Abstract
Homodimeric structure of cytochrome bc1, a common component of biological energy conversion systems, builds in four catalytic quinone oxidation/reduction sites and four chains of cofactors (branches) that, connected by a centrally located bridge, form a symmetric H-shaped electron transfer system. The mechanism of operation of this complex system is under constant debate. Here, we report on isolation and enzymatic examination of cytochrome bc1-like complexes containing fused cytochrome b subunits in which asymmetrically introduced mutations inactivated individual branches in various combinations. The structural asymmetry of those forms was confirmed spectroscopically. All the asymmetric forms corresponding to cytochrome bc1 with partial or full inactivation of one monomer retain high enzymatic activity but at the same time show a decrease in the maximum turnover rate by a factor close to 2. This strongly supports the model assuming independent operation of monomers. The cross-inactivated form corresponding to cytochrome bc1 with disabled complementary parts of each monomer retains the enzymatic activity at the level that, for the first time on isolated from membranes and purified to homogeneity preparations, demonstrates that intermonomer electron transfer through the bridge effectively sustains the enzymatic turnover. The results fully support the concept that electrons freely distribute between the four catalytic sites of a dimer and that any path connecting the catalytic sites on the opposite sides of the membrane is enzymatically competent. The possibility to examine enzymatic properties of isolated forms of asymmetric complexes constructed using the cytochrome b fusion system extends the array of tools available for investigating the engineering of dimeric cytochrome bc1 from the mechanistic and physiological perspectives.
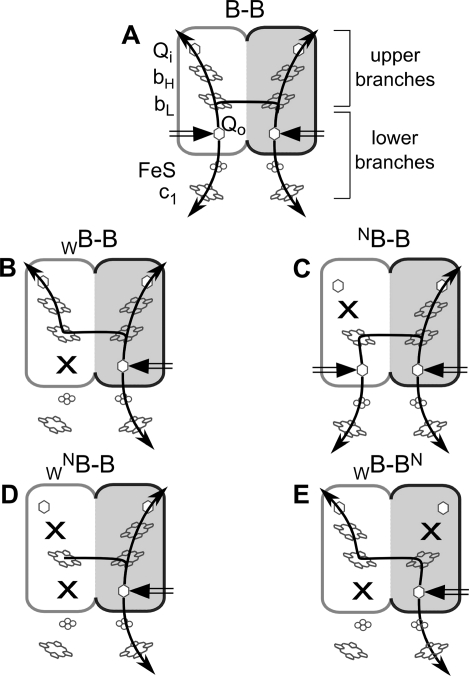
Cytochrome bc1 (mitochondrial complex III) is an integral component of many biological energy conversion systems. It operates according to the principles of Q-cycle in which the turnover of the enzyme leads to the net oxidation of quinol in the membrane and the reduction of cytochrome c outside the membrane with a vectorial transportation of protons across the membrane. The enzyme assembles as a homodimer. Each monomer contains three catalytic subunits—cytochrome c1, the iron–sulfur subunit, and cytochrome b—which together embed heme and iron–sulfur cofactors that assemble into two chains. Those chains integrate with the operation of two quinone oxidation/reduction sites, each on one side of the membrane. In addition, a two-heme bridge exists between the monomers in the core of the dimer. Overall, this makes up a rather complicated H-shaped system that displays high structural and spectroscopic symmetry. As depicted schematically in Figure 1A, each branch of H corresponds to one cofactor chain, whereas each upright of H (one lower and one upper branch) corresponds to one monomer of the dimer. Because of this complexity, the molecular mechanism of operation of cytochrome bc1 is under constant debate (for recent reviews see refs (1−3)).
Figure 1.
Asymmetric mutation patterns in cytochrome bc1-like complexes containing fused cytochrome b subunit (B–B). The two halves of the fusion protein, each corresponding to one cytochrome b, are shown as white and gray rounded rectangles, respectively. Crosses indicate position of knockout mutations N and W, which refer to H212N and G158W point mutations in cytochrome b, respectively. Black arrows indicate functional branches. Black double arrow indicates electron entry point at the Q0 site.
