Abstract
Objective To determine whether dietary n-3 long chain polyunsaturated fatty acid (LCPUFA) supplementation of pregnant women with a fetus at high risk of allergic disease reduces immunoglobulin E associated eczema or food allergy at 1 year of age.
Design Follow-up of infants at high hereditary risk of allergic disease in the Docosahexaenoic Acid to Optimise Mother Infant Outcome (DOMInO) randomised controlled trial.
Setting Adelaide, South Australia.
Participants 706 infants at high hereditary risk of developing allergic disease whose mothers were participating in the DOMInO trial.
Interventions The intervention group (n=368) was randomly allocated to receive fish oil capsules (providing 900 mg of n-3 LCPUFA daily) from 21 weeks’ gestation until birth; the control group (n=338) received matched vegetable oil capsules without n-3 LCPUFA.
Main outcome measure Immunoglobulin E associated allergic disease (eczema or food allergy with sensitisation) at 1 year of age.
Results No differences were seen in the overall percentage of infants with immunoglobulin E associated allergic disease between the n-3 LCPUFA and control groups (32/368 (9%) v 43/338 (13%); unadjusted relative risk 0.68, 95% confidence interval 0.43 to 1.05, P=0.08; adjusted relative risk 0.70, 0.45 to 1.09, P=0.12), although the percentage of infants diagnosed as having atopic eczema (that is, eczema with associated sensitisation) was lower in the n-3 LCPUFA group (26/368 (7%) v 39/338 (12%); unadjusted relative risk 0.61, 0.38 to 0.98, P=0.04; adjusted relative risk 0.64, 0.40 to 1.02, P=0.06). Fewer infants were sensitised to egg in the n-3 LCPUFA group (34/368 (9%) v 52/338 (15%); unadjusted relative risk 0.61, 0.40 to 0.91, P=0.02; adjusted relative risk 0.62, 0.41 to 0.93, P=0.02), but no difference between groups in immunoglobulin E associated food allergy was seen.
Conclusion n-3 LCPUFA supplementation in pregnancy did not reduce the overall incidence of immunoglobulin E associated allergies in the first year of life, although atopic eczema and egg sensitisation were lower. Longer term follow-up is needed to determine if supplementation has an effect on respiratory allergic diseases and aeroallergen sensitisation in childhood.
Trial registration Australian New Zealand Clinical Trials Registry ACTRN12610000735055 (DOMInO trial: ACTRN12605000569606).
Introduction
The prevalence of allergic diseases in Australia and other industrialised countries has increased over the past 30 years and is now estimated to be at least 20%.1 2 3 The cost to healthcare systems and the burden to families are high.4 5 The pattern of allergic disease differs with age; the incidence of food allergy and atopic dermatitis peaks by 1 year of age, whereas asthma and allergic rhinitis continue to rise until around 15 years of age.6 The increase in allergic disease has occurred too rapidly (within one to two generations) to be a result of genetic changes in the population, so it is likely to be related to environmental changes. In this context, strategies to reduce the burden of disease through prevention will be of enormous importance.
The period of increase in allergic diseases has coincided with a substantial shift in dietary intake of fatty acids to favour n-6 (omega 6) fatty acids over n-3 (omega 3) fatty acids, leading to speculation that the change in dietary fatty acid balance may be linked to the increased prevalence of childhood allergic disease.7 8 Diets rich in n-6 fatty acids, through increased consumption of vegetable oils rich in linoleic acid (18:2n-6), lead to a predominance of arachidonic acid (20:4n-6) in tissues. Arachidonic acid gives rise to eicosanoids such as prostaglandin E2, which can enhance the synthesis of T helper type 2 cytokines and immunoglobulin E antibodies—the hallmark of atopic responses to allergens. When diets are high in n-3 long chain polyunsaturated fatty acids (LCPUFA) (for example in fish), these are readily incorporated into cellular phospholipids, displacing arachidonic acid in the process. This leads to a range of biochemical and immunological changes, including reduction of prostaglandin E2 synthesis, alteration of receptor expression and activity, and reduced pro-inflammatory cytokine responses.9 10 Plausible mechanisms thus exist by which diets high in n-3 LCPUFA may modulate the development of immunoglobulin E mediated allergic disease and regulate immune responses.
