1 mg/kg SID46371153 plus renal IR (creatinine, 3.0±0.2 mg/dl; n=6). Moreover, treating mice with C3-exotoxin (creatinine, 1.6±0.2 mg/dl; n=4) or Y27632 (creatinine, 1.9±0.2 mg/dl; n=4) alone protected against renal IR injury (creatinine in vehicle-treated mouse with renal IR, 2.4±0.2 mg/dl; n=4) mimicking the renal protective effects of S1P2R blockade with JTE-013 (0.1 mg/kg; creatinine, 1.5±0.2 mg/dl; n=6).
Regulation of SK1 mRNA and Protein Expression with S1P2R Modulation In Vivo
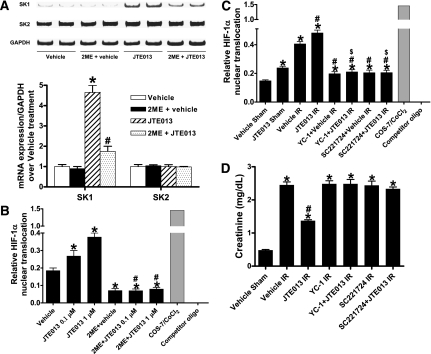
Because a prior study suggested that deletion or downregulation of S1P2R led to an induction of SK1 in mouse embryonic fibroblasts,22 and because induction of SK1 has been shown to protect against cardiac and renal IR injury,23–25 we tested whether SK1 mRNA and protein expression are induced with selective S1P2R blockade. Figure 5, A and B shows that kidneys from mice treated with JTE-103 (0.1 mg/kg) had significantly increased SK1 mRNA and protein expression in 6 hours with no changes in SK2 expression. Consistent with these findings, kidneys from S1P2R−/− mice or wild-type mice treated with S1P2R siRNA show significantly and selectively increased SK1 mRNA and protein expression (Figure 5, C–F). In contrast, activation of S1P2R with SID46371153 downregulated SK1 mRNA and protein expression in the kidney (Figure 5, G and H).
Figure 5.
S1P2R blockade upregulates renal SK1 expression in mice. Renal sphingosine kinase mRNA (A, C, E, and G) and protein (B, D, F, and H) expression in wild-type mice treated with JTE-013 (0.1 mg/kg intraperitoneally 6 hours before [A and B]; n=4), wild-type mice treated with S1P2R siRNA (50 μg intravenously 48 hours before [C and D]; n=4), S1P2R−/− mice (E and F; n=4) and wild-type mice treated with SID46371153 (a selective S1P2R agonist; 1 mg/kg, intraperitoneally 6 hours before [G and H]; n=4). I. Renal S1P lyase protein expression in wild-type mice treated with JTE-013 (0.1 mg/kg intraperitoneally 6 hours before sham operation or renal IR) or with vehicle (n=3–4). S1P2R deletion or blockade significantly and selectively increased SK1 expression compared with vehicle-treated mice (n=4–6 per group) without affecting S1P lyase expression. Conversely, S1P2R agonist significantly decreased SK1 expression compared with vehicle-treated mice. *P<0.05 versus respective control mice (vehicle, scramble, or wild-type). Error bars represent 1 SEM.
We also measured plasma and kidney S1P levels with HPLC.15,23 We determined that both plasma (13.5±2 pmol/μl; n=4) and kidney (10.2±2.8 pmol/mg protein; n=4) S1P increased significantly after renal IR compared with values in sham-operated animals (plasma S1P, 0.6±0.3pmol/μl; n=4; P<0.05; kidney S1P, 1.4±0.2 pmol/mg protein; n=4; P<0.05). Furthermore, consistent with increased SK1 mRNA and protein expression, selective S1P2R blockade with JTE-013 treatment also increased plasma (1.7±0.3 pmol/μl; P<0.05) and renal (3.7±0.8 pmol/mg protein; n=4; P<0.05) S1P levels compared with values in vehicle-treated sham-operated mice.
Renal S1P levels can also be modulated by changes in S1P lyase (an enzyme that degrades S1P to phosphoethanolamine and palmitaldehyde).26 Therefore, we also performed immunoblotting for S1P lyase in mouse kidney tissues subjected to sham-operation or to renal IR after vehicle or JTE-013 injection to test whether S1P degrading capacity changed after IR injury or with selective S1P2R blockade. We determined that mouse kidney S1P lyase protein expression did not change 5 hours after renal IR or with S1P2R blockade with JTE-013 (Figure 5I).
Inhibition of SK1 or S1P1R Signaling Prevents Renal Protection with S1P2R Blockade
Plasma creatinine increased significantly in wild-type, SK1−/−, and SK2−/− mice subjected to renal IR injury compared with sham-operated mice. JTE-013 protected wild-type mice and SK2−/− mice against renal IR injury (Figure 6A). In contrast, SK1−/− mice were not protected against renal IR injury after JTE-013 treatment. We also treated some wild-type mice with SKI-II (a selective but nonspecific SK inhibitor) or with CAY10621 (a SK1-specific inhibitor) before induction of renal IR injury. Vehicle, SKI-II, or CAY10621 administration had no detrimental effects on renal function in sham-operated mice (data not shown). SKI-II or CAY10621 treatment blocked the renal protective effects of JTE-013, providing additional evidence that renal protection with S1P2R blockade is mediated by the induction of SK1 activity (Figure 6B). We subsequently determined that JTE-013 (0.1 mg/kg) failed to protect against renal IR injury when mice were also treated with a selective S1P1R antagonist (W146, 0.1 mg/kg given intraperitoneally 20 minutes before ischemia and 20 minutes after reperfusion; Figure 6C). Therefore, it appears that additional S1P synthesized with JTE-013–mediated induction of SK1 protects against renal IR via pathways involving activation of S1P1R.
Figure 6.
