Abstract
Preeclampsia (PE) is a life-threatening hypertensive disorder of pregnancy. Elevated circulating endothelin-1 (ET-1) is associated with the disease. However the molecular basis of increased ET-1 production and its role in PE are unknown. This study aimed to investigate the causative factors, pathological role of elevated ET-1 production in PE, and the underlying mechanisms. In this study, we found that IgG from women with PE, in contrast to IgG from normotensive pregnant women, induced preproET-1 mRNA expression via angiotensin II type 1 receptor activation in kidneys and placentas in pregnant mice. The ET-A receptor-specific antagonist BQ123 significantly attenuated autoantibody-induced hypertension, proteinuria, and renal damage in pregnant mice, demonstrating that autoantibody-induced ET-1 production contributes to pathophysiology. Mechanistically, we discovered that IL-6 functioned downstream of TNF-α signaling, contributing to increased ET-1 production in pregnant mice. IL-6 blockade inhibited preeclamptic features in autoantibody-injected pregnant mice. Extending the data to human studies, we found that IL-6 was a key cytokine underlying ET-1 induction mediated by IgG from women with PE in human placental villous explants and that endothelial cells are a key source of ET-1. Overall, we provide human and mouse studies showing that angiotensin II type I receptor-agonistic autoantibody is a novel causative factor responsible for elevated ET-1 production and that increased TNF-α/IL-6 signaling is a key mechanism underlying increased ET-1 production and subsequent maternal features. Significantly, our findings revealed novel factors and signaling cascades involved in ET-1 production, subsequent disease symptom development, and possible therapeutic intervention in the management of PE.
Preeclampsia (PE) is a life-threatening disease of late pregnancy characterized by hypertension and proteinuria (1–3). The condition affects ~8% of first pregnancies and accounts for over 80,000 premature births each year in the United States (~15% of total premature births), over $4 billion in medical costs (2), and immeasurable human suffering. By conservative estimates, this disease is responsible for over 75,000 maternal deaths annually worldwide. PE is also associated with intrauterine growth restriction, a dangerous condition that puts the fetus at risk for many long-term cardiovascular disorders (4, 5). Thus, preeclampsia is a leading cause of maternal and neonatal mortality and morbidity and has an acute and long-term impact on mothers and babies. Despite intense research efforts, the underlying cause of PE remains a mystery, and its clinical management is hampered by the lack of presymptomatic screening, reliable diagnostic tests, and effective therapy. Thus, by understanding the molecular pathways involved in the development of PE, we can expand the therapeutic strategies used to treat this disease.
Recent studies reported that preeclamptic women possess angiotensin II (Ang II) type I receptor-agonistic autoantibodies (AT1-AA) that bind to and activate Ang II type I receptors (AT1R) in multiple cellular systems (6, 7), provoking many biological responses relevant to the pathophysiology of the disorder. For example, these circulating autoantibodies increase rat cardiomyocyte contraction rate; increase plasminogen activator inhibitor-1 production, resulting in decreased trophoblast invasion; increase NADPH oxidase production in trophoblast cells; and elevate levels of the antiangiogenic factor soluble fms-like kinase-1 (sFlt-1), leading to decreased angiogenesis in endothelial cells (8–12). However, these studies were restricted to the use of in vitro cultured cell systems and, therefore, did not directly address the relevance of AT1-AA to hypertension and proteinuria, the defining features of PE. To formally examine the role of AT1-AA in the pathophysiology of PE, we recently demonstrated that the injection of pregnant mice with AT1-AA recapitulates the key features of PE: hypertension, proteinuria, renal and placental morphologic changes, and an increase in the antiangiogenic factor sFlt-1 (8). Extending these animal studies to human studies, we recently showed that AT1-AA are highly prevalent in PE (>95%) and that Ab titers strongly correlate with the severity of disease (13). Although animal and human studies revealed the pathophysiological role of AT1-AA in PE, the underlying pathogenic mechanisms associated with the disorder remain undefined.
A growing body of evidence indicates that an increased maternal inflammatory response is associated with PE and was speculated to contribute to the disease (14, 15). Some investigators hypothesized that the activation of leukocytes and upregulation of certain cytokines propagate a state of chronic inflammation in some pregnant women, which manifests in preeclamptic features (16, 17). Increases in TNF-α, IL-6, IFN-γ, and IL-2 are well established (18–21). In contrast, anti-inflammatory molecules, such as IL-10 and IL-4, are decreased (14, 22). Multiple studies demonstrated that increased inflammatory cytokine production may lead to endothelial dysfunction, increased placenta apoptosis, decreased angiogenesis, and kidney abnormalities that are relevant to the pathophysiology of the disease (14). Recently, we showed that IgG from women with PE contributes to increased TNF-α induction in PE, and increased TNF-α contributes to pathogenesis of the disease (23). However, it remains undefined how autoantibody-mediated elevation of TNF-α, a key proinflammatory cytokine, leads to the development of hypertension and proteinuria, major features of PE.
In addition to increased circulatory inflammatory cytokines, endothelin-1 (ET-1) is known to be elevated in the blood of preeclamptic women. The initial report that higher levels of ET-1 are observed in the circulation of women with PE compared with women with normal pregnancy (24) was confirmed and extended by many other groups (25–32). ET-1 is a 21-aa peptide and a key mediator of vascular tone and renal sodium homeostasis through the binding of ET-A and ET-B receptors. Elevated ET-1 signaling is associated with high blood pressure, atherosclerosis, pulmonary hypertension, cardiovascular disease, and renal disease (33). However, the factors and signaling pathways that cause an increase in ET-1 in PE are unknown, and the exact role of elevated ET-1 in the pathogenesis of the disease remains largely undefined. In this study, we explored whether AT1-AA is a causative factor responsible for increased ET-1 production, as well as its exact role in the pathogenesis of PE. Moreover, we assessed whether the altered immune network is an underlying mechanism for its elevation in PE.
Materials and Methods
Patients
Patients who were admitted to Memorial Hermann Hospital were identified by the obstetrical faculty of the University of Texas Medical School at Houston. Preeclamptic patients (n = 23) were diagnosed with severe disease based on the definition set by the National High Blood Pressure Education Program Working Group Report. The criteria of inclusion, including no previous history of hypertension, were reported previously (34). Control pregnant women were selected on the basis of having an un-complicated, normotensive (NT) pregnancy, with an expected normal-term delivery (n = 20). The research protocol was approved by the Institutional Committee for the Protection of Human Subjects.
Purification of the IgG fraction from patient sera
Purified IgG fractions were isolated from preeclamptic and NT patient sera, as previously described, using GammaBind G Sepharose (Amersham Biosciences, Piscataway, NJ) (34). Typically, 200 μl patient serum was applied to the column matrix and eluted with 1.8 ml buffer, according to the manufacturer's recommended protocol. IgG fractions from individual patients were used separately for the experiments reported in this article, and individual patient IgG preparations were not pooled. We previously showed that the same physiological responses (e.g., hypertension, proteinuria, increased sFlt-1) were achieved when pregnant mice were injected with total IgG or affinity-purified AT1-AA from women with PE (34, 35). In each case, the physiological response to Ab injection was blocked by coinjection with losartan (an AT1R antagonist) or an autoantibody-neutralizing 7-aa epitope peptide that corresponds to a site on the second extracellular loop of the AT1R that is commonly recognized by AT1-AA. Furthermore, features of PE were not observed in mice injected with total IgG from women with NT pregnancies. In contrast, features of PE are observed in pregnant mice injected with total IgG from women with PE. For these reasons, we routinely used total IgG, rather than affinity-purified AT1-AA, for the experiments reported in this article.