Two previous randomised trials have shown that fish oil treatment during pregnancy, compared with placebo, resulted in down regulation of cytokine responses (interleukin-5, interleukin-13, interferon-γ) to allergens in mononuclear cells in cord blood,11 12 as well as up regulation of transforming growth factor-β in cord blood.12 These data are supported by a recent Swedish study showing fewer infants with immunoglobulin E associated eczema in response to n-3 LCPUFA supplementation from the 25th week of gestation (4/52 (8%) v 15/63 (24%), P<0.05).13 However, the lack of clarity regarding randomisation processes in this study means that one cannot exclude the possibility of bias, and the small sample size means that a large degree of uncertainty is associated with the results, highlighting the need for larger, more definitive randomised trials. We designed our study to determine the effect of n-3 LCPUFA supplementation during pregnancy on immunoglobulin E associated allergies (eczema or food allergy) at 12 months of age in infants with high hereditary risk. We recruited participants from the Docosahexaenoic Acid to Optimise Mother Infant Outcome (DOMInO) trial, which is a double blind, multicentre, randomised controlled trial of n-3 LCPUFA supplementation, predominantly as docosahexaenoic acid, in pregnancy to evaluate symptoms of maternal depression and neurodevelopment of young children.14 We have already shown that n-3 LCPUFA supplementation did not change the prevalence of maternal postnatal depression and that the mean developmental scores of children did not differ at 18 months of age.14
Methods
Study design
Pregnant women were approached to enter the allergy follow-up after randomisation into the DOMInO trial.14 Briefly, women were randomly allocated a unique number that corresponded to treatment or control through a computer driven telephone randomisation service according to an independently generated randomisation schedule, with balanced variable sized blocks. Stratification was by centre and parity (first birth versus subsequent births). Women were eligible for the allergy follow-up if the unborn baby had a mother, father, or sibling with a history of any medically diagnosed allergic disease (asthma, allergic rhinitis, eczema) and they were enrolled from the Women’s and Children’s Hospital or Flinders Medical Centre in Adelaide. Only Adelaide based families participated in the allergy follow-up because this allowed optimal quality assurance for allergy testing and achievement of an adequate sample size was possible. Eligible women were given information about the allergy follow-up, and written informed consent was sought before delivery. Baseline characteristics, including maternal age, maternal smoking during pregnancy, and parental education, were recorded.
Dietary treatments
The dietary treatments for the DOMInO trial have been described previously.14 Briefly, women allocated to the n-3 LCPUFA group were asked to consume three 500 mg capsules of fish oil concentrate rich in docosahexaenoic acid daily, providing 800 mg of docosahexaenoic acid and 100 mg of eicosapentaenoic acid (20:5n-3; Incromega 500 TG, Croda Chemicals, East Yorkshire, UK); women in the control group were asked to take three 500 mg vegetable oil capsules without n-3 LCPUFA daily. Women took capsules from 21 weeks’ gestation until delivery. The vegetable oil capsules contained a blend of three non-genetically modified oils (rapeseed, sunflower, and palm) in equal proportions, which was designed to match the polyunsaturated, monounsaturated, and saturated fatty acid profile of the average Australian diet.15 All capsules were similar in size, shape, and colour and were donated by Efamol, Surrey, UK. Neither the women nor the research staff were aware of the treatment allocated. Women reported their adherence to supplementation at telephone calls at 28 and 36 weeks’ gestation. In addition, the concentration of individual LCPUFA in plasma phospholipids from cord blood was assessed as an independent biomarker of adherence.
Infant allergic disease outcome assessments
The primary outcome for the nested allergy follow-up at 1 year of age was diagnosis of immunoglobulin E associated allergic disease (eczema or food allergy with sensitisation). One of five medical practitioners (blinded to treatment group allocation) made the diagnosis by taking a structured history and doing a standardised clinical examination, and one of four experienced nurses did skin prick tests. The medical practitioners and nurses were specifically trained in the trial assessments and had quality assurance reviews every six months with one of the investigators (DJP).
We defined sensitisation as a positive skin prick test to at least one of the allergens assessed. We considered a test to be positive if the mean of the horizontal and perpendicular weal diameters was 3 mm or greater in size than that of the negative control site at 15 minutes. The allergens tested were cows’ milk, whole hens’ egg, wheat, tuna, peanut, grass pollen, perennial ryegrass, olive tree pollen, Alternaria tenuis, cat hair, and house dust mite (Dermatophagoides pteronyssinus). Glycerin and histamine (10 mg/mL) were used as negative and positive controls.
We defined eczema as the presence of eczema (criteria according to Hanifin and Rijka16) on medical review or a history of an itchy rash distributed to the facial, flexural, or extensor surface of the skin that had followed a fluctuating or chronic course. We defined immunoglobulin E associated eczema or atopic eczema as eczema with sensitisation, in which the infant had a positive skin prick test to at least one of the allergens assessed.
We defined immunoglobulin E associated food allergy as a history of immediate (within 60 minutes) skin rash (hives, rash, or swelling) with or without respiratory symptoms (cough, wheeze, stridor), gastrointestinal symptoms (abdominal pain, vomiting, loose stools), or cardiovascular symptoms (collapse) following ingestion of a food (such as egg, peanut) and a positive skin prick test to the implicated food.