S1P1R is critical for S1P2R antagonist-mediated renal protection. Plasma creatinine levels in mice subjected to sham surgery (n=4) or to renal ischemia and reperfusion are shown (IR; n=5–6 for each group). (A) Mice deficient in SK1 were not protected against renal IR with JTE-013. (B) Wild-type (WT) mice treated with a potent SK inhibitor (SKI-II; 50 mg/kg subcutaneously 15 minutes before ischemia and 30 minutes after reperfusion) or a selective SK1 inhibitor (CAY10621; 2 mg/kg intravenously 30 minutes before ischemia) were not protected against renal IR injury with JTE-013 treatment (0.1 mg/kg). (C) JTE-013 (0.1 mg/kg) failed to protect against renal IR injury in mice treated with a selective S1P1R antagonist (W146; 0.1 mg/kg intraperitoneally 20 minutes before ischemia and 20 minutes after reperfusion). #P<0.05 versus vehicle-treated mice to subjected to renal ischemia and reperfusion; $P<0.05 versus JTE-013–treated mice subjected to renal IR. Error bars represent 1 SEM.
Regulation of SK1 Expression in Human Proximal Tubule-2 Cells In Vitro by S1P2R Modulation and Rho Kinase
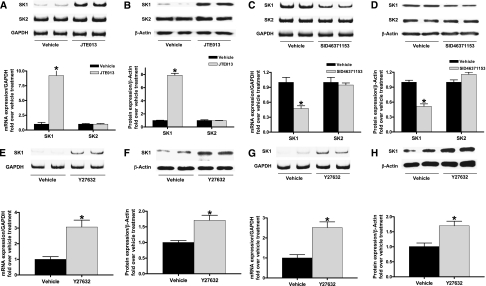
Figure 7, A–D shows that human proximal tubule-2 (HK-2) cells treated with JTE-013 (1 μM) or with SID46371153 (1 μM) for 6 hours significantly increased and decreased, respectively, SK1 mRNA and protein expression, with no changes in SK2 expression. We also show that a specific ROCK inhibitor, Y27632, significantly increased SK1 mRNA and protein expression in HK-2 cells (Figure 7, E and F) and in mouse kidney (Figure 7, G and H), mimicking the effects of a selective S1P2R antagonist (JTE-013).
Figure 7.
Inhibition of S1P2R or Rho kinase upregulates SK1 expression in human proximal tubule cells. SK mRNA (A and C) and protein (B and D) expression in HK-2 cells treated with vehicle, JTE-013, or SID SID46371153 for 6 hours (n=4 for each group). JTE-013 (a selective S1P2R antagonist; 1 μM) significantly and selectively induced SK1 mRNA (A) and protein (B) expression compared with vehicle treatment (0.001% DMSO). In contrast, SID46371153 (a selective S1P2R agonist; 1 μM) significantly and selectively reduced SK1 mRNA (C) and protein (D) expression compared with vehicle treatment (0.2% DMSO). (E–H) A specific Rho kinase (ROCK) inhibitor Y27632 increased SK1 mRNA and protein expression in HK-2 cells (E and F) and in mouse kidney (G and H) mimicking the effects of a selective S1P2R antagonist (JTE-013). *P<0.05 versus vehicle treatment. Error bars represent 1 SEM.
S1P2R Modulation Regulates HK-2 Cell Necrosis and Apoptosis In Vitro
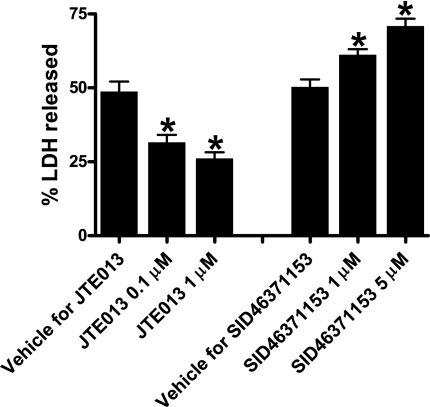
HK-2 cells pretreated with the selective S1P2R antagonist JTE-013 were protected against H2O2-induced necrosis (5 mM H2O2 for 2 hours; Figure 8) and apoptosis (20 ng/ml TNF-α and 10 μg/ml cycloheximide treatment for 16 hours; Figure 9). In contrast, a selective S1P2R agonist, SID46371153, potentiated H2O2-induced HK-2 cell necrosis (Figure 8). Moreover, SID46371153 potentiated apoptotic cell death in HK-2 cells (data not shown).
Figure 8.
S1P2R modulates necrosis of HK-2 cells. Lactate dehydrogenase (LDH) released after H2O2-induced necrosis in HK-2 cells (n=5–6 for each group, expressed as a percentage of total LDH released). Vehicle (DMSO in saline, 0.001% for JTE-013 and 0.2% for SID46371153), JTE-013 (0.1 or 1 μM), or SID46371153 (1 or 5 μM) was applied 30 minutes before H2O2 treatment. JTE-013 attenuated whereas SID46371153 significantly exacerbated LDH release in a dose-dependent manner. *P<0.05 versus respective vehicle treatment. Error bars represent 1 SEM.
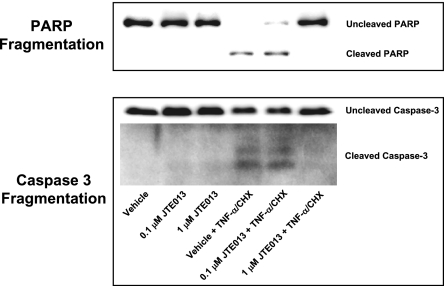
Figure 9.
S1P2R blockade attenuates HK-2 cell apoptosis induced by TNF-α (20 ng/ml) and cycloheximide (CHX; 10 μg/ml). Representative poly(adenosine diphosphate-ribose) polymerase (PARP) and caspase-3 fragmentation (n=4 for each group) as indices of HK-2 cell apoptosis. JTE-013 (0.1 or 1 μM) was applied 30 minutes before induction of apoptosis, and cells were harvested 16 hours later. S1P2R receptor blockade reduced PARP and caspase fragmentation in HK-2 cells.
Critical Role of HIF-1α in S1P2R Antagonist–Induced Upregulation of SK1 and In Vivo Renal Protection
Because HIF-1α is known to be involved in SK1 induction in human endothelial cells,27 we tested the hypothesis that JTE-013 induces SK1 via an HIF-1α–dependent mechanism in HK-2 cells. When HK-2 cells were pretreated with 10 μM of 2-methoxyestradiol (a potent HIF-1α inhibitor) 30 minutes before exposure to JTE-013 (1 μM for 6 hours), induction of mRNA encoding SK1 was significantly attenuated (Figure 10A) without affecting SK2 expression.