One potent approach to address the mechanistic problems related with a symmetry of cytochrome bc1 has recently been described in experiments designed to test conditions when this symmetry was broken.4−6 Our own studies used a model system based on a fusion protein that replaced two cytochromes b in the dimer in purple bacterium, Rhodobacter (Rb.) capsulatus.6 With this system we introduced mutations that inactivated individual branches in various combinations to expose all major electron transfer paths within a dimer for kinetic testing (Figure 1). This revealed that upon inactivation of one or two branches in one-half of the fusion protein (corresponding to partial or full inactivation of one monomer) (Figure 1B–D) the enzyme was still active and supported catalytically relevant electron transfer. This was consistent with studies that, with a help of a two-tag system in Paracoccus denitrificans, reported the enzymatic activity of the asymmetric form in which only one catalytic site was inhibited by mutation.4
In addition, our studies demonstrated that upon cross-inactivation of the lower branch in one half and the upper branch in the another half of the fusion protein (Figure 1E) electron transfer through the two-heme bridge between the two halves takes place on a catalytically relevant time scale.6 This indicated that the H-shaped structural arrangement of cytochrome bc1 should in fact be considered as a functional H-shaped electron transfer system that connects all four quinone oxidation/reduction sites. An independent proposal for the existence of the intermonomer electron transfer in cytochrome bc1 has been presented based on other studies on Rb. capsulatus which used a two-tag system to introduce asymmetric mutations and generate the heterodimeric cross-inactivated form.5
While the experiments using asymmetric forms of cytochrome bc1 mark a major step forward toward understanding of the engineering of the dimer, it is clear that further progress in this area will call for systematic analysis of various asymmetric electron transfer paths under a broad range of experimental conditions. Toward this goal, here we report on isolation, spectroscopic, and enzymatic characterization of isolated complexes containing fused cytochrome b with asymmetrically introduced mutations. The number of successfully isolated asymmetric variants was sufficient to present comparison of the maximum turnover rates of the forms that examined all the electron transfer paths of the dimer. This provided new mechanistic insights regarding operation of the dimer.
Materials and Methods
Isolation and Preparation of Proteins and Electrophoresis
The chromatophore membranes of WT (native form of cytochrome bc1) and the B–B derivatives (B–B denotes cytochrome bc1-like complexes in which two cytochromes b were fused into one subunit cytochrome bb) were prepared from semiaerobically grown cultures of Rb. capsulatus as described.6,7 Membranes, containing a mixture of inhibitors PMSF (phenylmethylsulfonyl fluoride), benzamidine, and 6-aminocaproic acid, were diluted to a final protein concentration 10 mg/mL and solubilized with DDM (n-dodecyl-β-d-maltoside) (1 mg protein:1.3 mg detergent) for 30 min at 4 °C. The mixture was ultracentrifuged (45 min, 45000g), and the supernatant was loaded onto a Strep-tag column (IBA-Biotechnology). All purification steps were performed at 4 °C.
The affinity chromatography was performed according to the protocol for Strep-tag purification supplied by the manufacturer (IBA), with the following modifications.7 Typically 3–5 mL of DDM-solubilized membranes was loaded onto the 1 mL Strep-tag sepharose column (IBA) pre-equilibrated with a washing buffer (100 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1 mM EDTA) containing additionally 20% glycerol and 0.01% DDM (Buffer WG). The column was washed with 2–3 column volumes of Buffer WG. The absorbed proteins were eluted with 3 column volumes of Buffer WG containing 2.5 mM desthiobiotin. The samples were taken directly for enzymatic activity assays (performed immediately after each isolation) or concentrated using Amicon Ultra 100 K centrifugal units for EPR measurements.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described.8 The gels were stained with Coomassie blue.
CW-EPR Measurements
Continuous wave (CW) spectra at X-band of the FeS cluster in purified cytochrome bc1 and various B–B complexes were measured as described in ref (9). The samples of protein (concentrated to approximately 15–20 μM cytochrome c1) were in elution buffer from the Strep-tag column (100 mM Tris buffer, pH 8, 150 mM NaCl, 0.01% DDM, 20% glycerol, 1 mM EDTA, 2.5 mM desthiobiotin), to which sodium ascorbate (at 1 mM final concentration) and stigmatellin (at 100 μM final concentration) were added. The samples were incubated for 5 min before freezing in liquid nitrogen. All spectra were recorded using the same parameters.