Introduction of human Ab into pregnant mice and blood pressure measurement
All animal studies were reviewed and approved by the Animal Welfare Committee, University of Texas Houston Health Science Center. Purified IgGs were isolated from preeclamptic or NT patient sera, and their introduction into pregnant mice was carried out, as previously described (34). Briefly, ~85 C57BL/6J pregnant mice (18–22 g; Harlan, Indianapolis, IN) were used in our study. Experiments were performed in the following groups of mice: pregnant mice with double injection of IgG purified from NT controls (n = 10) or preeclamptic patients (n = 14). In addition, some of the pregnant mice were treated with BQ123, an antagonist of ET-A receptor, by osmotic minipump (100 nmol/kg/d; n = 9) (36); anti–IL-6 injection (1 mg/wk; n = 10) (37); or anti–TNF-α injection (0.6 μg/g body weight/d; n = 9) (Abcam, Cambridge, MA) (23). Some animals also received losartan (0.24 mg, a generous gift from Merck & Co., Rahway, NJ) or an autoantibody-neutralizing 7-aa peptide (AFHYESQ) corresponding to an epitope on the second extracellular loop of the AT1R (1.5 mg). All of the mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and concentrated IgG (~800 μg), purified from 200 μl NT controls (n = 10) or patients’ serum (n = 14), was introduced into pregnant mice on gestation days 13 and 14 by orbital sinus injection. This stage of mouse pregnancy was selected because it is comparable to early-onset PE in humans and is also a time at which we can reliably determine whether a mouse is pregnant. The systolic blood pressure of all mice was measured at the same time daily (±1 h) by a carotid catheter-calibrated tail-cuff system, and the mice were kept warm using a warming pad (AD Instruments). Urine was collected for analysis using metabolic cages (Nalgene). All mice were sacrificed at gestation day 18 prior to delivery, and their serum and organs, including placentas and kidneys, were collected.
RNA isolation, semiquantitative PCR, and real-time PCR
RNA isolation and real-time PCR were performed, as described (38). PreproET-1 primer sequences and PCR conditions were as described (39); sense primer, 5′-TTCCCGTGATCTTCTCTCTGC-3′ and antisense primer, 5′-CTGCACTCCATTCTCAGCTCC-3′. IL-6 primer sequence and PCR conditions were as previously described (8): sense primer, 5′-TGGGAAATCGTGGAAATGAG-3′ and antisense primer, 5′-CTCTGAAGGACTCTGGCTTTG-3′. Real-time PCR for mouse AT1R expression was performed using SYBR green PCR mix (Sigma) under the following conditions: 95°C for 2 min for 1 cycle, 95°C for 10 s, 52°C for 15 s, and 72°C for 15 s, and fluorescence was quantified following 72°C for 15 s for 40 cycles. Mouse AT1R primer sequences used were sense, 5′-AACAGCTTGGTGGTGATCGTC-3′ and antisense; 5′-CATAGCGGTATAGACAGCCCA-3′. β-actin was used as an internal control, and primer sequences were as described (8). PreproET-1 mRNA expression was represented by the ratio of preproET-1 mRNA/β-actin mRNA.
Histological analysis
The kidneys of sacrificed pregnant mice were harvested, fixed, and processed, as previously described (8). Briefly, the tissues were fixed in 4% formaldehyde solution (Fisher Scientific) for 36–48 h at room temperature, washed with PBS 2× for 30 min, dehydrated, infiltrated, and embedded in paraffin. Four-micron serial sections were cut from blocks and stained with H&E by standard techniques or were left unstained for further analysis.
Transmission electron microscopy
Upon sacrifice, kidneys were removed immediately. Tissue samples were cut into 1-mm3 cubes and fixed in 3% glutaraldehyde overnight. The samples were rinsed and exposed to 1% osmium tetroxide, dehydrated, and embedded in an araldite-epon mixture. Semi-thin tissue sections were prepared (0.6 mm) and stained with uranyl acetate and lead citrate. The prepared samples were examined with a JEOL 1210 transmission electron microscope (JEOL).
Human placental villous explants treated with IgG purified from human serum
Human placental tissue was obtained from women with normal-term pregnancies delivered by elective cesarean section for breech presentation or a recurring indication in otherwise uncomplicated pregnancies, as described previously (8). Informed consent was obtained from the patients, and the study had the approval of the University of Texas-Houston Medical School Committee for the Protection of Human Subjects. Placental villi were isolated, cultured, and treated, as described (8, 40). After 72 h of treatment, the concentrations of ET-1 and IL-6 in cell culture media were determined by ELISA. To ensure viability, all placental explants were prepared and processed within 30 min of delivery. Routine histological analysis of placental explants at the termination of in vitro experiments showed no evidence of apoptosis or necrosis.
ELISA for sFlt-1, soluble endoglin, IL-6, and ET-1 measurement
Soluble endoglin (sEng), sFlt-1, and IL-6 levels in culture medium and/or mouse plasma were determined using ELISA kits (R&D Systems, Minneapolis, MN). ET-1 levels in culture medium were determined using a ELISA kit (ALPCO Diagnostics, Salem, NH).
CD-31 and ET-1 staining and quantification
Anti-CD31 Ab and anti–ET-1 Ab (BD Pharmingen, Franklin Lakes, NJ) were used to detect their expression in mouse placenta and human villous explants, as described (23, 41). Quantification of the immunohistochemical staining was performed using Image-Pro Plus software (Media Cybernetics, Bethesda, MD). The density of the brown staining (positive for CD-31 and ET-1) was measured. The densities of 10 areas/placenta were averaged, and the SEM is indicated (n = 4 placentas for each category).
Treatment of human endothelial cells and trophoblast cells
HUVECs (Cascade Biologics, Portland, OR) and immortalized human trophoblast cells (HTR-8) were cultured with endothelial cell growth medium complete medium (Cambrex, Walkersville, MD) or RPMI 1640 medium overnight, respectively. The next day, cells were transferred to serum-free medium and treated with purified IgG (1:10 dilution) from NT or preeclamptic pregnant women. Anti-human IL-6–neutralizing Ab was added to cells at 2.5 μg/ml for 30 min before treatment. After 24 h of treatment, the cell-culture media were harvested, and the concentration of ET-1 was determined by ELISA, as described above.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical significance of the difference between the mean values of multiple groups was tested by oneway ANOVA, followed by Tukey–Kramer posttests. Comparisons of multiple groups of blood pressure of pregnant mice at different time points were assessed by two-way repeated-measures ANOVA, followed by Bonferroni posttests. A p value < 0.05 was considered significant. Statistical analysis was carried out using GraphPad Prism 4 software (GraphPad Software, San Diego, CA).