Figure 10.
HIF-1α mediates S1P2R antagonist-induced upregulation of sphingosine kinase-1 and in vivo renal protection. (A) Induction of SK1 by S1P2R blockade is mediated by HIF-1α. Representative reverse transcriptase PCR bands and densitometric quantification of SK mRNA (band intensities normalized to glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) in HK-2 cells treated with vehicle (0.001% DMSO; n=4) or JTE-013 (1 μM; n=4) for 6 hours. We blocked HIF-1α function with a post-transcriptional inhibitor 2-methoxyestradiol (2ME; 10 μM) given 30 minutes before vehicle or JTE-013 treatment. Inhibition of HIF-1α with 2-methoxyestradiol significantly attenuated the increase in SK1 mRNA with JTE-013 treatment. (B) HIF-1α DNA binding activity in nuclear extracts from HK-2 cells treated with vehicle or JTE-013 (n=4) for 6 hours. JTE-013 increases nuclear translocation of HIF-1α, which is inhibited by 2-methoxyestradiol (10 μM, given 30 minutes before JTE-013 treatment). (C) JTE-013 increases HIF-1α nuclear translocation in kidneys of mice subjected to sham operation (n=4) or 30 minutes of renal ischemia followed by 24 hours of reperfusion (IR) (n=4). Pretreatment with a selective HIF-1α inhibitor, YC-1 (50 mg/kg intraperitoneally), or SC221724 (20 mg/kg intraperitoneally), blocked the increase in mouse kidney HIF-1α DNA binding. (D) YC-1 (50 mg/kg intraperitoneally) or SC221724 (20 mg/kg intraperitoneally) prevented renal protection with JTE-013 (n=4–6 in each group). Plasma creatinine was measured from 24 hours after reperfusion. *P<0.05 versus vehicle treatment (A and B) or vehicle sham (C and D); #P<0.05 versus JTE-013 treatment (A and B) or vehicle IR (C and D); $P<0.05 versus JTE-013 IR (C). Error bars represent 1 SEM. COS-7, African Green Monkey kidney fibroblast cell line.
We next tested whether inhibition of S1P2R directly increases nuclear HIF-1α and subsequent DNA binding in HK-2 cells and in mouse kidneys (Figure 10, B and C). In HK-2 cells, a selective S1P2R antagonist, JTE-013, dose-dependently increased nuclear HIF-1α and DNA binding, which was completely inhibited by 10 μM of 2-methoxyestradiol (given 30 minutes before JTE-013 treatment; Figure 10B). We also showed that JTE-013 increases nuclear HIF-1α in sham-operated mouse kidneys (Figure 10C). Renal IR (30-minute renal ischemia and 24-hour reperfusion) increased nuclear HIF-1α, which was further increased with JTE-013 (Figure 10C). Treating mice with a selective HIF-1α inhibitor, YC-1 (50 mg/kg given intraperitoneally), and SC221724 (20 mg/kg given intraperitoneally) completely prevented the increase in mouse kidney HIF-1α DNA binding. Finally, we showed that mice pretreated with a selective HIF-1α inhibitor, YC-1 (50 mg/kg given intraperitoneally), or SC221724 (20 mg/kg given intraperitoneally) were not protected against renal IR injury with JTE-013 treatment (Figure 10D). Taken together, these data demonstrate a critical role of increased nuclear HIF-1α in mediating the renal protective effects of S1P2R inhibition.
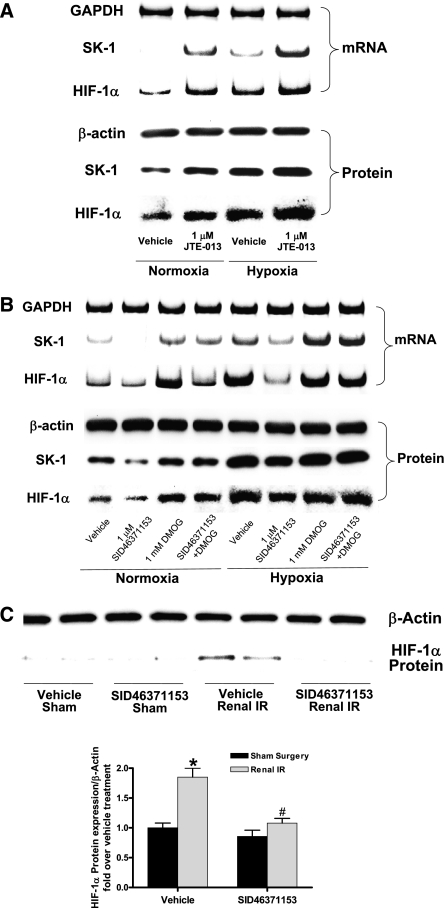
The next series of experiments were performed to determine whether S1P2R modulates nuclear translocation of HIF-1α by affecting existing HIF-1α protein or by synthesizing new HIF-1α. HK-2 cells were subjected to 24 hours of normoxia or to hypoxia (2% O2). A selective S1P2R antagonist (JTE-013; 1 μM) directly increased renal tubular HIF-1α mRNA and protein expression under both normoxic and hypoxic conditions (Figure 11A). In addition, a selective S1P2R agonist (SID46371153; 1 μM) decreased HIF-1α mRNA expression in normoxic HK-2 cells. SID46371153 also reduced HIF-1α mRNA and protein expression in hypoxic HK-2 cells (Figure 11B). JTE-013 increased and SID46371153 attenuated hypoxia-driven increases in SK1 mRNA and protein expression (Figure 11, A and B). A prolyl hydroxylase inhibitor dimethyloxaloylglycine (DMOG) (1 mM) reversed the SID46371153-mediated decreases in SK1 and HIF-1α protein expression in HK-2 cells. Finally, SID46371153 reduced the induction of HIF-1α in mouse kidneys subjected to renal IR (Figure 11C). Preventing this reduction of HIF-1α expression with DMOG injection (5 mg per mouse intraperitoneally; creatinine, 2.36±0.2 mg/dl; n=4) blocked the exacerbation of renal injury with SID46371153 (creatinine, 3.0±0.2 mg/dl; n=6). Therefore, these data suggest that S1P2R directly modulates new synthesis of HIF-1α that directly contributes to increased SK1 synthesis and renal protection.