Measurements of Enzymatic Activity
Steady-state enzymatic activity of cytochrome bc1 and various B–B complexes was assayed by measuring the DBH2 (2,3-dimethoxy-5-methyl-6-decyl-1,4-benzohydroquinone)-dependent reduction of mitochondrial cytochrome c, as described in ref (10). All measurements were performed using freshly isolated protein complexes at room temperature in 50 mM Tris, pH 8.0 containing 0.01% DDM. The course of each assay was as follows. A cuvette containing appropriate concentration of cytochrome c in total volume 0.74 mL was inserted into spectrophotometer equipped with magnetic mixer, and the degree of reduction of cytochrome c was followed by monitoring absorbance at 550 nm. The background of nonenzymatic reduction of cytochrome c was measured for 20 s after injection of 5 μL of 3 mM DBH2 in DMSO to obtain final concentration 20 μM. Afterward, 5 μL of 1 μM of appropriate form of cytochrome bc1 or B–B complexes (the concentration was determined from the amount of reduced cytochrome c1) was injected into cuvete to obtain final concentration 6.7 nM. The turnover rate for each concentration of cytochrome c was estimated from the slope of initial absorbance increase immediately after addition of the enzyme, and the background rate was subtracted. Each data point represents the average turnover rate determined from 3–4 repetitions for independent cell harvests and isolations of enzyme. The maximum turnover rates (Vmax) for 20 μM DBH2 were determined from fitting the Michaelis–Menten equation to averaged experimental data points.
Results and Discussion
Spectral Properties and Subunit Composition of Isolated Asymmetric B–B Complexes
In this study, we used the family of asymmetric B–B complexes originally described in ref (6). Those complexes contained point mutation corresponding to G158W or H212N in cytochrome b (denoted as W or N, respectively). G158W (W) prevented the substrate binding at the Q0 site11 inactivating the lower branch of the H-shaped electron transfer system (Figure 1B), while H212N (N) prevented heme bH assembly12 inactivating the upper branch of this system (Figure 1C). Figure 1 shows that permutations of these two mutations expose all possible electron-transfer paths within the H-shaped electron transfer system for kinetic testing.
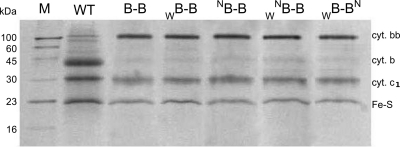
Figure 2 compares the electrophoretic profiles of B–B complexes that were isolated from the membranes using Strep-tag affinity chromatography and were subsequently used in the enzymatic activity assays (summarized in Figure 4 and Table 1). It is clear that highly pure samples were obtained, and in all cases the fused cytochrome bb replaced cytochrome b subunits present in the native cytochrome bc1 dimer. As expected, this cytochrome is 2 times larger and is accompanied by the two remaining catalytic subunits of cytochrome bc1: cytochrome c1 and the FeS subunit.
Figure 2.
SDS-page analysis of B–B complexes containing mutations W and N in various asymmetric combinations. Samples of cytochrome bc1 (WT) and various B–B complexes were isolated by Strep-tag affinity chromatography. M, marker (from IBA Biotechnology).
Figure 4.
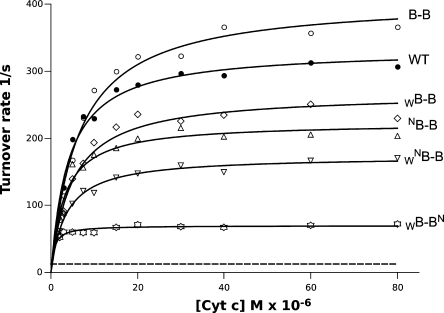
Comparison of enzymatic activities of cytochrome bc1 and various B–B complexes. Plots show dependence of the turnover rate vs concentration of cytochrome c (Cyt c) in 50 mM Tris, pH 8, 0.01% DDM, 20 μM DBH2. Fitting of the measured data points to the Michaelis–Menten kinetics yielded the values of Vmax listed in Table 1. Broken line shows the estimated level of activity of WB–BN in which heme bL–bL electron transfer is assumed not to occur on a catalytic time scale. It was calculated as 0.5 × (VWmax + VNmax), where VWmax and VNmax denote the values of Vmax determined for the isolated cytochromes bc1 containing mutations G158W and H212N, respectively.