Results
PreproET-1 mRNA levels induced by AT1R activation in pregnant mice injected with IgG purified from preeclamptic women
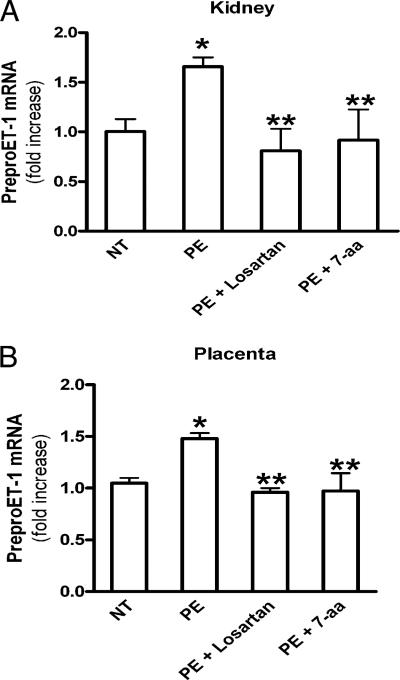
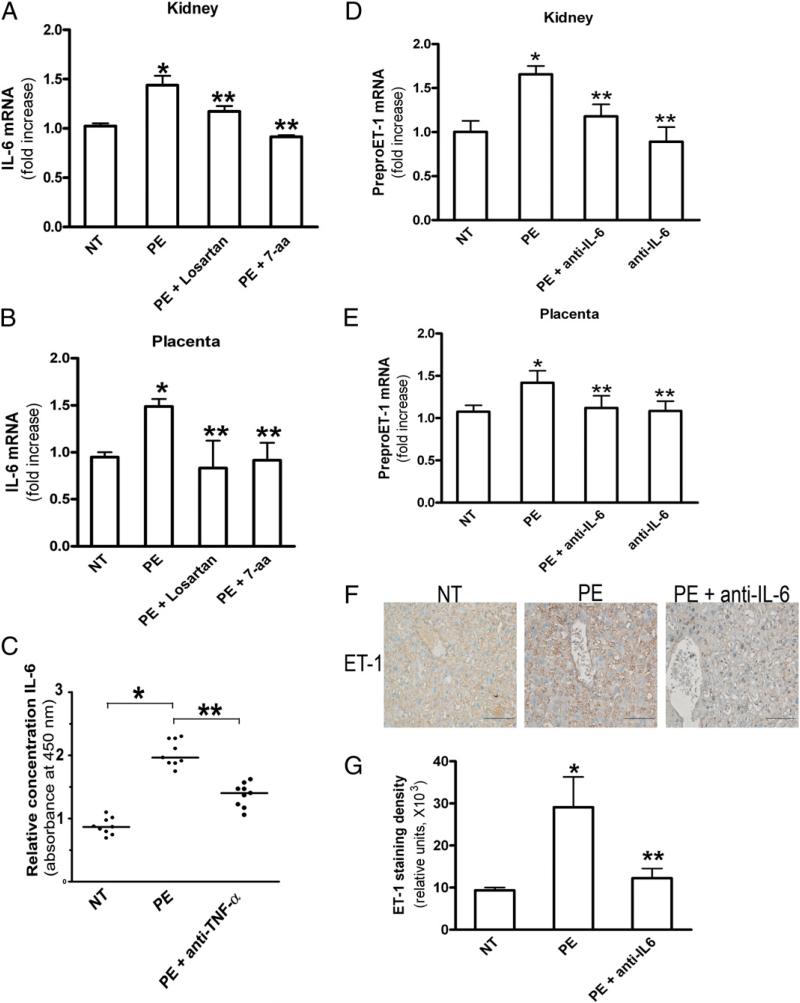
The factors responsible for elevated ET-1 in preeclamptic women are unknown. To determine whether AT1-AA underlies increased ET-1 production, IgG purified from NT pregnant women (NT-IgG) or preeclamptic women (PE-IgG) was introduced into pregnant mice at gestation days 13 and 14 by retro-orbital injection, as previously described (34). It is very difficult to accurately measure circulating ET-1 levels in mice because of its short t1/2, high-affinity binding with its receptor, and the small blood volume collected (42). For these reasons, we chose to determine preproET-1 mRNA levels as a well-accepted measure reflecting ET-1 production (43). Kidneys and placentas were collected at gestation day 18, and levels of ET-1 mRNA expression were measured using quantitative RT-PCR. We found that the expression level of preproET-1 mRNA in the kidneys of PE-IgG–injected pregnant mice was increased ~80% compared with that of the mice injected with NT-IgG (Fig. 1A). In placentas, a 50% increase in preproET-1 mRNA expression levels in PE-IgG–injected pregnant mice was observed compared with that of the pregnant mice injected with NT-IgG (Fig. 1B). Next, to assess whether the autoantibody-mediated increase in preproET-1 mRNA expression levels in mouse kidney and placenta were mediated via AT1R activation, pregnant mice were coinjected with losartan, an AT1R blocker, or an autoantibody-neutralizing 7-aa epitope peptide. When PE-IgG was coinjected into pregnant mice with losartan or the autoantibody-neutralizing 7-aa epitope peptide, the autoantibody-mediated induction of preproET-1 mRNA expression in kidneys and placentas was significantly inhibited. These results indicated that PE-IgG, by specifically activating the AT1R, is responsible for the upregulation of preproET-1 mRNA expression in kidneys and placentas in pregnant mice. We did not notice any significant changes in AT1R expression in kidneys or placentas in response to PE-IgG (Supplemental Fig. 1), only changes in receptor activation, as described above
FIGURE 1.
PreproET-1 mRNA levels are increased in kidneys and placentas of autoantibody-injected pregnant mice via AT1R activation. PreproET-1 mRNA levels were elevated in the kidney (A) and placenta (B) of PE-IgG–injected pregnant mice but not in NT-IgG–injected pregnant mice. Coinjection of losartan or autoantibody-neutralizing 7-aa epitope peptide resulted in decreased preproET-1 mRNA levels in the kidneys and placentas of PE-IgG–injected pregnant mice. n = 7–12 for each variable. *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment.
PE-IgG–induced hypertension is blocked by ET-A receptor antagonist in pregnant mice
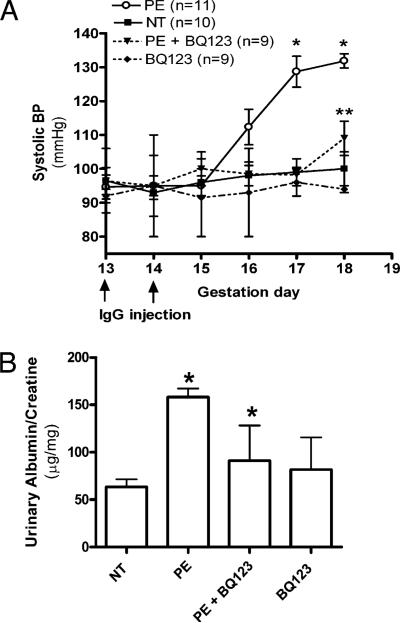
To assess the role of autoantibody-induced ET-1 in the pathogenesis of PE, we treated PE-IgG–injected pregnant mice with an ET-A receptor-specific antagonist, BQ123, which was delivered by an osmotic minipump implanted s.c. ET-A is the receptor responsible for the hypertensive effects of ET-1. We found that autoantibody-induced hypertension was almost completely blocked by BQ123 (decrease from 136 ± 6 to 109.4 ± 5.0 mm Hg; p < 0.05). There was no significant blood pressure change in pregnant mice treated with BQ123 alone (Fig. 2A). These findings provided in vivo evidence of the contributory role of PE-IgG–induced ET-1 in hypertension, a key clinical feature of PE.
FIGURE 2.
ET-A antagonist BQ123 reduces PE-IgG–induced PE-like features. The key features of PE, hypertension (A) and proteinuria (B), present in the PE-IgG–injected pregnant mice were reduced by treatment with the ET-A antagonist BQ123 (n = 9–12 for each variable). *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment.
In vivo effect of ET-A antagonist on proteinuria seen in PE-IgG–injected pregnant mice
Next, we assessed the effect of ET-A antagonism on proteinuria, another key maternal feature of PE. PE-IgG–injected pregnant mice were treated with the ET-A receptor antagonist, as described above. Urine samples (24 h) were collected at postinjection day 5, and albumin and creatinine were measured by ELISA. We found that BQ123 treatment significantly reduced urinary protein from 158 ± 9.2 to 91.3 ± 37.2 μg albumin/mg creatinine (p < 0.05). There was no significant effect on the ratio of albumin to creatinine in pregnant mice treated with BQ123 alone (Fig. 2B). Overall, these findings provided in vivo animal evidence of the contributory role of PE-IgG–induced ET-1 production to proteinuria seen in PE.
PE-IgG–induced ET-1 production contributes to renal impairment in pregnant mice
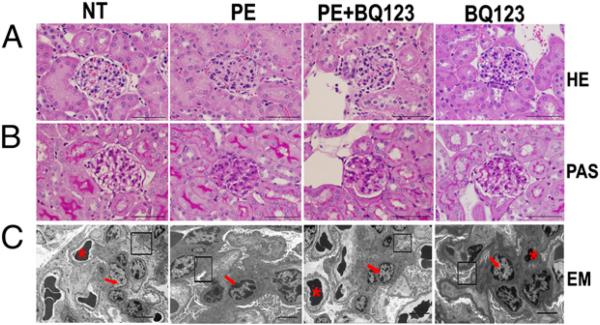
Our previous study (34) showed that AT1-AA induces kidney damage, including endotheliosis, a pathognomonic alteration in preeclamptic women. Thus, it is possible that elevated ET-1 may also contribute to renal impairment in autoantibody-injected pregnant mice. H&E staining revealed that the glomeruli of PE-IgG–injected pregnant mice were smaller and hypercellular compared with those of mice injected with NT-IgG, whose glomeruli were open and easily distinguished (Fig. 3). In contrast, renal impairment was significantly reduced, although not completely eliminated, in PE-IgG–injected pregnant mice receiving BQ123 treatment. The glomeruli of mice injected with NT-IgG did not display any renal morphologic changes. Electron microscopic analysis indicated that glomerular endothelial damage was present in PE-IgG–injected mice (Fig. 3C). The glomeruli of these mice showed endothelial swelling, resulting in the occlusion of capillary loop spaces. Treatment of PE-IgG–injected pregnant mice with BQ123 significantly prevented the kidney damage, resulting in capillary spaces that were completely open compared with the glomeruli of mice injected with NT-IgG, whose capillary spaces were wide and showed no swelling or obstruction (Fig. 3). Taken together, our studies demonstrated that elevated ET-1 underlies renal damage and dysfunction in PE-IgG–induced PE.