Figure 11.
S1P2R modulates renal proximal tubular HIF-1α synthesis. (A and B) HK-2 cells were subjected to normoxia (95% room air plus 5% CO2) or to hypoxia (2% O2, 93% N2, and 5% CO2) for 24 hours (representative of three experiments). A selective S1P2R antagonist (JTE-013; 1 μM) increased renal tubular HIF-1α mRNA and protein expression under both normoxic and hypoxic conditions (A). A selective S1P2R agonist (SID46371153; 1 μM) decreased HIF-1α mRNA expression in normoxic renal proximal tubule cells (B). SID46371153 also reduced HIF-1α mRNA and protein expression in hypoxic renal proximal tubule cells. Hypoxia increased SK1 mRNA and protein expression, and this increase was potentiated by JTE-013 and attenuated by SID46371153 (A and B). A proxyl hydroxylase inhibitor DMOG (1 mM) reversed the SID46371153-mediated decreases in SK1 and HIF-1α expression in HK-2 cells. (C) Mice were subjected to 30 minutes of renal ischemia and 5 hours of reperfusion. SID46371153 reduced the induction of HIF-1α in mouse kidneys subjected to renal IR (representative of four experiments). *P<0.05 versus sham operation group; #P<0.05 versus vehicle-treated renal IR group. Error bars represent 1 SEM.
Selective Regulation of S1P2R and SK1 in Proximal Tubule Cells after Renal IR or JTE-013 Treatment
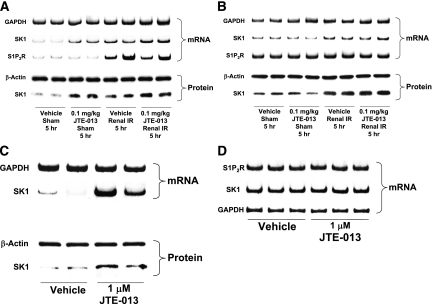
Next, we wanted to determine the specific kidney cell type that induces S1P2R and SK1 in response to renal IR or selective S1P2R antagonism. Mice were injected with vehicle or with 0.1 mg/kg JTE-013, then subjected to sham operation or to renal IR (Figure 12). We isolated proximal tubules (with Percoll density gradient separation) and renal endothelial cells (with Dynabeads coupled to CD144 antibody). We determined that renal IR induced SK1 in both renal proximal tubule cells (Figure 12A) and endothelial cells (Figure 12B). However, JTE-013 increased SK1 mRNA and protein expression only in mouse proximal tubules (Figure 12A) and not in renal endothelial cells (Figure 12B). Renal IR also did not induce S1P2R mRNA in mouse kidney endothelial cells (Figure 12B). In addition, we also observed a robust induction of SK1 mRNA and protein in freshly isolated mouse proximal tubules from naive mice treated with 1 μM JTE-103 for 6 hours (Figure 12C). Finally, JTE-013 failed to induce SK1 and S1P2R in immortalized human endothelial cells (EA.hy926 cells) (Figure 12D).
Figure 12.
Renal IR or S1P2R antagonist modulates S1P2R and SK1 in proximal tubule cells but not in endothelial cells. (A and B) Mice were injected with vehicle or with 0.1 mg/kg JTE-013, then subjected to sham operation or to renal IR (representative of three experiments). We isolated proximal tubules (with Percoll density gradient separation) and renal endothelial cells (with Dynabeads coupled to CD144 antibody). Renal IR induced SK1 in renal proximal tubule cells (A) and in endothelial cells (12). However, a selective S1P2R antagonist, JTE-013, increased SK1 mRNA and protein expression only in mouse proximal tubules (A). JTE-013 or renal IR failed to induce S1P2R mRNA in mouse kidney endothelial cells (B). (C) We also observed a robust induction of SK1 mRNA and protein in freshly isolated mouse proximal tubules from naive mice treated with 1 μM JTE-013 for 6 hours. (D) JTE-103 failed to induce SK1 and S1P2R in immortalized human endothelial cells (EA.hy926 cells).
Discussion
S1P is an endogenous lysophospholipid ligand that regulates cellular necrosis, apoptosis, and inflammation in many cell types by binding to cell surface G-protein–coupled high-affinity S1P receptors.28,29 Five identified S1PRs produce distinct as well as overlapping intracellular signaling events. S1P1R, S1P2R, and S1P3R in particular are widely expressed in many cell types, including the brain, liver, heart, kidney, and immune system.28 S1P1R couples exclusively through Gi pathway, whereas S1P2R and S1P3R can couple with Gi, Gq, or G12/13 pathways.18 Of five S1P receptor subtypes, the S1P1R has been the most extensively studied. Activation of the S1P1R protects against cardiac and hepatic IR injury11,13,15 and reduces systemic and tissue inflammation by directly affecting lymphocyte egress.29,30 In the kidney, activation of the S1P1R with SEW2871 or with FTY720 protects against renal IR injury by producing anti-inflammatory effects on infiltrating pro-inflammatory T lymphocytes,13,31 as well as by eliciting direct cytoprotective effects on renal proximal tubule cells.32 However, other S1P receptor subtypes are also expressed in the kidney in addition to the S1P1R. Renal proximal tubule cells, endothelial cells, and immune cells present in the kidney also express S1P2R, S1P3R, and S1P4R subtypes.9,14 In particular, unlike the well characterized renal protective effects of S1P1R, to our knowledge the physiologic and pathophysiologic roles of S1P2R modulation of renal IR injury have never been studied previously.
In this study, we provide direct evidence for a detrimental role of renal S1P2R activation in IR injury. We complemented pharmacologic S1P2R blockade with genetic (S1P2R−/−), as well as gene deletion (with siRNA knockdown of S1P2R) studies. All three approaches of S1P2R inhibition produced a similar degree of renal protection after IR, as well as selective induction of SK1, implicating it as a mechanism of renal protection. Previous studies of S1P2R physiology and pharmacology were limited by lack of a selective S1P2R agonist. In this study, we used a novel and highly selective agonist for the S1P2R (SID46371153). SID46371153 was developed from high-throughput screening of nearly 100,000 compounds and was found to be selective for the S1P2R.33 In addition, the activity of SID46371153 was inhibited by the S1P2R selective antagonist JTE-013.