When the infants were 6 months of age, a research nurse or research assistant telephoned parents and asked them a set of standard questions to prospectively monitor symptoms of allergic disease, and these data sheets were available to the medical practitioners to facilitate their 1 year of age assessments. These phone calls also provided an opportunity to update family contact details to maximise follow-up and collect information on possible confounding variables at both 6 and 12 months of age, including details of infant feeding and rejection of foods, use of antibiotics, number of other children in the home, and presence of a dog or cat as a pet. Other possible confounding variables such as mode of delivery were available from the DOMInO trial dataset.
Sample size and statistical analysis
We calculated the sample size for the allergy follow-up by assuming an incidence of atopic eczema or food allergy in high risk Australian infants at 1 year of age of 14%.11 With 328 children in each treatment group, we would be able to detect an absolute reduction of 7% (relative reduction of 50%) in the incidence of atopic eczema or food allergy from 14% to 7% with 80% power (α=0.05). Both parents and the healthcare sector consider such a reduction to be important, and it was realistic compared with the 67% and 59% relative reductions in child allergy outcomes in response to fish oil treatment during pregnancy reported by Furuhjelm et al and Olsen et al.13 17
We did all analyses according to the intention to treat principle. We used multiple imputations to deal with missing data (both outcomes and covariates), with 50 complete datasets imputed for analysis. We imputed data and combined results by using the mi and mi_analyze procedures in SAS version 9.2. We did imputation sequentially (baseline variables, post-randomisation variables, outcomes) by using the parametric regression method for continuous and count variables and the logistic regression method for binary and ordinal variables.18 In addition to a primary imputed analysis, we did sensitivity analyses on the original data and on imputed data created using different seeds and different imputation models. All approaches produced similar results, so we present only the results of the primary imputed analysis here. Web appendix A gives further details of the imputation approach, the amount of missing data, and the results of sensitivity analyses.
We used linear regression models to analyse continuous outcomes, with treatment effects expressed as mean differences. We used negative binomial regression models to analyse count outcomes, with the effect of treatment expressed as a ratio of means. We analysed binary outcomes by using log binomial regression models, with treatment effects expressed as relative risks. We analysed rare binary outcomes by doing Fisher’s exact tests on the original (unimputed) data. We did both unadjusted and adjusted analyses, with adjustment for the stratification variables of centre and parity as well as the pre-specified baseline variables of infant sex and maternal history of allergic disease. We considered the adjusted analyses to be the primary analyses.
We also compared potential confounding variables measured after randomisation between groups. We used Fisher’s exact tests and Wilcoxon tests based on the original unimputed data to assess binary and continuous confounders respectively. We assessed statistical significance at the two sided P<0.05 level. We used SAS version 9.2 for all analyses.
Results
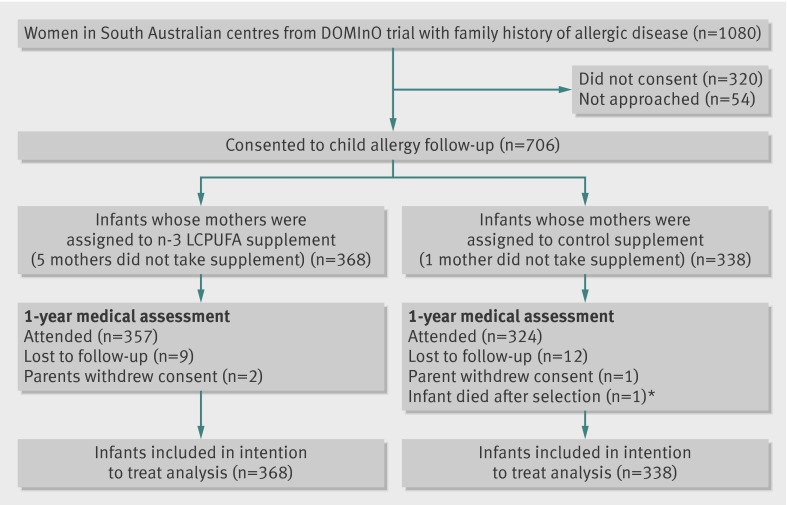
The figure shows the study profile. Enrolment for the allergy follow-up began on 20 March 2006 and ended on 8 May 2008. We enrolled 234/368 (64%) of the n-3 LCPUFA supplementation group and 216/338 (64%) of the control group from the Women’s and Children’s Hospital. Data collection at 1 year of age was completed on 26 August 2009. In total, 681/706 (96.5%) infants attended their 1 year medical review and 666/706 (94.3%) had skin prick tests results. The baseline demographic and clinical characteristics of the participating infants were comparable between the two groups (table 1).