FIGURE 3.
Autoantibody-induced renal damage is alleviated by ET-A antagonist BQ123. A, H&E staining reveals that extensive endothelial swelling, narrowing, and occlusion of capillary lumens of PE-IgG–injected pregnant mice is significantly prevented in pregnant mice treated with BQ123. The kidneys of NT-IgG–injected mice or mice injected with BQ123 alone are unremarkable. Scale bar, 200 μm. B, Periodic acid-Schiff–stained sections from mice injected with PE-IgG show swollen endothelial cells without periodic acid-Schiff–positive material in the cytoplasm, and swelling was diminished by BQ123 treatment. No marked cellular proliferation or increase in mesangial matrix was observed in these mice. These changes were not evident in mice following injection of NT-IgG or BQ123 treatment alone. Scale bar, 200 μm. C, Transmission electron microscopy demonstrates that the glomerular endothelial cell swelling and destruction observed in PE-IgG–injected mice were reduced in the BQ123-treated group and were not evident in the pregnant mice injected with NT-IgG or BQ123 treatment alone. Original magnification ×1500. Scale bar, 10 μm. n = 4–10 for each variable. *Capillary space; arrow, endothelial cell nucleus; box, intact podocytes.
IgG from preeclamptic women stimulates excess ET-1 secretion by human placental villous explants via AT1R activation
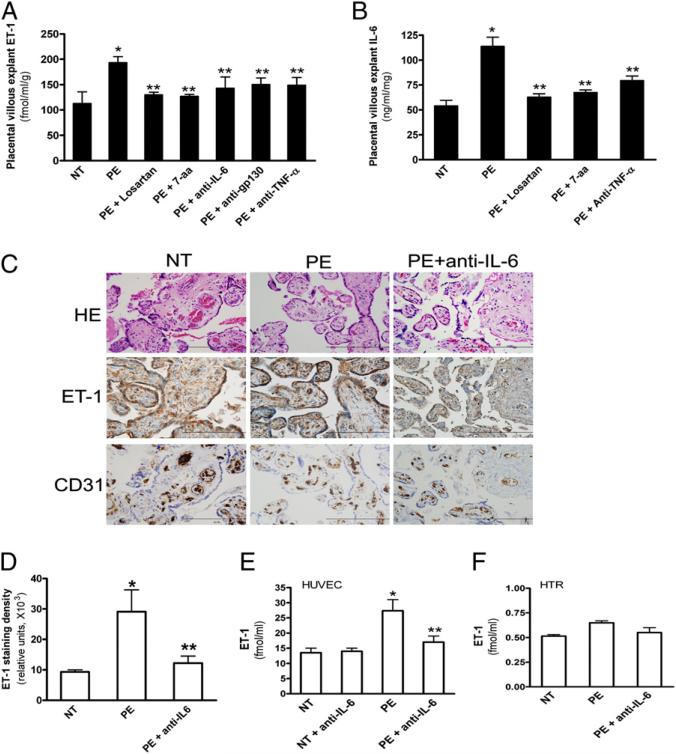
Because ET-1 mRNA levels are elevated in mouse placentas of autoantibody-injected pregnant mice (Fig. 1B), the placenta is likely a major organ responsible for the production of autoantibody-induced ET-1. To assess the direct role of PE-IgG in ET-1 production in humans, we took advantage of human placental villous explants. Similar to in vivo studies from pregnant mice, human placental villous explants incubated with PE-IgG showed an increase in secreted ET-1, whereas ET-1 was not induced in explants incubated with NT-IgG (Fig. 4A). AT1R activation was required for ET-1 secretion, because coincubation of PE-IgG with losartan or the autoantibody-neutralizing 7-aa epitope peptide attenuated the induction of ET-1 levels. Overall, we extended our mouse in vivo study to in vitro human studies showing that PE-IgG is capable of inducing ET-1 secretion via AT1R activation from human placental villous explants.
FIGURE 4.
AT1R activation induces ET-1 secretion from PE-IgG–treated human placental villous explants via the TNF-α/IL-6–signaling cascade. A, Culturing human villous explants with PE-IgG resulted in increased ET-1 secretion. Coculturing the explants with PE-IgG and losartan (5 μM), the autoantibody-neutralizing 7-aa epitope peptide (1 μM), or anti–TNF-α, anti–IL-6, or anti-gp130 (1 μM) reduced elevated ET-1 secretion. *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment. B, IL-6 levels were induced by PE-IgG but not NT-IgG. PE-IgG–induced IL-6 production was significantly decreased in the presence of losartan, autoantibody-neutralizing 7-aa epitope peptide, or anti-TNF-α, indicating that IL-6 is downstream of TNF-α and contributes to PE-IgG–induced ET-1 production. Six different placentas were collected and treated with IgG (n = 3–6 patient IgG for each category). *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment. C, At the end of treatment, human placental villous explants were collected, fixed, and stained with H&E and anti-human ET-1 and anti-human CD31 Ab. Scale bar, 100 μm. D, Expression of ET-1 was quantified using Image-Pro Plus image-analysis software. Cell-culture super-natants from HUVECs (E) and HTR-8 human trophoblast cells (F) were collected for ET-1 measurements by ELISA. Data are expressed as mean ± SEM of at least four experiments performed in duplicate (n = 3–6 patient IgG for each category). *p < 0.001 versus villous explants treated with NT–IgG, **p < 0.05 versus villi treated with PE-IgG.
TNF-α and IL-6 function downstream of the AT1R and contribute to PE-IgG–induced ET-1 production in human placental villous explants
In an effort to determine the specific factors involved in PE-IgG–induced ET-1 production, we screened a series of candidates using the human placental villous explant culture system. Among the candidates tested, we found that anti–TNF-α, anti–IL-6, and anti-gp130 (IL-6 coreceptor) Abs significantly reduced AT1-AA–induced ET-1 secretion from the human placental villous explants (Fig. 4A). However, anti–TNF-α, anti–IL-6, or anti-gp130 alone had no effect on ET-1 secretion (Fig. 4A). These studies revealed that TNF-α and IL-6 are likely novel inflammatory cytokines functioning downstream of the AT1R responsible for PE-IgG–mediated induction of ET-1 production in PE.
Elevated TNF-α is responsible for increased IL-6 production in PE-IgG–treated human placental villous explants
IL-6 and TNF-α are elevated in the circulation of preeclamptic women (19, 44). Our previous studies showed that PE-IgG is capable of inducing the synthesis and secretion of TNF-α in human placental villous explants (23). However, whether PE-IgG can induce IL-6 production remains unknown. ELISA analysis revealed that PE-IgG also significantly increased IL-6 secretion from cultured human placental villous explants (Fig. 4B). Losartan or the autoantibody-neutralizing 7-aa epitope peptide significantly inhibited the PE-IgG–mediated induction of IL-6 secretion in human placental villous explants (Fig. 4B), indicating that AT1R activation was required. To our surprise, ELISA measurement showed that anti–TNF-α treatments significantly inhibited PE-IgG–stimulated IL-6 secretion from cultured human placental villous explants (Fig. 4B), indicating that TNF-α functions downstream of the AT1R and is responsible for PE-IgG–induced IL-6 secretion from human placental villous explants.
Human endothelial cells are a major source contributing to PE-IgG–induced ET-1 production via IL-6 signaling
Next, to determine which cell types are responsible for autoantibody-induced ET-1 production, we assessed ET-1 expression in human placental villous explants. Immunohistochemistry studies showed that ET-1 was mainly expressed in endothelial cells and trophoblast cells (Fig. 4C). Quantitative image analysis of ET-1 immunostaining demonstrated significantly increased immunore-activity in the placental villous explants treated with PE-IgG compared with those treated with NT-IgG. IL-6–neutralizing Ab treatment significantly decreased ET-1 immunostaining in the placental villous explants treated with PE-IgG (Fig. 4C, 4D), indicating that IL-6 is major cytokine responsible for autoantibody-induced ET-1 production in endothelial cells and/or trophoblast cells in human villous explants. Thus, to directly test whether endothelial and/or trophoblast cells are responsible for autoantibody-induced ET-1 production via IL-6 signaling, we treated primary human endothelial cells (HUVEC) and immortalized human trophoblast cells (HTR-8) with NT-IgG or PE-IgG in the presence or absence of IL-6–neutralizing Ab. We found that PE-IgG induced ET-1 production in human endothelial cells but not in HTR-8 cells and that IL-6 blockade significantly reduced autoantibody-induced ET-1 production (Fig. 4E, 4F). Overall, these studies provided direct evidence that endothelial cells are the key cell types contributing to PE-IgG–mediated ET-1 production via IL-6 signaling.