Global gene deletion studies have shown that unlike S1P1R, S1P2R is not necessary for normal vascular development.34 However, S1P2R deficiency results in deafness and balance abnormality due to defects in hair cells and stria vascularis. Several prior studies in other organs and cell types suggested a pro-inflammatory and pro-apoptotic role for S1P2R activation.17,35,36 Blockade of S1P2R significantly inhibited H2O2-induced permeability in the rat lung perfused model,37 and HEK-293 cells overexpressing the S1P2R were prone to cell rounding and apoptosis.38 Moreover, S1P2R signaling modulates mast cell function, including degranulation and cytokine/chemokine release.39 The S1P2R also stimulates pro-inflammatory macrophage activities, including macrophage retention and inflammatory cytokine secretion.40
Activation of the S1P2R in endothelial cells resulted in ROCK- and phosphatase and tensin homolog–dependent disruption of adherens junctions and increased paracellular permeability.16 Our studies also support an import role for proximal tubular Rho and ROCK activation in S1P2R-mediated exacerbation of renal injury after IR. In addition, specific inhibitor of Rho or ROCK mimicked the renal protective effects of S1P2R antagonist JTE-013. Finally, we show in this study that inhibition of ROCK mimics S1P2R antagonist–mediated induction of SK1. Therefore, we propose that S1P2R-mediated activation of Rho and ROCK may lead to increased renal injury after IR by downregulation of cytoprotective SK1. Because several studies also suggested a protective role for S1P2R activation by reducing cardiac and hepatic IR injury,41,42 it is likely that S1P2R produces tissue and cell type–specific modulation of injury and inflammation.
We demonstrate a selective induction of SK1 mRNA and protein with S1P2R blockade in renal proximal tubule cells in vivo and in HK-2 cells in vitro without affecting S1P degrading enzyme S1P lyase. Our findings agree with findings in mouse embryonic fibroblasts, where S1P2R deletion resulted in a marked upregulation of SK1.22 We also demonstrate that induction of renal tubular SK1 plays a critical role in renal protection induced with S1P2R blockade because JTE-013 failed to protect against renal IR in mice treated with selective SK1 inhibitors (SKI-II or CAY10621) or mice deficient in SK1. Furthermore, we showed that activation of the S1P2R led to downregulation of renal tubular SK1, providing a potential mechanism of exacerbated renal IR injury with S1P2R activation. In addition, our findings imply that tonic S1P2R activation by endogenous S1P may reduce additional S1P generation by decreasing SK1 synthesis. Finally, our data suggest that although both endothelial and renal proximal tubule cells may make more S1P in response to renal IR, only the proximal tubule cells upregulate S1P12R to respond to increased renal S1P.
SK1 is a well known mediator of tissue protection, growth, and survival.22,25,43 In acute lung injury, overexpression of SK1 was protective.44 Furthermore, in cardiac IR injury, SK1 activation plays a potent cytoprotective role and SK1-deficient myocytes had increased injury after ischemia.25 Overexpression of SK1 would indeed increase the synthesis of endogenous S1P in the renal cortex and in proximal tubules. All five S1PR subtypes bind to S1P, with similar nanomolar affinities.45 For example, S1P1R, S1P2R, and S1P3R bind to S1P with a mean Kd of approximately 25±18, 18±7, and 20±9 nM, respectively. However, because S1P1R is the most abundant S1P receptor subtype expressed in the kidney,14 we hypothesized that increased SK1-mediated S1P synthesis directly mediates renal protection via S1P1R activation. Our results support this hypothesis because a selective S1P1R antagonist (W146) blocked the renal protective effect of JTE-013. In the kidney, direct S1P1R activation with a selective agonist32 or enhanced activation of S1P1R secondary to increased SK1 activity24 produces protection against IR in mice. Our findings also imply that S1P2R activation by enhanced S1P generation may in turn limit additional S1P formation by reducing SK1 synthesis.
Collectively, our data support a critical role for HIF-1α in SK1 induction and renal tubular protection with S1P2R inhibition. We showed in this study that a selective HIF-1α blocker (2-methoxyestradiol) prevented JTE-013–mediated HIF-1α nuclear translocation as well as induction of SK1 in HK-2 cells. 2-methoxyestradiol is a natural metabolite of estrogen that is known to inhibit HIF-1α at the level of translation, independent of effects of estrogen receptors.46 Furthermore, in vivo administration of selective HIF-1α transcriptional inhibitors (YC-1 or SC221724) also blocked kidney HIF-1α nuclear translocation and renal protection afforded by JTE-13. HIF-1 is a heterodimeric transcription factor composed of an α and a β subunit.47,48 Under normoxic conditions, prolyl-hydroxylation and ubiquitination of the oxygen-dependent degradation domain of HIF-1α results in rapid HIF-1α degradation. With hypoxia or ischemia, HIF-1α stabilizes and interacts with HIF-1β, forming the HIF-1 heterodimer. Nuclear translocation of HIF-1 allows binding to the hypoxia-responsive element with subsequent induction of several cytoprotective genes, including vascular endothelial growth factor and erythropoietin. Consistent with this, previous studies have demonstrated that HIF-1α activation protects against renal IR injury.47
Mechanistically, our results suggest that S1P2R can directly modulate new HIF-1α mRNA and protein synthesis. We therefore propose that increased nuclear HIF-1α binding with JTE-103 is due to increased synthesis of HIF-1α rather than by affecting degradation or nuclear translocation of existing HIF-1α protein. Increased synthesis and enhanced nuclear translocation of HIF-1α with S1P2R inhibition may lead to a novel therapeutic approach in attenuating AKI due to renal IR or hypoperfusion. Our data suggest that blockade or deletion of S1P2R removes the tonic inhibition of HIF-1α with subsequent SK1 induction (Figure 13).
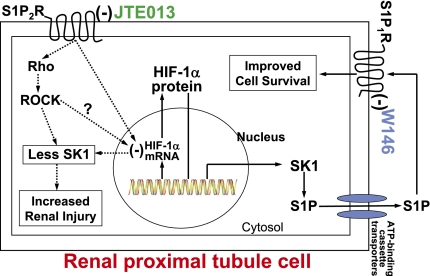
Figure 13.