Flow diagram of trial. DOMInO=Docosahexaenoic Acid to Optimise Mother Infant Outcome; LCPUFA=long chain polyunsaturated fatty acids. *Death was stillbirth and unrelated to objective of study
Table 1.
Baseline demographic and clinical characteristics. Values are numbers (percentages) unless stated otherwise
| Characteristic | n-3 LCPUFA (n=368) | Control (n=338) |
|---|---|---|
| Mean (SD) mother’s age at trial entry (years) | 29.6 (5.7) | 29.5 (5.6) |
| Parity zero | 150 (41) | 131 (39) |
| Maternal smoking during pregnancy | 47 (13) | 45 (13) |
| Mother completed secondary education | 232 (63) | 222 (66) |
| Father completed secondary education | 196 (53) | 182 (54) |
| Maternal history of allergic disease: | 257 (70) | 236 (70) |
| Eczema | 92 (25) | 63 (19) |
| Asthma | 156 (42) | 148 (44) |
| Allergic rhinitis | 133 (36) | 121 (36) |
| Paternal history of allergic disease: | 207 (56) | 178 (53) |
| Eczema | 53 (14) | 37 (11) |
| Asthma | 122 (33) | 86 (25) |
| Allergic rhinitis | 108 (29) | 109 (32) |
| Both parents with history of allergic disease | 109 (30) | 97 (29) |
| Infant’s sex male | 169 (46) | 168 (50) |
LCPUFA=long chain polyunsaturated fatty acid.
Concentrations of docosahexaenoic acid and eicosapentaenoic acid in the plasma phospholipids of cord blood from women in the n-3 LCPUFA group were greater than those for the control group (docosahexaenoic acid: median 7.5% v 6.2% total phospholipid fatty acids, P<0.001; eicosapentaenoic acid: median 0.54% v 0.27% total phospholipid fatty acids, P<0.001 based on a comparison of mean log transformed values). The concentration of total n-3 LCPUFA in the cord blood was higher in women in the n-3 LCPUFA group (median 8.8% v 7.2% total phospholipid fatty acids, P<0.001 based on a comparison of mean log transformed values), whereas the concentration of arachidonic acid in cord blood was lower in women in the n-3 LCPUFA group compared with control (mean 14.6% v 16.4%, P<0.001 based on a comparison of means). At 28 weeks’ gestation, 284/368 (77%) of mothers in the n-3 LCPUFA group and 270/338 (80%) of mothers in the control group reported that they had missed zero to three capsules a week (from a total of 21 capsules a week). Fewer than 2% of mothers in each group chose not to take any capsules.
Infant allergic disease outcomes
We found no difference in the overall percentage of infants diagnosed as having immunoglobulin E associated allergic disease (eczema or food allergy with sensitisation) at 1 year of age between the n-3 LCPUFA and control groups (32/368 (9%) v 43/338 (13%); unadjusted relative risk 0.68, 95% confidence interval 0.43 to 1.05, P=0.08; adjusted relative risk 0.70, 0.45 to 1.09, P=0.12) (table 2). However, the percentage of infants with atopic eczema at 1 year of age was lower in the n-3 LCPUFA group than in the control group, whereas the percentage of infants with food allergy did not differ between groups (table 2). The incidence of food allergy was about 3%, and most food allergies were to egg (13/706 (1.8%)—6/368 (1.6%) in the n-3 LCPUFA group and 7/338 (2.1%) in the control group; unadjusted relative risk 0.78, 0.26 to 2.29, P=0.65; adjusted relative risk 0.79, 0.27 to 2.30, P=0.66). The percentage of infants with non-immunoglobulin E associated allergic disease (without sensitisation) did not differ between groups (table 2). Web appendix B shows other infant allergic disease outcomes not presented in table 2 but analysed according to the statistical analysis plan. Respiratory manifestations of allergic disease were rare and did not differ between groups (web appendix B).
Table 2.
Clinical allergy assessment outcomes at 12 months of age.
| Outcome | n-3 LCPUFA* (n=368) | Control* (n=338) | Unadjusted relative risk (95% CI) | Unadjusted P value | Adjusted† relative risk (95% CI) | Adjusted† P value |
|---|---|---|---|---|---|---|
| Allergic disease with sensitisation: | 32 (9) | 43 (13) | 0.68 (0.43 to 1.05) | 0.08 | 0.70 (0.45 to 1.09) | 0.12 |
| Eczema with sensitisation | 26 (7) | 39 (12) | 0.61 (0.38 to 0.98) | 0.04 | 0.64 (0.40 to 1.03) | 0.06 |
| Food allergy with sensitisation | 11 (3) | 11 (3) | 0.94 (0.40 to 2.22) | 0.88 | 0.96 (0.41 to 2.25) | 0.93 |
| Sensitisation with/without allergic disease: | 53 (14) | 67 (20) | 0.73 (0.52 to 1.02) | 0.07 | 0.75 (0.53 to 1.04) | 0.08 |
| Egg sensitisation | 34 (9) | 52 (15) | 0.61 (0.40 to 0.91) | 0.02 | 0.62 (0.41 to 0.93) | 0.02 |
| Peanut sensitisation | 15 (4) | 22 (7) | 0.62 (0.33 to 1.18) | 0.15 | 0.63 (0.34 to 1.19) | 0.16 |
| Cows’ milk sensitisation‡ | 6 (2) | 3 (1) | 0.51 | |||
| Allergic disease without sensitisation | 63 (17) | 53 (16) | 1.10 (0.79 to 1.55) | 0.57 | 1.10 (0.79 to 1.55) | 0.57 |
LCPUFA=long chain polyunsaturated fatty acid.