Table 1. Maximum Turnover Rates for Isolated Cytochrome bc1 and Various B–B Complexes.
| namea | Vmax[s–1]b | namea | Vmax[s–1]b |
|---|---|---|---|
| WT | 333 ± 8 | WNB–B | 173 ± 4 |
| B–B | 408 ± 12 | WB–BN | 69.8 ± 1.4 |
| WB–B | 267 ± 10 | H212N | 20.7 ± 0.6 |
| NB–B | 223 ± 8 | G158W | 5.0 ± 0.07 |
Letter code corresponds to schemes of Figure 1. WT, wild type cytochrome bc1.
Vmax for 20 μM DBH2.
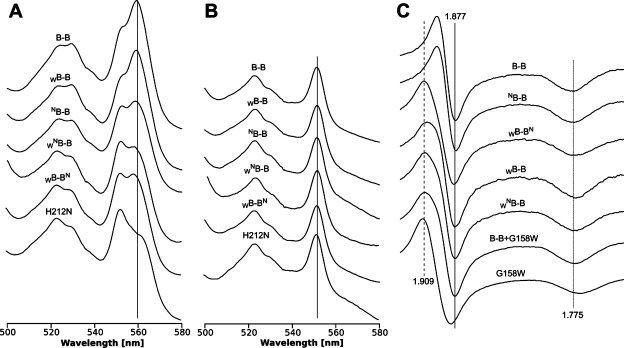
Figure 3 summarizes spectroscopic properties of those B–B complexes in the isolated forms. The optical spectra in the α region (Figure 3A,B) were recorded to check for the presence of reduced hemes b (560 nm) (Figure 3A) and high potential hemes c1 (552 nm) (Figure 3B). From the shape of the spectra (relative amplitudes of 552 and 560 nm) it is clear that B–B and WB–B retain the native amounts of hemes in the preparations, while NB–B, WNB–B, and WB–BN show reduced amounts of heme B, as expected for the loss of one heme imposed by the presence of one N mutation in the complex. This represents a spectroscopic signature implying the structural asymmetry of the isolated complexes with one N. We note slight variations in the amount of hemes B between the preparations shown in Figure 3. These variations are observed when complexes (especially the mutated complexes that in isolated form tend to be less stable than the native complex) are purified using Strep-tag column and relate to the possibility of partial dissociation of subunits not-containing Strep-tag during the purification procedure.7
Figure 3.
Spectroscopic proof of structural asymmetry imposed by mutations N and W in isolated B–B complexes. A–C compare spectra of pure B–B complexes isolated using Strep-tag affinity chromatography. A and B, optical spectra of hemes in isolated complexes reduced by dithionite (A) or ascorbate (B). Bottom spectra show the reference for cytochrome bc1 containing H212N in cytochrome b (isolated by Strep-tag). C, X-band continuous wave EPR spectra of the FeS cluster in isolated complexes. All samples were treated with stigmatellin. B–B+G158W represents the sum of the normalized to gy amplitude spectra of B–B and G158W. G158W is the reference spectrum for cytochrome bc1 with G158W in cytochrome b (isolated by Strep-tag). Vertical dashed line shows the g = 1.909, which corresponds to the first inflection point of microwave absorption of gy transition of the cluster for G158W mutant, while solid line shows g = 1.877, which corresponds to the second inflection point of absorption of gy transition of the cluster of B–B form (as well the native cytochrome bc1). Forms WB–B, WNB–B, and WB–BN have the first and second inflections at g = 1.909 and g = 1.877, which confirms their asymmetry with respect to the mutation W. Form NB–B has the inflection points at the same g values as B–B. The dotted line approximates position of gx transition (g = 1.775).