A schematic of proposed cellular mechanisms of renal protection with S1P2R inhibition in renal proximal tubules. Our data suggest that selective S1P2R blockade with JTE-013 results in increased HIF-α mRNA and protein synthesis and SK1 induction leading to new S1P synthesis with subsequent S1P1R activation. Consistent with this hypothesis, selective S1P1R blockade with W146 inhibits the renal protective effects of JTE-013. Our findings also imply that tonic S1P2R stimulation directly reduces HIF-α mRNA synthesis, leading to attenuated HIF-1α transcription factor activity. Moreover, tonic S1P2R activation by endogenous S1P places brake on additional S1P generation by reducing SK1 synthesis and activity. Finally, we showed that inhibition of Rho ROCK signaling mimics S1P2R antagonist–mediated induction of SK1 and also attenuates S1P2R agonist–mediated exacerbation of renal function. Therefore, we also propose that S1P2R-mediated activation of Rho and ROCK may lead to increased renal injury after IR. (−) represents inhibition.
In summary, we demonstrate in this study that S1P2R in the kidney modulates renal IR injury by regulating renal proximal tubular SK1 synthesis via Rho kinase and HIF-1α (Figure 13). Clinically, pretreatment (before renal ischemia or hypoperfusion) or post-treatment is an excellent option for the frequent clinical scenarios where patients are subjected to renal injury during surgical procedures (e.g., kidney transplantation, open heart surgery, aorto-vascular surgery, or liver transplantation). We showed here that a selective S1P2R blockade with JTE-013 provided significant renal protection when given immediately before renal ischemia or even 30 minutes after reperfusion. Collectively, these findings may have important clinical implications because they imply that S1P2R antagonism might be a viable therapeutic option in attenuating the effects of renal IR.
Concise Methods
Materials
Full drug names used in this study are listed in Supplemental Material.
Murine Model of Renal IR Injury
After Columbia University Institutional Animal Care and Use Committee approval, we subjected male C57BL/6 (Harlan, Indianapolis, IN) as well as S1P2R−/−, SK1−/−, and SK2−/− mice34,49,50 (congenically derived on a C57BL/6 background for approximately 10 generations and kindly provided by Dr. Richard L. Proia, National Institutes of Health, Bethesda, MD) to 20 or 30 minutes of renal IR, as described elsewhere.23,51,52 To test the effects of specific S1P receptor subtype antagonists on renal function after IR injury, we treated mice with intraperitoneal W146, JTE-013, or CAY10444, selective inhibitors for S1P1R, S1P2R, and S1P3R, respectively (0.01–0.1 mg/kg), 10 minutes before and 30 minutes after renal ischemia or sham operation. We also tested whether a single injection of JTE-013 could also provide renal protection after IR injury. In a separate cohort of mice, a single dose of JTE-013 (0.1 mg/kg) was given intraperitoneally 10 minutes before ischemia or 30 or 60 minutes after reperfusion.
To test the effects of SK inhibition, the SK inhibitors SKI-II or CAY10621 were administered to mice subjected to renal IR injury. SKI-II (50 mg/kg) was administered subcutaneously 15 minutes before ischemia and 4 hours after reperfusion. CAY10621, an SK1-specific inhibitor with no inhibitory effect on SK2, was administered subcutaneously (2 mg/kg) 30 minutes before ischemia.53,54 A separate cohort of mice was treated with a selective S1P2R agonist (SID46371153; 0.1–1 mg/kg) 15 minutes before renal IR injury.33 To test the effects of selective S1P1R inhibition on JTE-013–mediated renal protection, some mice were also treated with a selective S1P1R antagonist (W146; 0.1 mg/kg intraperitoneally) 20 minutes before ischemia and 20 minutes after reperfusion. To inhibit Rho or Rho kinase in vivo, some mice were intraperitoneally injected with C3 exotoxin (a specific inhibitor of Rho; 0.2 mg/kg 1 hour before ischemia) or Y27632 (a Rho kinase inhibitor; 10 μg/kg 1 hour before ischemia). To inhibit HIF-1α prolyl hydroxylase activity in vivo, we treated the mice with DMOG (5 mg intraperitoneally 30 minutes before renal ischemia).
For in vivo inhibition of HIF-1α transcriptional activity, mice were treated with YC-1 (50 mg/kg intreperitoneally 24 hours and 15 minutes before renal ischemia) or with SC221724 (20 mg/kg interperitoneally 1 hour before ischemia and 10 minutes before reperfusion). We collected kidneys at 5 hours and plasma at 24 hours after renal IR injury to examine the severity of renal dysfunction.
Measurement of Renal Function
Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). This method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens well known to the Jaffe method.55
Histologic detection of necrosis
Morphologic assessment was performed by an experienced renal pathologist (V.D.), who was unaware of the treatment that each animal had received. An established grading scale of necrotic injury (Renal Injury Score, 0–4) to the proximal tubules was used for the histopathologic assessment of IR-induced damage, as outlined by Jablonski and colleagues19 and as described previously in our studies.23,52
Detection of Renal Tubular Apoptosis
We detected apoptosis after renal IR with TUNEL staining as described elsewhere15 using a commercially available in situ cell death detection kit (Roche, Indianapolis, IN) according to the instructions provided by the manufacturer.
Reverse Transcription PCR
Semiquantitative reverse transcriptase PCR assays for pro-inflammatory mRNAs, as well as SK1, SK2 and S1P1R-5 mRNA, were performed as described elsewhere.15,56 Table 1 lists the primer sequences utilized in this study.
Table 1.