*All data are based on analysis of 50 imputed datasets; values are numbers (percentages).
†Adjusted for centre, parity, maternal history, and sex.
‡Fisher’s exact test using original data; adjusted/imputed analyses not done owing to rarity of outcomes (n-3 LCPUFA group, n=349; control group, n=317).
Infants were most commonly sensitised to egg (12.2% of all infants). The percentage of infants introduced to egg by 1 year of age was 86% (315/368) in the n-3 LCPUFA group and 86% (292/338) in the control group. More infants were sensitised to egg in the control group than in the n-3 LCPUFA group (table 2), and we detected more infants in the control group who repeatedly spat out or refused to eat egg (18/338 (5%) v 9/368 (2%), P=0.06) (web appendix B). The overall rate of sensitisation to peanut was 5.2% and did not differ between groups (table 2). The frequency of sensitisation to all other allergens tested was less than 2% (tables 2 and 3).
Table 3.
Sensitisation to other allergen skin prick test extracts. Values are numbers (percentages) unless stated otherwise
| Extract | n-3 LCPUFA (n=349) | Control (n=317) | P value* |
|---|---|---|---|
| Wheat | 2 (0.6) | 2 (0.6) | >0.99 |
| Fish | 3 (0.9) | 0 (0.0) | 0.25 |
| Ryegrass pollen | 1 (0.3) | 1 (0.3) | >0.99 |
| Olive tree pollen | 0 (0.0) | 0 (0.0) | NA |
| Dermatophagoides pteronyssinus | 1 (0.3) | 1 (0.3) | >0.99 |
| Cat hair | 7 (2.0) | 5 (1.6) | 0.78 |
| Alternaria tenuis | 4 (1.2) | 1 (0.3) | 0.38 |
LCPUFA=long chain polyunsaturated fatty acid; NA=not applicable.
*Fisher’s exact test using original data; adjusted/imputed analyses not done owing to rarity of outcomes.
Other infant clinical outcomes
No anaphylactic reactions were reported. The percentage of infants with any hospital admissions during the first year of life (67/368 (18%) in n-3 LCPUFA group versus 74/338 (22%) in the control group; unadjusted relative risk 0.82, 0.61 to 1.11, P=0.20; adjusted relative risk 0.82, 0.61 to 1.11, P=0.20) and the total number of hospital admissions per child (mean 0.25 in the n-3 LCPUFA group versus 0.35 in the control group; unadjusted ratio of means 0.72, 0.50 to 1.03, P=0.08; adjusted ratio of means 0.73, 0.51 to 1.04, P=0.08) did not differ between groups. Similarly, we found no differences between the groups for the number of infants visiting a doctor because they were unwell (336/368 (91%) in the n-3 LCPUFA group versus 310/338 (92%) in the control group; unadjusted relative risk 1.00, 0.95 to 1.04, P=0.89; adjusted relative risk 1.0, 0.95 to 1.04, P=0.85) or the number of visits to a doctor per child (mean 4.55 in the n-3 LCPUFA group versus 4.28 in the control group; unadjusted ratio of means 1.06, 0.93 to 1.22, P=0.38; adjusted ratio of means 1.06, 0.93 to 1.22, P=0.38). The percentage of infants with respiratory tract infections (65/368 (18%) in the n-3 LCPUFA group versus 66/338 (20%) in the control group; unadjusted relative risk 0.90, 0.66 to 1.23, P=0.50; adjusted relative risk 0.91, 0.67 to 1.23, P=0.53) did not differ between groups. We also found no difference between the groups for the number of infants exposed to antibiotic treatment during the first 12 months of life (214/368 (58%) in the n-3 LCPUFA group versus 190/338 (56%) in the control group; unadjusted relative risk 1.03, 0.91 to 1.18, P=0.61; adjusted relative risk 1.04, 0.91 to 1.18, P=0.55). Ear infections were the most common reason for antibiotic use in infancy (99/368 (27%) in the n-3 LCPUFA group versus 84/338 (25%) in the control group; unadjusted relative risk 1.08, 0.84 to 1.39, P=0.53; adjusted relative risk 1.10, 0.86 to 1.41, P=0.44).