AT1R activation-induced IL-6 production is inhibited by TNF-α blockade in PE-IgG–injected pregnant mice
Similar to human in vitro studies, we demonstrated that IL-6 mRNA levels were significantly upregulated in kidneys and placentas of autoantibody-injected pregnant mice and that IL-6 induction was significantly inhibited by losartan or autoantibody-neutralizing 7-aa epitope peptide treatment (Fig. 5A, 5B). In addition, we found that IL-6 levels were significantly elevated in the circulation of autoantibody-injected pregnant mice and that its increased production was significantly inhibited by TNF-α blockade in these mice (Fig. 5C). Thus, our studies revealed the PE-IgG–mediated TNF-α induction contributes to increased IL-6 production in pregnant mice.
FIGURE 5.
IL-6 functions downstream of TNF-α signaling responsible for PE-IgG–induced ET-1 production in pregnant mice. IL-6 mRNA levels were elevated in kidneys (A) and placentas (B) of PE-IgG–injected pregnant mice but not in NT-IgG–injected pregnant mice. Coinjection of losartan or the autoantibody-neutralizing 7-aa epitope peptide inhibited PE-IgG–mediated induction of IL-6 mRNA levels in pregnant mice (n = 8 for each variable). C, IL-6 levels were elevated in the blood of PE-IgG–injected, but not NT-IgG–injected, pregnant mice. The IL-6 induction was inhibited by anti–TNF-α Ab in autoantibody-injected pregnant mice (n = 8–10 for each variable). IL-6 blockade inhibited PE-IgG-induced preproET-1 mRNA in kidneys (D) and placentas (E) of pregnant mice (n = 10–14 for each variable). For A–E, *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment. F, ET-1 staining of the placentas of mice injected with NT-IgG and PE-IgG with or without neutralizing IL-6 Ab. Scale bar, 50 μm. G, Quantification of ET-1 staining (n = 8 mice for each category). Scores are mean ± SEM. *p < 0.01 versus NT-IgG injection, **p < 0.05 versus PE-IgG injection.
IL-6 blockade significantly attenuates increased preproET-1 mRNA levels in kidneys and placentas of PE-IgG–injected pregnant mice
To assess the in vivo significance of increased IL-6 on PE-IgG–induced preproET-1 mRNA production, we coinjected pregnant mice with PE-IgG and mouse anti–IL-6–neutralizing Ab. At embryonic day 18, the mice were sacrificed, and preproET-1 mRNA levels were measured by quantitative RT-PCR. We found that the PE-IgG–mediated increase in preproET-1 mRNA expression in kidneys and placentas from autoantibody-injected pregnant mice was significantly inhibited by anti–IL-6 Abs (Fig. 5D, 5E). However, treatment with IL-6–neutralizing Ab alone had no effect on preproET-1 mRNA expression (Fig. 5D, 5E). Consistent with our findings from human placental villous explants, immunostaining of mouse placenta revealed that ET-1 was mainly expressed in the endothelial cells (Fig. 5F). Quantitative image analysis showed that PE-IgG significantly increased immunostaining of ET-1 in the mouse placenta and that IL-6–neutralizing Ab significantly inhibited its elevation (Fig. 5G). These findings provide in vivo evidence that elevated IL-6 contributes to autoantibody-mediated induction of ET-1 in pregnant mice.
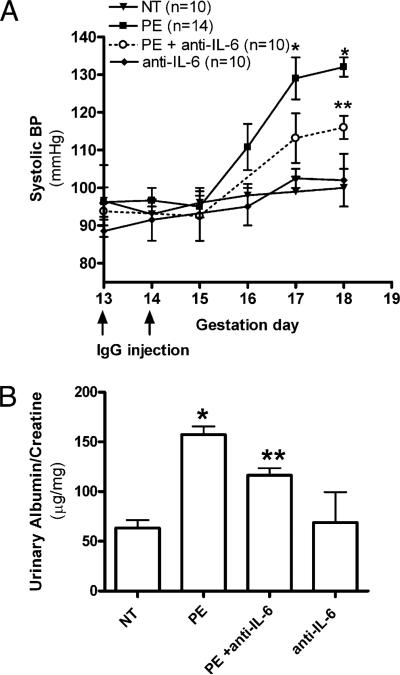
Contributory role of elevated IL-6 in hypertension and proteinuria in PE-IgG–injected pregnant mice
To elucidate the critical role of IL-6 in the pathogenesis of PE, we monitored the effects of anti–IL-6 treatment on the key preeclamptic features seen in autoantibody-injected pregnant mice. We found that the key diagnostic features, hypertension and proteinuria, were significantly attenuated, but not completely prevented, in animals coinjected with PE-IgG and IL-6–neutralizing Ab compared with pregnant mice injected with PE-IgG alone (Fig. 6). By embryonic day 18, neutralization of IL-6 reduced hypertension from 136.8 ± 4.1 to 112.0 ± 3.8 mm Hg and urinary protein from 157.2 ± 8.5 to 116.3 ± 7.1 μg albumin/mg creatinine (p < 0.05). Pregnant mice injected with NT-IgG retained their baseline blood pressure and normal renal function. These findings provided direct evidence that IL-6 contributes to key maternal features of PE seen in autoantibody-injected pregnant mice.
FIGURE 6.
IL-6 blockade inhibits PE-IgG–induced preeclamptic-like features. The key features of PE, hypertension (A) and proteinuria (B), present in the PE-IgG–injected pregnant mice, were significantly reduced by treatment with neutralizing anti-mouse IL-6 Ab (n = 10–14 for each variable). *p < 0.05 versus NT-IgG treatment, **p < 0.05 versus PE-IgG treatment.
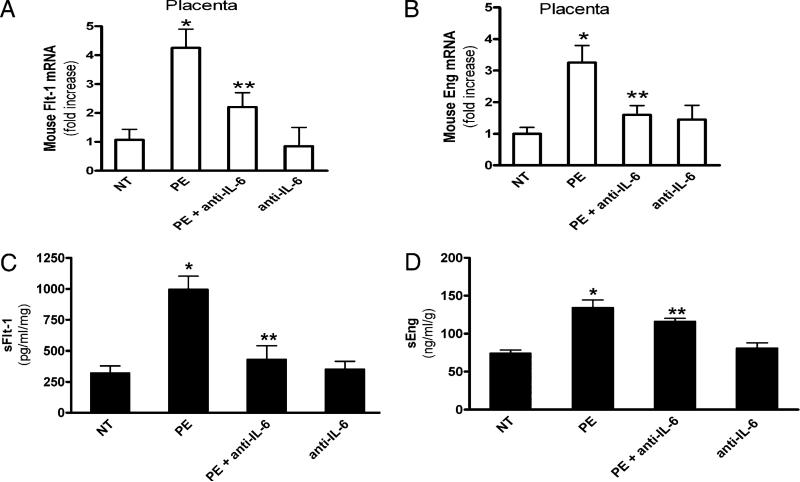
Effect of increased IL-6 on PE-IgG–induced sFlt-1 and sEng secretion in pregnant mice and cultured human villous explants
In addition to ET-1, sFlt-1 and sEng are key antiangiogenic factors that are elevated in PE (45, 46). Our earlier studies found that blocking PE-IgG–induced TNF-α signaling inhibited the induction of sFlt-1 and sEng (23). Because we found that PE-IgG–mediated IL-6 elevation is downstream of TNF-α signaling (Fig. 4), it is possible that PE-IgG–mediated IL-6 induction plays a role in autoantibody-induced sFlt-1 and sEng production. Similar to ET-1, we found that increased sFlt-1 and sEng mRNA expression in the placenta of autoantibody-injected pregnant mice was significantly decreased by IL-6–neutralizing Ab (Fig. 7A, 7B). Consistent with our mouse studies, we found that the induction of sFlt-1 and sEng was inhibited by the presence of human IL-6–neutralizing Ab (Fig. 7C, 7D) in human placenta villous explants, indicating a contributory role for IL-6 signaling in PE-IgG–mediated sFlt-1 and sEng induction in humans.