The structural asymmetry imposed by the presence of one W per complex was verified by the EPR spectra of the FeS cluster (Figure 3C). To avoid complications arising from the fact that the quinone content may vary in isolated cytochrome bc1 preparations, which consequently will affect the shape of the EPR spectrum,11,13 we have compared the spectra of the samples treated with stigmatellin. Binding of this inhibitor results in a specific change in the spectrum that does not depend on the quinone content or whether the enzyme is purified in detergent solution containing glycerol or in membranes.14,15 Furthermore, the spectrum of the G158W mutant treated with stigmatellin differs significantly from the corresponding spectrum of the wild type (Figure 3C) (manuscript in preparation). We used this feature in our comparative analysis, which revealed that the shape of the EPR spectra of stigmatellin-treated B–B complexes containing only one W represents a sum of two different spectral components corresponding to the stigmatellin spectra of wild type and symmetric mutant G158W with equal contributions (Figure 3C and Figure S1 of the Supporting Information). Because the EPR spectrum of the FeS cluster in cytochrome bc1 is in general considered to be a sensor of the interaction of this subunit with cytochrome b subunit which also reflects the status of the Q0 site, the spectra presented in Figure 3C provide strong evidence of the proper asymmetric assembly of the complexes in isolated form. This type of clear spectroscopic evidence has not been provided for any other so far reported isolation of asymmetric cytochrome bc1 constructs.4,5
Enzymatic Activities of Isolated Asymmetric B–B Complexes
Figure 4 presents the results of the enzymatic activity assays preformed with the isolated B–B forms that inactivated various branches of the H-shaped electron transfer system. All those forms refer to the samples that display electrophoretic profiles and exhibit spectroscopic properties as described in Figures 2 and 3. Fitting of a Michaelis–Menten equation to a dependence of the measured enzymatic turnover rate on the concentration of substrate cytochrome c (lines in Figure 4) yielded the values of Vmax that were summarized in Table 1.
We found that B–B without any additional mutation, as in Figure 1A (all four branches are available for electron transfer), displays a high enzymatic activity (highest Vmax) which is in the same range, if not exceeding that, measured for the native enzyme. However, when one lower or one upper branch is inactivated (WB–B, NB–B) as in Figure 1B,C, the Vmax drops to about 60% that of the Vmax of B–B. A slightly larger drop (to about 40%) is observed when both the upper and the lower branch on the same side are inactivated (WNB–B) (as in Figure 1D) and the complex is forced to use only one half, corresponding to just one monomer of dimeric cytochrome bc1. An even larger drop in Vmax is observed when the upper and the lower branches across are inactivated (WB–BN) (Figure 1E), in which case the complex is forced to use the heme bL–bL connection between the two halves. The level of activity is however still significantly higher than a level of activity estimated for a theoretical case for WB–BN in which heme bL–bL electron transfer would not to occur on a catalytic time scale (Figure 4, broken line). It is also higher than the level of activity observed in the symmetrically inactivated mutants G158W or H212N (Table 1).
From the comparison shown in Figure 4 and Table 1, it is clear that all the B–B forms corresponding to the cytochrome bc1 with partial or full inactivation of one monomer (i.e., WB–B, NB–B, WNB–B), despite the 40–60% drop in Vmax vs B–B, retain high enzymatic activity. This convincingly implicates that the native dimer of cytochrome bc1 remains operational even with one monomer inactive. Furthermore, the decrease in Vmax by an approximately factor of 2 is consistent with a general prediction valid for the kinetic model assuming independent operation of two monomers.
From the comparison of Figure 4 and Table 1, it is also clear that the cross-inactivated B–B form corresponding to cytochrome bc1 with disabled complementary parts of each monomer (WB–BN) is enzymatically active. This first report documenting enzymatic activity of cross-inactivated form purified to homogeneity from membranes implicates that the path relying on heme bL to bL electron transfer as the only route connecting the Q0 site with the Qi site can efficiently sustain the catalytic steady state turnover of cytochrome bc1.