Possible confounding variables
Table 4 shows characteristics of infant feeding measured after randomisation. More infants in the n-3 LCPUFA group than in the control group were initially breast fed, whereas more infants in the control group than the n-3 LCPUFA group were given cows’ milk protein formula in the first six months of life (table 44). We found no differences between the groups for other possible confounding variables that may influence allergic disease, including the number of infants born by caesarean delivery (104/368 (28%) in the n-3 LCPUFA group compared with 98/338 (29%) in the control group, P=0.83), the age when eggs were introduced (median 8.0 (interquartile range 6.0-10.0) months for both groups, P=0.42), the age when fish was introduced (median 9.0 (7.0-10.0) months for both groups, P=0.90), the age when nuts were introduced (median 10.0 (8.0-11.0) months for both groups, P=0.65), the number of other children in the home (median 1.0 for both groups, P=0.56), and having a cat or dog as a pet (241/366 (66%) in the n-3 LCPUFA group compared with 208/334 (62%) in the control group, P=0.33).
Table 4.
Infant feeding related post-randomisation characteristics. Values are numbers (percentages) unless stated otherwise
| Characteristic | n-3 LCPUFA | Control | P value* |
|---|---|---|---|
| Initiation of breast feeding | 348/362 (96) | 303/333 (91) | 0.005 |
| Any breast feeding at 6 months of age | 171/362 (47) | 144/333 (43) | 0.29 |
| Ever fed formula | 326/367 (89) | 307/334 (92) | 0.17 |
| Median (interquartile range) age formula introduced (months) | 1.0 (0.0-4.0) | 1.0 (0.0-4.0) | 0.76 |
| Use of cows’ milk protein formula in first 6 months | 260/361 (72) | 265/332 (80) | 0.02 |
| Use of hydrolysed protein formula in first 6 months | 11/361 (3) | 8/332 (2) | 0.61 |
| Median (interquartile range) age solids introduced (months) | 5.0 (4.0-5.0) | 5.0 (4.0-5.0) | 0.053 |
LCPUFA=long chain polyunsaturated fatty acid.
*Groups compared using Fisher’s exact tests for binary outcomes and Wilcoxon tests for continuous outcomes.
Discussion
Our study showed that a prenatal dose of 900 mg/day of n-3 LCPUFA had no effect on the composite outcome of immunoglobulin E associated allergic disease, comprising eczema or food allergy, in the first year of life. However, treatment with n-3 LCPUFA during pregnancy reduced the percentage of infants with atopic eczema and reduced the percentage of infants with sensitisation to eggs. We did not detect any effect of n-3 LCPUFA supplementation during pregnancy on allergic disease without sensitisation (that is, non-immunoglobulin E associated allergy).
Comparison with other studies
Our observations that infants exposed to n-3 LCPUFA during pregnancy were less likely to develop atopic eczema and had a lower propensity for sensitisation are consistent with the postulate that the immunomodulatory effects of n-3 LCPUFA are mediated through mechanisms associated with the atopic (immunoglobulin E associated) responses.8 9 10 Additionally, our finding that fewer infants in the n-3 LCPUFA group were sensitised to egg compared with control is consistent with the findings of previous smaller trials by Dunstan et al and Furuhjelm et al.11 13 As many children with egg allergy are known to develop asthma and allergic respiratory disease,19 longer term follow-up of the children in our study is important to determine whether the lower rates of egg sensitisation in the n-3 LCPUFA group translate to lower aeroallergen sensitisation and fewer children with respiratory allergic diseases at a later time. Of interest are the findings by Olsen et al,17 who showed that n-3 LCPUFA supplementation during pregnancy reduced asthma in adolescence. However, the allergy outcomes from this trial were obtained through linkage to a national registry of doctors’ visits.17 The expected event rates in Olsen et al were low, and whether diagnoses were made according to standard definitions is not known.17 The planned allergy assessments of the children in our study at 3 and 6 years of age will be important in documenting the progression of allergic disease and whether n-3 LCPUFA supplementation during pregnancy has long term effects.
Strengths and limitations
Strengths of our study are the concealed randomisation, the large sample size, and the minimal rate of attrition. Furthermore, women included in our trial had comparable demographic characteristics to Australian women who gave birth in 2006-7,20 21 suggesting generalisability of the results to the wider population.