FIGURE 7.
IL-6 signaling contributes to PE-IgG–mediated induction of antiangiogenic factors in mouse placentas and human villous explants. Quantitative RT-PCR was used to quantify Flt-1 (A) and endoglin (B) mRNA abundance from placentas of mice injected with NT-IgG or PE-IgG in the presence or absence of IL-6–neutralizing Ab (n = 8 mice for each group). Data are expressed as mean ± SEM. *p < 0.05 versus mice injected with NT-IgG, **p < 0.05 versus preeclamptic IgG injection. C and D, Normal human placental villous explants were collected and treated with IgG from women with PE or NT pregnant individuals in the presence or absence of IL-6–neutralizing Ab for 72 h. At the end of treatment, explant culture supernatants were collected for sFlt-1 (C) and sEng (D) measurements by ELISA. Data are expressed as mean 6 SEM of at least four experiments performed in duplicate (n = 4–6 patient IgG for each category). *p < 0.001 versus villous explants treated with NT IgG, **p < 0.05 versus villi treated with preeclamptic IgG.
Discussion
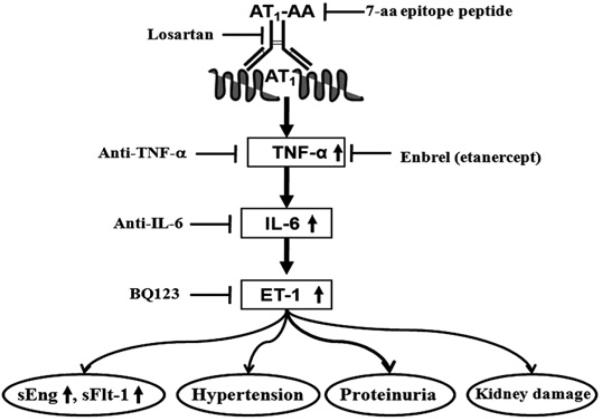
In this study, we provided in vivo evidence from animal models and in vitro evidence from human placental villous explants showing that PE-IgG is a novel causative factor that contributes to increased ET-1 production and that increased ET-1 production contributes to clinical manifestations of PE in pregnant mice, including hypertension, proteinuria, and renal damage. Mechanistically, we demonstrated that increased IL-6 functions downstream of TNF-α to mediate autoantibody-induced ET-1 production from endothelial cells. These studies led us to further discover that IL-6 is a key cytokine responsible for the induction of ET-1, as well as for the induction of antiangiogenic factors sFlt-1 and sEng and, thereby, plays an important role in the autoantibody-induced hypertension and proteinuria in pregnant mice (Fig. 8). Although research reported in this article was conducted using total IgG, we showed that the pathophysiological effects of PE-IgG required AT1R activation (i.e., inhibited by losartan or autoantibody-neutralizing 7-aa epitope peptide). We showed in earlier studies that these pathogenic autoantibodies in total PE-IgG can be highly enriched by affinity chromatography using a peptide sequence corresponding to the 27-aa sequence of the second extracellular loop of the AT1R. Thus, the results that we obtained using total IgG presumably reflect the activity of those autoantibodies that bind to and activate the AT1R. Overall, the mouse and human studies provided strong evidence that autoantibody-mediated AT1R activation induces ET-1 production via IL-6 signaling and that the increased ET-1 production contributes to the clinical manifestation of PE (Fig. 8).
FIGURE 8.
Working model presenting signaling pathways and mediators accounting for autoantibody-induced features of PE. AT1R activation via autoantibodies found in the serum of preeclamptic women leads to ET-1 induction via elevated TNF-α–IL-6–signaling cascade and contributes to hypertension, proteinuria, renal damage, and sFlt-1 and sEng induction, key features seen in PE. This implies that blockade of AT1R activation, inflammatory cytokine signaling, or ET-A receptor activation may be potential therapeutic strategies in the management of this serious disorder of pregnancy.
ET-1 is elevated in the blood of preeclamptic women (24–32). However, the causative factors for its elevation remain elusive. Ang II is known to stimulate ET-1 production (47); however, Ang II levels are not elevated in PE beyond that occurring in un-complicated pregnancies (48, 49). Likely candidates are the angiotensin receptor-activating autoantibodies present in women with PE (50). Supporting this hypothesis, in this study, we found that autoantibody from preeclamptic women specifically activates AT1R to induce preproET-1 mRNA production in kidneys and placentas in pregnant mice. Consistent with our animal studies, we provided additional evidence that autoantibody from preeclamptic women induces ET-1 production in human placental villous explants via AT1R activation. Moreover, we demonstrated that endothelial cells are a major site responsible for autoantibody-induced ET-1 production. Overall, human and mouse studies suggested that autoantibody existing in preeclamptic women contributes to ET-1 induction in PE.
ET-1 is a potent vasoconstrictor functioning via the ET-A receptor to stimulate vascular smooth muscle contraction and hypertension associated with disease (51). Using our autoantibody-induced model of PE in pregnant mice, we demonstrated that the ET-A receptor-specific antagonist BQ123 attenuates autoantibody-induced elevated blood pressure in pregnant mice, indicating the contributory role of increased ET-1 to hypertension in this animal model of PE. Consistent with our adoptive-transfer studies, LaMarca et al. (52) recently showed that an ET-A receptor antagonist blocked hypertension in pregnant rats injected with AT1-AA purified from a transgenic rat model of PE. Together, the results with pregnant mice injected with human AT1-AA and pregnant rats infused with AT1-AA generated from double-transgenic rats provide strong in vivo evidence that increased ET-1 contributes to autoantibody-induced hypertension. In addition, the important role for ET-1 signaling in PE is strongly supported by several other animal studies. For example, in an animal model of PE in pregnant rats generated by reducing uterine perfusion pressure (RUPP), the administration of an ET-A receptor antagonist blocked hypertension (53, 54). Similarly, ET-A receptor antagonism blocked hypertension in pregnant rats chronically infused with TNF-α (55). These animal studies provide additional evidence for the importance of elevated ET-1 in hypertension associated with PE. Notably, earlier studies showed that sera from the RUPP model induced ET-1 production from human endothelial cells (56) and its induction was inhibited by an AT1R blocker, suggesting that there is a circulating factor existing in the sera of the RUPP model capable of activating AT1R to induce ET-1. Supporting this finding, we have provided mouse and human evidence that AT1-AA exist in the circulation of preeclamptic women and are likely the circulating factors that activate AT1R to induce ET-1 production in PE. Although animals are not known to spontaneously develop PE, a number of valuable experimentally induced animal models of PE in rodents have been developed. In combination with in vitro studies using placental villous explants, multiple animal model studies have shown that AT1-AA contribute to the induction of ET-1 production via AT1R activation and that elevated ET-1 underlies hypertension in animal models of PE.
Proteinuria and renal damage are major features of PE that are also observed in the autoantibody-induced model of PE in pregnant mice (34). In our study, we showed that PE-IgG induces ET-1 gene expression in kidneys and that an ET-A–specific antagonist blocks proteinuria and kidney impairment. These results indicated that increased ET-1 functions via ET-A receptor activation to contribute to kidney damage and abnormalities in autoantibody-injected pregnant mice. Hypertension causes renal damage, and renal abnormalities also lead to hypertension by increased water and sodium retention. Thus, reduction in hypertension and renal dysfunction by ET-A antagonism in our adoptive-transfer animal model of PE suggests that elevated ET-1 may contribute to renal and vascular abnormalities associated with PE. Although renal damage may be secondary to hypertension, it is also possible that elevated ET-1 in the kidney directly induces renal damage and dysfunction, because ET-1 is known to increase apoptosis and podocyte injury in the kidney. For example, overexpression of human ET-1 in mice induces glomerulosclerosis, even in the absence of hypertension (57), suggesting that the detrimental role of elevated ET-1 signaling in the kidney is blood pressure independent. In addition, in an experimental proliferative nephritis animal model, which is characterized by glomerular damage without hypertension, proteinuria was prevented by ET-A receptor antagonism (58), again suggesting that elevated ET-1 is directly linked to renal damage. Animal preclinical studies demonstrated that endothelin blockade successfully prevents systemic and renal damage in connective tissue disease (59, 60). These findings suggest a novel concept that elevated ET-1 may directly lead to renal damage and indicate that ET-1 likely contributes to organ damage and dysfunction, independent of systemic blood pressure.