The Vmax of WB–BN is however lower than those obtained for WB–B, NB–B, and WNB–B. At this stage, deciding what causes the maximum turnover rate to be low is difficult. Because the B–B construct should only be treated as a model (not an exact replica of the native dimer), some distortions from the native structure comprise one group of possibilities,7 particularly when dealing with the isolated forms. Such distortions could, for example, modify a distance between two hemes bL and/or their electrochemical properties. Besides structural effects, mechanistic reasons can also be envisaged. For example, the “pressure” of reverse reaction,10,16,17 which may be more pronounced in WB–BN when electrons have to travel through two hemes bL before reaching heme bH and the Qi site, might influence the measured overall rate.
Because the measured rate in WB–BN is significantly lower than the level in the native (and B–B) protein, one should weigh up a possibility that the overall rate is affected by a small fraction of enzymatically active forms that are not WB–BN but contaminate isolated preparations used for enzymatic activities assays. The potential risk of such contamination is associated with recombination processes intrinsic to systems based on coexpression of two copies of the same gene. However, our calculations indicate that those types of contaminations would have to be at the level of at least 14% to obscure kinetics (see Supporting Information). In light of the results presented in ref (7) and estimated frequency of reversions, this is very unlikely. In addition, we should emphasize that the measured enzymatic activities of isolated complexes fully corroborate with the results of flash-induced electron transfer measurements in membranes,6 which by definition are much less sensitive to the presence of small amounts of any types of contaminants. In this case, unlike in enzymatic steady state turnover measurements, the amplitude of signal is directly proportional to the concentration of protein in the sample, and the accumulation of signal that would originate from small fractions of highly active variants is not possible. If we were to continue considering the case discussed above, the assumed enzymatic activity solely due to presence of 14% of active contaminants would correspond to the flash-activated kinetics in which the amplitude reaches only 14% of signal of B–B protein. This clearly was not the case.6
Relation to Other Systems
Studies based on kinetic analysis of isolated complexes of asymmetric forms of cytochrome bc1 allow us now to advance to the new level of understanding of the dimeric function of this enzyme. Necessary for this is a coherent picture derived from comprehensive analyses of all the major electron transfer paths of the dimer using pure complexes isolated with protocols that by themselves do not affect the enzymatic properties of the enzyme. A recently described alternative approach to introduce asymmetric mutations in cytochrome bc1 is based on a two-tag system that is intrinsically heterogenic and cannot guarantee with any certainty that the lengthy isolation protocols yield preparations retaining functionally relevant properties. Of particular concern in this regard is the finding that of the two isolations of asymmetric cytochrome bc1 with the two tags system reported to date, only one yielded complexes that were enzymatically active.4 In another instance, the complexes at the final stage of preparation were inactive and consequently all kinetic analysis must have relied on data that derived from the mixture comprising both the homo- and heterodimeric forms.5
With our one-step purification of the fusion protein described here we have demonstrated that the isolated asymmetric B–B forms can be purified to homogeneity and, moreover, are pure and enzymatically active fractions that bear spectroscopic signatures confirming their structural asymmetry. Importantly, the number of B–B variants meeting these criteria was sufficient to test all the electron transfer paths of the dimer. This level of experimental clarity which opens the door to cytochrome bc1 mechanism, physiology, and its regulation to-date remains in the experimental domain of the singular fusion system.
Mechanistic Implications
At this stage, the enzymatic analysis of isolated complexes of B–B variants we have presented here complements our previous kinetic analysis on B–B complexes in membranes.6 We demonstrated now that the B–B forms corresponding to the cytochrome bc1 with partial or full inactivation of one monomer (WB–B, NB–B, WNB–B) or cross-inactivation of complementary parts of each monomer (WB–BN) all retained enzymatic activity when isolated from membranes and purified to homogeneity. This result fully supports the concept that electrons freely distribute between the four quinone catalytic sites of a dimer and that any path connecting the sites of the opposite sides of the membrane (i.e., Q0 with Qi) is enzymatically competent.6 We believe that this not only holds true for the uncoupled conditions tested here (and in other asymmetric work done so far) but also extends to the physiological conditions when the enzyme operates under the membrane potential. However, the examination of the behavior of the asymmetric enzymes that would include the coupled conditions is certainly needed.