A limitation of the study is that we used a conservative definition to define food allergy. Infants were diagnosed as having food allergy only if they were sensitised and had a clear clinical history of an immediate allergic reaction to the offending food. We did not have resources to do oral food challenges for the infants with suspected food allergy (sensitisation to the offending food and a history of repeatedly spitting out or refusing to eat this food), and we may have underestimated the true frequency of food allergy, as may be demonstrated with egg allergy. Although only about 2% of infants had confirmed egg allergy in our trial (1.6% in the n-3 LCPUFA group and 2.1% in the control group), a further 4% (2.4% in the n-3 LCPUFA group and 5.3% in the control group) had a suspected egg allergy. A recent Australian cohort study estimated the prevalence of egg allergy to be 8.9% at 1 year of age on the basis of challenge with raw egg,22 which approximates the total confirmed and suspected egg allergy in our control group (7.4% v 4.1% in the n-3 LCPUFA group; web appendix B).
Another potential limitation of our study was the observation that the initiation of breast feeding and the use of cows’ milk protein formula in the first six months differed between the groups. As these were post-randomisation variables, we could not adjust for them in the statistical analyses; however, we found no relation between the initiation of breast feeding and atopic eczema or between breast feeding and egg sensitisation in exploratory analyses. Furthermore, very few infants (total of 9/706; 6/368 (2%) in the n-3 LCPUFA group and 3/338 (1%) in the control group) in this study were sensitised to cows’ milk protein, despite more infants in the control group having been fed cows’ milk based formula in the first six months of life. Three quarters of the infants in our trial had been exposed to cows’ milk based formula before 6 months of age, and 99% of infants had been introduced to cows’ milk by 12 months of age. Collectively, these data suggest that the small imbalance between groups for breast feeding and formula feeding in the first 6 months of life did not influence the outcomes of the trial.
Conclusions and policy implications
Many of the commercially available prenatal supplements provide low doses of n-3 LCPUFA (<300 mg/day compared with the 900 mg/day used in our trial) on the basis of consensus guidelines and recommendations that have largely focused on improving outcomes of pregnancy and enhancing developmental outcomes in early childhood.23 24 However, from an immunological standpoint, the doses available in many commercial supplements are unlikely to suppress the concentration of arachidonic acid in cord blood25; they are therefore unlikely to result in the changes in prostaglandins and cytokines associated with higher dose n-3 LCPUFA supplementation.26 27 28 Even with the higher dose supplementation used in our study, we did not show an overall reduction in immunoglobulin E associated allergic disease at 1 year of age, although our data suggest that infant atopic eczema and sensitisation to egg are reduced by n-3 LCPUFA supplementation during pregnancy. Clearly, further follow-up studies are needed to definitively determine whether prenatal n-3 LCPUFA supplementation is beneficial as an allergy prevention strategy.
What is already known on this topic
The prevalence of allergic diseases in industrialised countries is estimated to be at least 20%
The period of increase in allergic diseases has coincided with a substantial shift in dietary intake of fatty acids to favour n-6 fatty acids over n-3 fatty acids
Several mechanistic studies have suggested that higher intakes of n-3 long chain polyunsaturated fatty acids (LCPUFA) during pregnancy modulate the neonatal immune response
What this study adds
n-3 LCPUFA supplementation in pregnancy did not reduce the overall incidence of immunoglobulin E associated allergies in the first year of life, although atopic eczema and egg sensitisation were lower
Longer term follow-up of these children will determine the effectiveness of n-3 LCPUFA supplementation in pregnancy as a preventive strategy for allergic diseases
We thank the families who participated and the following investigators and research team members who supported the data collection: A Anderson, E Griffith, S Warcup, F Tan, D Tye, P Quinn, P Di Giovine, M O’Grady, C Oates, A Scott, W Helbers, N Naccarella, H Loudis, and M Crabb. We also acknowledge the support of the Docosahexaenoic Acid to Optimise Mother Infant Outcome (DOMInO) Trial Steering Committee: M Makrides, R A Gibson, A J McPhee, L Yelland, J Quinlivan, and P Ryan.
Contributors: MM, MSG, SLP, and RAG were responsible for the study concept and design. DP, MM, TS, MSG, SLP, and RH were involved in acquisition of data. All authors analysed and interpreted data. DP, MM, and TS drafted the manuscript. All authors revised the manuscript critically for important intellectual content. DP, MM, and TS did the statistical analysis. MM, MSG, SLP, RAG, and DP were involved in obtaining funding. DP, MM, MSG, and RH supervised the study. MM is the guarantor.
Funding: The study was supported by grants from the Australian National Health and Medical Research Council (ID 399389) and Australian Egg Corporation Limited. Treatment and placebo capsules were donated by Efamol, UK.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: all authors had support from the National Health and Medical Research Council (NHMRC) of Australia and the Australian Egg Corporation for the submitted work; MSG has received honorariums from Nutricia; SLP has received grants from the NHMRC and honorariums from the Nestlé Nutrition Institute and Fonterra and has been paid for lectures by the World Allergy Association and the American Academy of Asthma, Allergy and Immunology; RH has been paid for expert testimony from Analysis Plus and Rodika Research Services and has received grants from Commonwealth Serum Laboratories, Vaxine, GlaxoSmithKline, and Healthed; RAG has received grants from the NHMRC and honorariums from Fonterra; MM has received grants from the NHMRC and honorariums from Fonterra, Nestlé Nutrition Institute, and Nutricia.