Multiple factors and signaling pathways are involved in ET-1 elevation. By screening a series of candidates, we identified that increased inflammatory cytokines, such as TNF-α and IL-6, are key proinflammatory cytokines responsible for PE-IgG–induced ET-1 production in human placental villous explants and in pregnant mice. In addition, we further discovered that TNF-α blockade attenuated the induction of IL-6, indicating that IL-6 is likely a downstream mediator of PE-IgG–induced ET-1 production. IL-6 is known to be increased in the blood of preeclamptic women and was speculated to be associated with the pathophysiology of PE (21, 61–63). For example, IL-6 is increased in the RUPP pregnant rat (64). Infusion of IL-6 results in hypertension and renal dysfunction in pregnant rats but not in non-pregnant rats, suggesting that elevated IL-6 contributes to hypertension and kidney dysfunction in pregnant rats (64). It is of interest that previous in vitro studies showed that mesangial cells produce increased amounts of IL-6 in response to Ang II or PE-IgG, suggesting that elevated IL-6 may be responsible for renal dysfunction seen in PE (12). In this study, using human in vitro studies and mouse in vivo studies, we demonstrated for the first time, to our knowledge, that IL-6 is induced by PE-IgG and that IL-6 blockade inhibits PE-IgG–induced ET-1 elevation and significantly attenuates autoantibody-induced hypertension and proteinuria. Overall, our results suggested that the autoantibody-mediated induction of ET-1 in endothelial cells is an autocrine process involving IL-6. Of note, our recent studies demonstrated the important role of elevated TNF-α signaling in autoantibody-induced features of PE (23). However, the mechanism by which AT1R activation-mediated TNF-α induction leads to preeclamptic features remained unclear until our present studies. We provide direct in vivo evidence in mice and in vitro human studies with placental explants that TNF-α signaling through IL-6 underlies ET-1 production and that elevated ET-1 is a key effector responsible for autoantibody-induced hypertension, proteinuria, and renal damage (Fig. 8). To critically evaluate the roles and functional relationships of TNF-α, IL-6, and ET-1, as well as the antiangiogenic factors sFlt-1 and sEng, in PE, it will be necessary to determine the temporal relationships between the increase in the abundance of these factors during pregnancy and the onset of symptoms of PE.
It is widely held view that enhanced inflammatory responses are associated with PE and contribute to pathophysiological features of the disease (1). It is also recognized that women with medical conditions associated with chronic inflammation (obesity, essential hypertension, diabetes mellitus, and autoimmune diseases) are at increased risk for developing PE (2). A particularly informative example in the autoimmune category is that of the antiphospholipid syndrome, characterized by recurrent fetal loss and intrauterine growth retardation. Girardi and colleagues (65, 66) conducted adoptive-transfer experiments to show that antiphospholipid Abs cause fetal rejection and intrauterine growth restriction when injected into pregnant mice and that this is associated with a proinflammatory response. PE has been reported in a high percentage of pregnant women with well-documented antiphospholipid autoantibodies. It will be of interest to determine whether these women also harbor AT1-AA.
Numerous studies demonstrated a role for IL-6 in increased blood pressure. For example, plasma levels of IL-6 are strongly associated with hypertension in humans and can be reduced by administration of Ang II receptor antagonists (67–69). Animal studies showed that infusion of IL-6 induces hypertension in pregnant rats (64, 70) and that the hypertension resulting from RUPP is accompanied by increased IL-6 (64). Additional evidence for a role of IL-6 in hypertension comes from studies of Coles et al. (71) and Lee et al. (72) using IL-6–deficient mice. Lee et al. showed that the Ang II-induced hypertension in wild-type mice is accompanied by a 10-fold increase in plasma IL-6 levels to ~25 pg/ml. Both groups showed that Ang II-induced hypertension was significantly inhibited in IL-6–deficient mice. Altogether, there is compelling evidence for a role for IL-6 in Ang II-induced hypertension, findings consistent with a role for IL-6 in AT1-AA–induced hypertension in our Ab-injection model of PE in pregnant mice. Trophoblast cells are capable of producing and responding to IL-6 (73–76). Trophoblast cells have a JAK/STAT-signaling pathway that functions downstream of IL-6R activation. JAK/STAT signaling, downstream of IFN-γ stimulation, is responsible for increased sFlt-1 production by human corneal fibroblasts. Thus, we speculate that IL-6 stimulates sFlt-1 and sEng production by trophoblast cells in an autocrine manner by JAK-STAT signaling. It is possible that increased IL-6 contributes to Ab-mediated hypertension in women with PE via IL-6–induced stimulation of sFlt-1 production by trophoblasts.
In conclusion, our studies identified a causative factor and novel mechanisms underlying increased ET-1 production in a mouse model of PE and revealed an important role for increased inflammatory cytokine-mediated ET-1 production in the pathophysiology of PE. It is impossible to treat PE using ET-A receptor blockers because of teratogenic effects (77, 78). However, because of the important role of increased inflammatory cytokines in ET-1 production and pathogenesis in PE, targeting the TNF-α and IL-6–signaling cascade is a novel therapeutic possibility.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL076558, HD34130, and RC4HD067977; American Heart Association Grant 10GRNT3760081; the March of Dimes (6-FY06-323); and the Texas Higher Education Coordinating Board.
Abbreviations used in this article
- Ang II
angiotensin II
- AT1-AA
angiotensin II type I receptor-agonistic autoantibodies
- AT1R
angiotensin II type I receptors
- ET-1
endothelin-1
- NT
normotensive
- NT-IgG
IgG purified from normotensive pregnant women
- PE
preeclampsia
- PE-IgG
IgG purified from preeclamptic women
- RUPP
reduced uterine perfusion pressure
- sEng
soluble endoglin
- sFlt-1
soluble fms-like kinase-1
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Cooper DW. Pathogenesis and genetics of preeclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 3.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000;71(5, Suppl.):1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4(2B):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- 6.Fu ML, Leung PS, Wallukat G, Bergström G, Fu H, Schulze W, Herlitz H. Agonist-like activity of antibodies to angiotensin II receptor subtype 1 (AT1) from rats immunized with AT1 receptor peptide. Blood Press. 1999;8:317–324. doi: 10.1080/080370599439544. [DOI] [PubMed] [Google Scholar]
- 7.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 8.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavoie JL, Bianco RA, Sakai K, Keen HL, Ryan MJ, Sigmund CD. Transgenic mice for studies of the renin-angiotensin system in hypertension. Acta Physiol. Scand. 2004;181:571–577. doi: 10.1111/j.1365-201X.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 10.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta. 2003;24:181–190. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- 11.Dechend R, Viedt C, Müller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 12.Bobst SM, Day MC, Gilstrap LC, III, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am. J. Hypertens. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol. Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am. J. Obstet. Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 16.Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in preeclampsia. J. Reprod. Immunol. 2003;59:153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 17.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin. Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin. Exp. Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 20.Visser W, Beckmann I, Bremer HA, Lim HL, Wallenburg HC. Bioactive tumour necrosis factor alpha in pre-eclamptic patients with and without the HELLP syndrome. Br. J. Obstet. Gynaecol. 1994;101:1081–1082. doi: 10.1111/j.1471-0528.1994.tb13587.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Lewis DF, Gu Y, Zhao S, Groome LJ. Elevated maternal soluble Gp130 and IL-6 levels and reduced Gp130 and SOCS-3 expressions in women complicated with preeclampsia. Hypertension. 2011;57:336–342. doi: 10.1161/HYPERTENSIONAHA.110.163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahm P, Rao DS, Donatucci CF. Antiandrogens in the treatment of priapism. Urology. 2002;59:138. doi: 10.1016/s0090-4295(01)01492-3. [DOI] [PubMed] [Google Scholar]
- 23.Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-alpha signaling. Hypertension. 2010;55:1246–1253. doi: 10.1161/HYPERTENSIONAHA.110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J. Clin. Endocrinol. Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 25.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am. J. Obstet. Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 26.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am. J. Obstet. Gynecol. 1992;166:962–968. doi: 10.1016/0002-9378(92)91372-h. [DOI] [PubMed] [Google Scholar]