Reverse transcriptase PCR primers used in this study
| Primers | Species | Sequences (Sense/Antisense) | Product Size (bp) | Cycle Number | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| S1P1R | Mouse | 5′-CGGTGTAGACCCAGAGTCCT-3′ 5′-AGCAGCAGATGAGAATGAAC-3′ | 393 | 20 | 64 |
| S1P2R | Mouse | 5′-AAAACCAACCACTGGCTGTC-3′ 5′-GAGTGGAACTTGCTGTT-3′ | 317 | 25 | 60 |
| S1P3R | Mouse | 5′-AAGCCTAGCGGGAGAGAAAC-3′ 5′-GGCAATCAAAACCATCAGGT-3′ | 452 | 22 | 64 |
| S1P4R | Mouse | 5′-GCAGAAGTCTCCACGTCCTC-3′ 5′-GCTGAGTGACCGAGAAGTCC-3′ | 403 | 23 | 62 |
| S1P5R | Mouse | 5′-ACACCAAATGCCCAGCTTAC- 3′ 5′-ACCAAGAGCACAGCCAAGTTC-3′ | 335 | 32 | 62 |
| SK1 | Mouse | 5′-GATGCATGAGGTGGTGAATG-3′ 5′-GCCCACTGTGAAACGAATC-3′ | 337 | 22 | 64 |
| SK2 | Mouse | 5′-ACTGCTCGCTTCTTCTCTGC-3′ 5′-ACCATTGAGGGACAGGTCAG-3′ | 437 | 23 | 68 |
| TNF-α | Mouse | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ | 290 | 24 | 65 |
| ICAM-1 | Mouse | 5′-TGTTTCCTGCCTCTGAAGC-3′ 5′-CTTCGTTTGTGATCCTCCG-3′ | 409 | 21 | 60 |
| MCP-1 | Mouse | 5′-ACCTGCTGCTACTCATTCAC-3′ 5′-TTGAGGTGGTTGTGGAAAAG-3′ | 312 | 22 | 60 |
| MIP-2 | Mouse | 5′-CCAAGGGTTGACTTCAAGAAC-3′ 5′-AGCGAGGCACATCAGGTACG-3′ | 282 | 28 | 60 |
| S1P2R | Human | 5′-GCCTAGCCAGTTCTGAAAGC-3′ 5′-GAGTGGAACTTGCTGTT-3′ | 249 | 22 | 58 |
| HIF-1α | Human | 5′-CAGCTATTTGCGTGTGAGGA-3′ 5′-CCAAGCAGGTCATAGGTGGT-3′ | 400 | 26 | 64 |
| SK1 | Human | 5′-ATCTCCTTCACGCTGATGC-3′ 5′-GTGCAGAGACAGCAGGTTCA-3′ | 330 | 26 | 66 |
| SK2 | Human | 5′-GGAGGAAGCTGTGAAGATGC-3′ 5′-GCAGGTCAGACACAGAACGA-3′ | 482 | 22 | 66 |
| GAPDH | Mouse and human | 5′-ACCACAGTCCATGCCATCAC-3′ 5′-CACCACCCTGTTGCTGTAGCC-3′ | 450 | 16 | 65 |
Respective anticipated reverse transcriptase PCR product size, PCR cycle number for linear amplification, and annealing temperatures used for each primer.
siRNA Preparation and Delivery to Mice In Vivo
A chemically synthesized 21-nucleotide siSTABLE (stability-enhanced siRNA) sequence specific for S1P2R (5′-UAACUCCCGUGCAGUGGUUUU-3′) were custom made and purchased from Dharmacon Research (Lafayette, CO) in 2′-hydroxyl, annealed, desalted, and dialyzed duplex form for in vivo use. The double-stranded sequence for S1P2R siRNA was injected as 50 μg of siRNA (100 μL) intravenously 48 hours before renal ischemia.
Induction of HK-2 Cell Necrosis, Apoptosis, and Hypoxia
Necrotic injury in HK-2 cells was induced with exposure to 5 mM H2O2 for 2–4 hours and lactate dehydrogenase released into cell culture media was measured as described elsewhere.57,58 To induce apoptosis, HK-2 cells were exposed to TNF-α (10 ng/ml) plus cycloheximide (5 μg/ml) for 16 hours as described elsewhere.59 Some cells were pretreated with vehicle (diluted DMSO), JTE-013 (0.1–1 μM), or SID46371153 (0.1–1 μM) 30 minutes before induction of cell death. HK-2 cells treated with vehicle (0.2% DMSO), SID46371153 (1 μM), DMOG (1 mM), or SID46371153 plus DMOG and subjected to hypoxia (2% O2, 93% N2, and 5% CO2) or normoxia (95% room air in 5% CO2) for 24 hours.
Immunoblotting Analyses
Mouse kidney cortical tissues (including corticomedullary junction), HK-2 cell lysates, or freshly isolated mouse kidney proximal tubules (Percoll gradient separation) were collected for immunoblotting analyses of HIF-1α, SK1, SK2, and β-actin (internal protein loading control) as described elsewhere.15 We also probed for these proteins in immortalized human umbilical vein endothelial cells (EA.hy926). Finally, we performed immunoblotting for S1P lyase in mouse kidney tissues subjected to sham operation or to renal IR after vehicle or JTE-013 injection.
HIF-1α DNA-Binding Assay
Nuclear extracts from mouse kidney tissues and HK-2 cells were prepared using the Transfactor Extraction Kit (BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer’s instructions. HIF-1α DNA-binding activity in nuclear extracts was determined using a TransFactor Family Colorimetric kit specific for HIF-1α (BD Biosciences Clontech) according to the manufacturer’s instructions. Nuclear extracts from COS-7 cells treated with CoCl2 (Active Motif, Carlsbad, CA) and nuclear extracts incubated with a HIF-1α–specific competitor oligonucleotide served as positive and negative controls, respectively.
Isolation of Mouse Kidney Proximal Tubules and Endothelial Cells
Mouse kidney proximal tubules were isolated using the methods described by Vinay and colleagues (collagenase digestion followed by Percoll density gradient separation).60 Renal endothelial cells were isolated by magnetic beads separation according to the manufacturer’s instruction (Invitrogen, Carlsbad, CA).
Determination of Kidney and Plasma S1P
Five hours after renal IR or sham operation, mouse plasma and kidneys (lysed in 150 mM Tris-HCl, 150 mM NaCl, 1% Triton-X, 10 mM EDTA, 1 mM Na3VO4, 100 mM NaF plus protease and phosphatase inhibitors) were collected for S1P measurement as described elsewhere.23,56
Statistical Analyses
The data were analyzed with a t test for comparing means between two groups. One-way ANOVA plus a Tukey post hoc multiple comparison test was used for comparing multiple groups. Two-way ANOVA plus a Bonferroni post-test was used to test the effects of sham operation or renal IR injury on different mouse strains or treatment groups. The ordinal values of the Renal Injury Scores were analyzed by the Mann–Whitney nonparametric test. In all cases, P<0.05 was taken to indicate statistical significance. All data are expressed throughout the text as mean ±SEM.