Ethical approval: Approval was granted by the human research ethics committees of each centre—Women’s and Children’s Hospital, Adelaide (REC 1657) and Flinders Medical Centre, Adelaide (REC 199045).
Data sharing: No additional data available.
Cite this as: BMJ 2012;344:e184
Web Extra. Extra material supplied by the author
Appendix A
Appendix B
References
- 1.Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368:733-43. [DOI] [PubMed] [Google Scholar]
- 2.International Study of Asthma and Allergies in Childhood Steering Committee (ISSAC). Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998;351:1225-32. [PubMed] [Google Scholar]
- 3.Robertson CF, Roberts MF, Kappers JH. Asthma prevalence in Melbourne schoolchildren: have we reached the peak? Med J Aust 2004;180:273-6. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clin Exp Allergy 2004;34:520-6. [DOI] [PubMed] [Google Scholar]
- 5.Kemp AS. Atopic eczema: its social and financial costs. J Paediatr Child Health 1999;35:229-31. [DOI] [PubMed] [Google Scholar]
- 6.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet 1995;346:1065-9. [DOI] [PubMed] [Google Scholar]
- 7.Black PN, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J 1997;10:6-12. [DOI] [PubMed] [Google Scholar]
- 8.Prescott SL, Calder PC. N-3 polyunsaturated fatty acids and allergic disease. Curr Opin Clin Nutr Metab Care 2004;7:123-9. [DOI] [PubMed] [Google Scholar]
- 9.Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. Fatty acids and lymphocyte functions. Br J Nutr 2002;87(suppl 1):S31-48. [DOI] [PubMed] [Google Scholar]
- 10.Gottrand F. Long-chain polyunsaturated fatty acids influence the immune system of infants. J Nutr 2008;138:1807-12S. [DOI] [PubMed] [Google Scholar]
- 11.Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 2003;112:1178-84. [DOI] [PubMed] [Google Scholar]
- 12.Krauss-Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Del Carmen Ramirez-Tortosa M, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol 2008;121:464-70,e6. [DOI] [PubMed] [Google Scholar]
- 13.Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 2009;98:1461-7. [DOI] [PubMed] [Google Scholar]
- 14.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 2010;304:1675-83. [DOI] [PubMed] [Google Scholar]
- 15.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PR. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003;38:391-8. [DOI] [PubMed] [Google Scholar]
- 16.Hanifin JM, Rijka G. Diagnostic features of atopic dermatitis. Acta Dermatovener (Stockholm) Suppl 1980;92:44-7. [Google Scholar]
- 17.Olsen SF, Osterdal ML, Salvig JD, Mortensen LM, Rytter D, Secher NJ, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr 2008;88:167-75. [DOI] [PubMed] [Google Scholar]
- 18.Rubin D. Multiple imputation for nonresponse in surveys. John Wiley and Sons, 1987.
- 19.Tariq SM, Matthews SM, Hakim EA, Arshad SH. Egg allergy in infancy predicts respiratory allergic disease by 4 years of age. Pediatr Allergy Immunol 2000;11:162-7. [DOI] [PubMed] [Google Scholar]
- 20.Chan A, Scott J, Nguyen A-M, Green P. Pregnancy outcome in South Australia 2007. Pregnancy Outcome Unit, Epidemiology Branch, Department of Health, 2008.
- 21.Chan A, Scott J, Nguyen A-M, Green P. Pregnancy outcome in South Australia 2006. Pregnancy Outcome Unit, Epidemiology Branch, Department of Health, 2007.
- 22.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol 2011;127:668-76,e1-2. [DOI] [PubMed] [Google Scholar]
- 23.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab 2009;55:97-122. [DOI] [PubMed] [Google Scholar]
- 24.Koletzko B, Cetin I, Brenna JT, for the Perinatal Lipid Intake Working Group. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98:873-7. [DOI] [PubMed] [Google Scholar]
- 25.Smuts CM, Borod E, Peeples JM, Carlson SE. High-DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids 2003;38:407-14. [DOI] [PubMed] [Google Scholar]
- 26.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor α and interleukin 1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996;63:116-22. [DOI] [PubMed] [Google Scholar]
- 27.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 2000;71:343-8S. [DOI] [PubMed] [Google Scholar]
- 28.Mantzioris E, Cleland LG, Gibson RA, Neumann MA, Demasi M, James MJ. Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr 2000;72:42-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A
Appendix B