- 27.Dekker GA, Kraayenbrink AA, Zeeman GG, van Kamp GJ. Increased plasma levels of the novel vasoconstrictor peptide endothelin in severe pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991;40:215–220. doi: 10.1016/0028-2243(91)90120-a. [DOI] [PubMed] [Google Scholar]
- 28.Kraayenbrink AA, Dekker GA, van Kamp GJ, van Geijn HP. Endothelial vasoactive mediators in preeclampsia. Am. J. Obstet. Gynecol. 1993;169:160–165. doi: 10.1016/0002-9378(93)90154-b. [DOI] [PubMed] [Google Scholar]
- 29.Mastrogiannis DS, O'Brien WF, Krammer J, Benoit R. Potential role of endothelin-1 in normal and hypertensive pregnancies. Am. J. Obstet. Gynecol. 1991;165:1711–1716. doi: 10.1016/0002-9378(91)90020-r. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi F, Constantino L, Machado R, Petronilho F, Dal-Pizzol F. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in preeclamptic women. J. Obstet. Gynaecol. Res. 2008;34:957–963. doi: 10.1111/j.1447-0756.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 31.Ariza AC, Bobadilla NA, Halhali A. [Endothelin 1 and angiotensin II in preeeclampsia]. Rev. Invest. Clin. 2007;59:48–56. [PubMed] [Google Scholar]
- 32.Aydin S, Benian A, Madazli R, Uludag S, Uzun H, Kaya S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;113:21–25. doi: 10.1016/S0301-2115(03)00368-3. [DOI] [PubMed] [Google Scholar]
- 33.Epstein BJ, Anderson S. Endothelin receptor antagonists as anti-hypertensives: the next frontier. Expert Rev. Cardiovasc. Ther. 2009;7:675–687. doi: 10.1586/erc.09.24. [DOI] [PubMed] [Google Scholar]
- 34.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic auto-antibodies in intrauterine growth restriction seen in preeclampsia. J. Exp. Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund AK, Goens MB, Nuñez BA, Walker MK. Characterizing the role of endothelin-1 in the progression of cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice. Toxicol. Appl. Pharmacol. 2006;212:127–135. doi: 10.1016/j.taap.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Liang B, Song Z, Wu B, Gardner D, Shealy D, Song XY, Wooley PH. Evaluation of anti-IL-6 monoclonal antibody therapy using murine type II collagen-induced arthritis. J. Inflamm. (Lond.) 2009;6:10. doi: 10.1186/1476-9255-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J. Clin. Invest. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou CC, Irani RA, Zhang Y, Blackwell SC, Mi T, Wen J, Shelat H, Geng YJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-alpha induction contributes to increased soluble endoglin production in preeclampsia. Circulation. 2010;121:436–444. doi: 10.1161/CIRCULATIONAHA.109.902890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Yen H, Chen CH, Soni R, Jasani N, Sylvestre G, Reznik SE. The endothelin-converting enzyme-1/endothelin-1 pathway plays a critical role in inflammation-associated premature delivery in a mouse model. Am. J. Pathol. 2008;173:1077–1084. doi: 10.2353/ajpath.2008.080257. [Published erratum appears in 2009 Am. J. Pathol. 174: 1120.]
- 42.Attinà T, Camidge R, Newby DE, Webb DJ. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart. 2005;91:825–831. doi: 10.1136/hrt.2004.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Granger JP. Endothelin. Am. J. Physiol. 2003;285:R298–R301. doi: 10.1152/ajpregu.00249.2003. [DOI] [PubMed] [Google Scholar]
- 44.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am. J. Reprod. Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 45.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 46.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 47.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- 48.de Jong CL, Dekker GA, Sibai BM. The renin-angiotensin-aldosterone system in preeclampsia. A review. Clin. Perinatol. 1991;18:683–711. [PubMed] [Google Scholar]
- 49.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am. J. Physiol. Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 50.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jüpner A, Baur E, Nissen E, Vetter K, Neichel D, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffrin EL. State-of-the-Art lecture. Role of endothelin-1 in hypertension. Hypertension. 1999;34:876–881. doi: 10.1161/01.hyp.34.4.876. [DOI] [PubMed] [Google Scholar]
- 52.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr., et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 54.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 55.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 56.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 57.Hocher B, Thöne-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, et al. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J. Clin. Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gómez-Garre D, Largo R, Liu XH, Gutierrez S, López-Armada MJ, Palacios I, Egido J. An orally active ETA/ETB receptor antagonist ameliorates proteinuria and glomerular lesions in rats with proliferative nephritis. Kidney Int. 1996;50:962–972. doi: 10.1038/ki.1996.397. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura T, Ebihara I, Fukui M, Tomino Y, Koide H. Effect of a specific endothelin receptor A antagonist on mRNA levels for extracellular matrix components and growth factors in diabetic glomeruli. Diabetes. 1995;44:895–899. doi: 10.2337/diab.44.8.895. [DOI] [PubMed] [Google Scholar]
- 60.Ortmann J, Nett PC, Celeiro J, Traupe T, Tornillo L, Hofmann-Lehmann R, Haas E, Frank B, Terraciano LM, Barton M. Endothelin inhibition delays onset of hyperglycemia and associated vascular injury in type I diabetes: evidence for endothelin release by pancreatic islet beta-cells. Biochem. Biophys. Res. Commun. 2005;334:689–695. doi: 10.1016/j.bbrc.2005.06.140. [DOI] [PubMed] [Google Scholar]
- 61.Silver RM, Schwinzer B, McGregor JA. Interleukin-6 levels in amniotic fluid in normal and abnormal pregnancies: preeclampsia, small-forgestational-age fetus, and premature labor. Am. J. Obstet. Gynecol. 1993;169:1101–1105. doi: 10.1016/0002-9378(93)90263-i. [DOI] [PubMed] [Google Scholar]
- 62.Nakabayashi M, Sakura M, Takeda Y, Sato K. Elevated IL-6 in midtrimester amniotic fluid is involved with the onset of preeclampsia. Am. J. Reprod. Immunol. 1998;39:329–334. doi: 10.1111/j.1600-0897.1998.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 63.Munno I, Chiechi LM, Lacedra G, Berardesca C, Patimo C, Marcuccio L, Nardelli P, Loizzi P. Evaluation of nonspecific immunity and plasma levels of interferon-gamma, interleukin-6 and tumor necrosis factor-alpha in preeclampsia. Immunopharmacol. Immunotoxicol. 1999;21:551–564. doi: 10.3109/08923979909007125. [DOI] [PubMed] [Google Scholar]
- 64.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48:711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 65.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salmon JE, Girardi G. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J. Reprod. Immunol. 2008;77:51–56. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fliser D, Buchholz K, Haller H, EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) Investigators Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 68.Manabe S, Okura T, Watanabe S, Fukuoka T, Higaki J. Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J. Cardiovasc. Pharmacol. 2005;46:735–739. doi: 10.1097/01.fjc.0000185783.00391.60. [DOI] [PubMed] [Google Scholar]
- 69.Vázquez-Oliva G, Fernández-Real JM, Zamora A, Vilaseca M, Badimón L. Lowering of blood pressure leads to decreased circulating interleukin-6 in hypertensive subjects. J. Hum. Hypertens. 2005;19:457–462. doi: 10.1038/sj.jhh.1001845. [DOI] [PubMed] [Google Scholar]
- 70.Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R1013–R1023. doi: 10.1152/ajpregu.00729.2003. [DOI] [PubMed] [Google Scholar]
- 71.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am. J. Pathol. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr., Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal R, Loganath A, Roy AC, Wong YC, Lindoff C, Ng SC. Increased expression of interleukin 6 in term compared to the first trimester human placental villi. Horm. Metab. Res. 2000;32:164–168. doi: 10.1055/s-2007-978615. [DOI] [PubMed] [Google Scholar]
- 74.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J. Soc. Gynecol. Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol. Cell Physiol. 2009;297:C1228–C1235. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 76.Langer B, Grima M, Coquard C, Bader AM, Schlaeder G, Imbs JL. Plasma active renin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstet. Gynecol. 1998;91:196–202. doi: 10.1016/s0029-7844(97)00660-1. [DOI] [PubMed] [Google Scholar]
- 77.Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drug. Exp. Biol. Med. (Maywood) 2006;231:653–695. [PubMed] [Google Scholar]
- 78.Kohan DE. Endothelin, hypertension and chronic kidney disease: new insights. Curr. Opin. Nephrol. Hypertens. 2010;19:134–139. doi: 10.1097/MNH.0b013e328335f91f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.