On the other hand, our result and the model that accommodates them are inconsistent with models that include a high level of allosteric cooperativity between the monomers intrinsic to mechanism.18−20 To provide an illustration of such inconsistency, consider a heterodimeric modified Q cycle model18 which postulates a number of obligatory events that force a specific electron transfer sequence involving dismutation of partial products formed sequentially in every monomer. According to this model, the complexes in which one monomer is partially or fully inactivated should lose the postulated multiturnover enzymatic competence. Furthermore, the cross-inactivated complexes relying just on a cross-dimer electron transfer should not be active either. In fact, it seems that this electron transfer sequence needs to be forbidden upon the multiple turnovers for the model to work. However, as the authors of this model propose, this sequence alone is sufficient to sustain the cytochrome bc1-dependent photosynthetic growth of bacterial cells.5
If indeed the cross-dimer electron transfer alone is capable of supporting the cytochrome bc1-dependent growth of the cells, it would further strengthen the concept, central to our model, of free distribution of electrons between the catalytic sites, as it would provide an ultimate proof of a built in redundancy which allows physiological function of the protein even after the operational damage of its parts. The only requirement that needs to be met is a preservation of the electronic communication between the sites supporting the Q cycle. While this flexibility is a clear advantage to redundancy (and possibly to suppression of ROS generation), there is evidence of further robustness of cytochrome bc1 as shown recently in plant chloroplasts.21
Glossary
Abbreviations
- Rb.
Rhodobacter
- DDM
n-dodecyl-β-d-maltoside
- FeS cluster; 2 iron
2 sulfur Rieske cluster
- DBH2
2,3-dimethoxy-5-decyl-6-methyl-1,4-benzohydroquinone
- B–B complex
cytochrome bc1-like complex with two cytochromes b fused into one subunit
- Vmax
maximum turnover rate.
Supporting Information Available
Additional Figure S1 and analysis of Vmax. This material is available free of charge via the Internet at http://pubs.acs.org.
Conflict of Interest Statement
The authors declare no competing financial interest.
This work was supported by The Wellcome Trust International Senior Research Fellowship to A.O. and START Programme from the Foundation for Polish Science to A.B. and M.C.
Author Contributions
† These authors contributed equally to this work.
Supplementary Material
References
- Cramer W. A.; Hasan S. S.; Yamashita E. (2011) The Q cycle of cytochrome bc complexes: A structure perspective. Biochim. Biophys. Acta 1807, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D. M., Nitschke W., and Cooley J. W. (2009) The cytochrome bc1 and related bc complexes: the Rieske/cytochrome b complex as the functional core of a central electron/proton transfer complex, in The Purple Phototrophic Bacteria (Hunter N., Daldal F., Thurnauer M. C., and Beatty J. T., Eds.) Springer, The Netherlands. [Google Scholar]
- Crofts A. R.; Holland J. T.; Victoria D.; Kolling D. R. J.; Dikanov S. A.; Gilbreth R.; Lhee S.; Kuras R.; Guergova Kuras M. (2008) The Q-cycle reviewed: how well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex?. Biochim. Biophys. Acta 1777, 1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani M.; Covian R.; Kleinschroth T.; Anderka O.; Ludwig B.; Trumpower B. L. (2010) Direct demonstration of half-of the sites reactivity in the dimeric cytochrome bc1 complex. J. Biol. Chem. 285, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciano P.; Lee D.