Disclosures
None.
Acknowledgments
We thank R. L. Proia for providing the S1P2R−/−, SK1−/−, and SK2−/− mice.
This work was supported by National Institutes of Health Grant R01 GM-067081.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sphingosine Lipids in the Resolution of Renal Ischemia and Reperfusion Injury,” on pages 187–189.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011050503/-/DCSupplemental.
References
- 1.Faubel S: Acute kidney injury and multiple organ dysfunction syndrome. Minerva Urol Nefrol 61: 171–188, 2009 [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT: Perioperative renal protection. Best Pract Res Clin Anaesthesiol 22: 193–208, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Aronson S, Blumenthal R: Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth 12: 567–586, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Jo SK, Rosner MH, Okusa MD: Pharmacologic treatment of acute kidney injury: Why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol 2: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Jones DR, Lee HT: Protecting the kidney during critical illness. Curr Opin Anaesthesiol 20: 106–112, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Hassoun HT, Santora R, Rabb H: Organ crosstalk: The role of the kidney. Curr Opin Crit Care 15: 481–487, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S: Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD: Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG: Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B: High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 114: 1403–1409, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhang Z, Lin X, Xu DS, Feng Y, Ding K: A polysaccharide, MDG-1, induces S1P1 and bFGF expression and augments survival and angiogenesis in the ischemic heart. Glycobiology 20: 473–484, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW: S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int 69: 1601–1608, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Park SW, Kim M, Chen SW, Brown KM, D’Agati VD, Lee HT: Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest 90: 1209–1224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoura A, Hla T: Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res 82: 221–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoki I, Takuwa N, Sugimoto N, Yoshioka K, Takata S, Kaneko S, Takuwa Y: Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun 346: 293–300, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH: International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev 62: 579–587, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, Tange J: An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35: 198–204, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Ikeda H, Satoh H, Yanase M, Inoue Y, Tomiya T, Arai M, Tejima K, Nagashima K, Maekawa H, Yahagi N, Yatomi Y, Sakurada S, Takuwa Y, Ogata I, Kimura S, Fujiwara K: Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology 124: 459–469, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Teraishi K, Kurata H, Nakajima A, Takaoka M, Matsumura Y: Preventive effect of Y-27632, a selective Rho-kinase inhibitor, on ischemia/reperfusion-induced acute renal failure in rats. Eur J Pharmacol 505: 205–211, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S: The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol 25: 4237–4249, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M, Kim M, Park SW, Pitson SM, Lee HT: Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol 31: 353–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M, Kim M, Kim N, D’Agati VD, Emala CW, Sr, Lee HT: Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol 293: F1827–F1835, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS: Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit 12: BR318–BR324, 2006 [PubMed] [Google Scholar]
- 26.Saba JD, Hla T: Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res 94: 724–734, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Schwalm S, Döll F, Römer I, Bubnova S, Pfeilschifter J, Huwiler A: Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun 368: 1020–1025, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Chae SS, Proia RL, Hla T: Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat 73: 141–150, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Allende ML, Dreier JL, Mandala S, Proia RL: Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG: Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int 71: 1223–1231, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen H: High throughput screening for S1P receptor agonists and antagonists, S1P2 agonist. The Scripps Molecular Screening Center Probe Report, 2011. Available at: http://mli.nih.gov/mli/?dl_id=720 Accessed December 7, 2007
- 34.Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL: Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem 282: 10690–10696, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T: Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol 31: 81–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T: Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest 117: 2506–2516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T: Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27: 1312–1318, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S: Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem 274: 4626–4632, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S: Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199: 959–970, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Okamoto Y, Inoki I, Yoshioka K, Du W, Qi X, Takuwa N, Gonda K, Yamamoto Y, Ohkawa R, Nishiuchi T, Sugimoto N, Yatomi Y, Mitsumori K, Asano M, Kinoshita M, Takuwa Y: Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest 120: 3979–3995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J,, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944–H2951, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B, Tran-Van-Nhieu J, Manin S, Poelstra K, Chun J, Carpentier S, Levade T, Mallat A,, Lotersztajn S. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J 21: 2005–2013, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Maceyka M, Payne SG, Milstien S, Spiegel S: Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta 1585: 193–201, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Wadgaonkar R, Patel V, Grinkina N, Romano C, Liu J, Zhao Y, Sammani S, Garcia JG, Natarajan V: Differential regulation of sphingosine kinases 1 and 2 in lung injury. Am J Physiol Lung Cell Mol Physiol 296: L603–L613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young N, Van Brocklyn JR: Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. ScientificWorldJournal 6: 946–966, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mooberry SL: Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat 6: 355–361, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Schödel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt KU, Willam C: HIF-prolyl hydroxylases in the rat kidney: Physiologic expression patterns and regulation in acute kidney injury. Am J Pathol 174: 1663–1674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, Arend M, Klaus SJ, Heyman SN: Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant 23: 3472–3478, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J: The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity 26: 287–297, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL: Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279: 52487–52492, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Park SW, Chen SW, Kim M, Brown KM, Kolls JK, D’Agati VD, Lee HT: Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Lab Invest 91: 63–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D'Agati VD,, Lee HT. Selective renal over-expression of human heat shock protein 27 reduces renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 299: F347–358, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puneet P, Yap CT, Wong L, Lam Y, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ: SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328: 1290–1294, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Wong L, Tan SS, Lam Y, Melendez AJ: Synthesis and evaluation of sphingosine analogues as inhibitors of sphingosine kinases. J Med Chem 52: 3618–3626, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Slot C: Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 17: 381–387, 1965 [DOI] [PubMed] [Google Scholar]
- 56.Park SW, Kim M, Chen SW, D’Agati VD, Lee HT: Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock 33: 31–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee HT, Kim M, Jan M, Emala CW: Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells. Am J Physiol Renal Physiol 291: F67–F78, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Lee HT, Emala CW: Adenosine attenuates oxidant injury in human kidney proximal tubular cells via A1 and A2a adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Lee HT, Kim M, Jan M, Penn RB, Emala CW: Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Vinay P, Gougoux A, Lemieux G: Isolation of a pure suspension of rat proximal tubules. Am J Physiol 241: F403–F411, 1981 [DOI] [PubMed] [Google Scholar]