-W.; Yang H.; Darrouzet E.; Daldal F. (2011) Intermonomer electron transfer between the low-potential hemes of cytochrome bc1. Biochemistry 50, 1651–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świerczek M.; Cieluch E.; Sarewicz M.; Borek A.; Moser C. C.; Dutton P. L.; Osyczka A. (2010) An electronic bus bar lies in the core of cytochrome bc1. Science 329, 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla M.; Borek A.; Sarewicz M.; Osyczka A. (2012) Fusing two cytochromes b of Rhodobacter capsulatus cytochrome bc1 using various linkers defines a set of protein templates for asymmetric mutagenesis. Protein Eng., Des. Sel. 25, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osyczka A.; Dutton P. L.; Moser C. C.; Darrouzet E.; Daldal F. (2001) Controlling the functionality of cytochrome c1 redox potentials in the Rhodobacter capsulatus cytochrome bc1 complex through disulfide anchoring of a loop and a β-branched amino acid near the heme-ligating methionine. Biochemistry 40, 14547–14556. [DOI] [PubMed] [Google Scholar]
- Sarewicz M.; Dutka M.; Froncisz W.; Osyczka A. (2009) Magnetic interactions sense changes in distance between heme bL and iron-sulfur cluster in cytochrome bc1. Biochemistry 48, 5708–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek A.; Sarewicz M.; Osyczka A. (2008) Movement of the iron-sulfur head domain of cytochrome bc1 transiently opens the catalytic Qo site for reaction with oxygen. Biochemistry 47, 12365–12370. [DOI] [PubMed] [Google Scholar]
- Ding H.; Moser C. C.; Robertson D. E.; Tokito M. K.; Daldal F.; Dutton P. L. (1995) Ubiquinone pair in the Qo site central to the primary energy conversion reactions of cytochrome bc1 complex. Biochemistry 34, 15979–15996. [DOI] [PubMed] [Google Scholar]
- Osyczka A.; Moser C. C.; Daldal F.; Dutton P. L. (2004) Reversible redox energy coupling in electron transfer chains. Nature 427, 607–612. [DOI] [PubMed] [Google Scholar]
- Ding H.; Robertson D. E.; Daldal F.; Dutton P. L. (1992) Cytochrome bc1 complex [2Fe-2S] cluster and its interaction with ubiquinone and ubihydroquinone at the Qo site: a double-occupancy Qo site model. Biochemistry 31, 3144–3158. [DOI] [PubMed] [Google Scholar]
- Sharp R. E.; Moser C. C.; Gibney B. R.; Dutton P. L. (1999) Primary steps in the energy conversion reaction of the cytochrome bc1 complex Qo site. J. Bioenerg. Biomembr. 31, 225–233. [DOI] [PubMed] [Google Scholar]
- Sharp R. E.; Gibney B. R.; Palmitessa A.; White J. L.; Dixon J. A.; Moser C. C.; Daldal F.; Dutton P. L. (1999) Effect of inhibitors on the ubiquinone binding capacity of the primary energy conversion site in the Rhodobacter capsulatus cytochrome bc1 complex. Biochemistry 38, 14973–14980. [DOI] [PubMed] [Google Scholar]
- Cieluch E.; Pietryga K.; Sarewicz M.; Osyczka A. (2010) Visualizing changes in electron distribution in coupled chains of cytochrome bc1 by modifying barrier for electron transfer between the FeS cluster and heme c1. Biochim. Biophys. Acta 1797, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarewicz M.; Borek A.; Cieluch E.; Świerczek M.; Osyczka A. (2010) Discrimination between two possible reaction sequences that create potential risk of generation of deleterious radicals by cytochrome bc1. Implications for the mechanism of superoxide production. Biochim. Biophys. Acta 1797, 1820–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley J. W.; Lee D.-W.; Daldal F. (2009) Across membrane communication between the Qo and Qi active sites of cytochrome bc1. Biochemistry 48, 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covian R.; Trumpower B. L. (2008) Regulatory interactions in the dimeric cytochrome bc1 complex: the advantages of being a twin. Biochim. Biophys. Acta 1777, 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkidjanian A. Y. (2007) Proton translocation by the cytochrome bc1 complexes of phototrophic bacteria: introducing the activated Q-cycle. Photochem. Photobiol. Sci. 6, 19–34. [DOI] [PubMed] [Google Scholar]
- Malnoe A.; Wollman F.-A.; de Vitry C.; Rappaport F. (2011) Photosynthetic growth despite a broken Q-cycle. Nature Commun. 2, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.