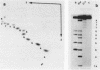
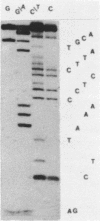
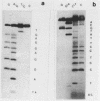
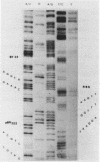
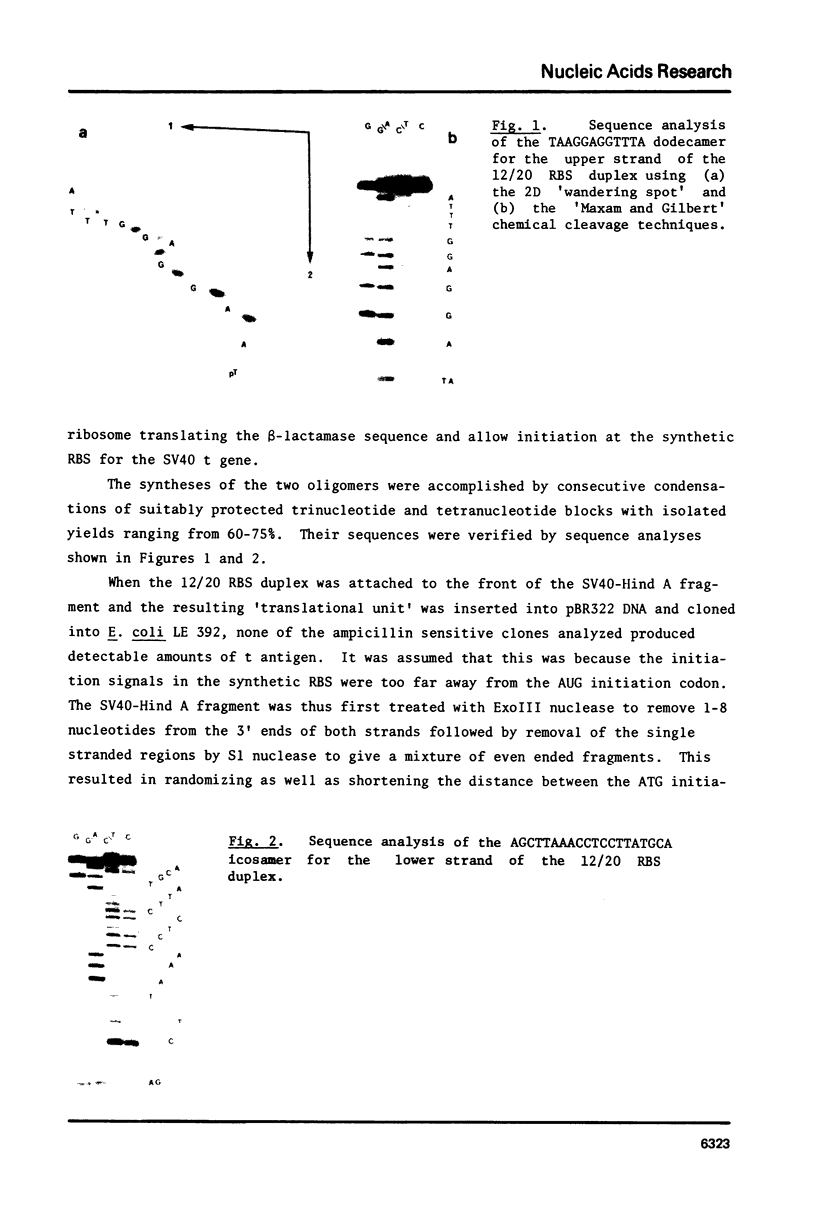
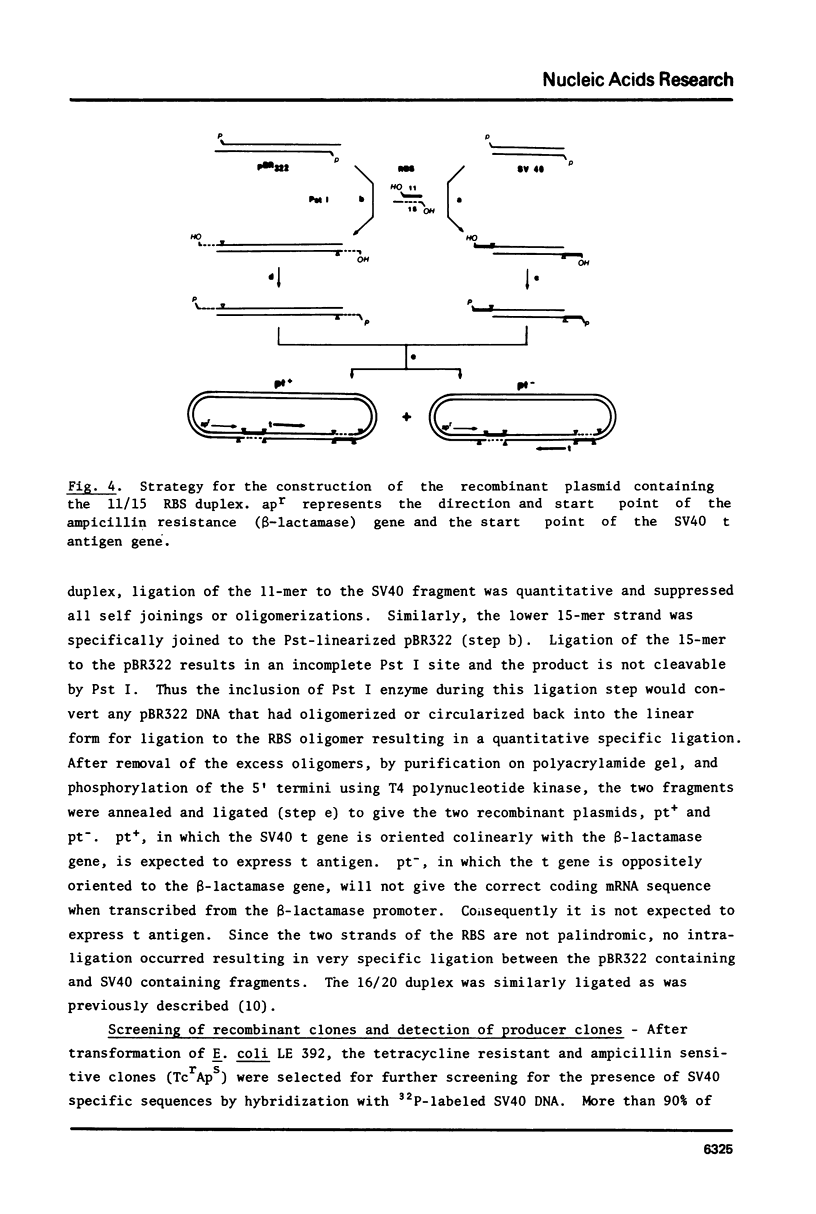
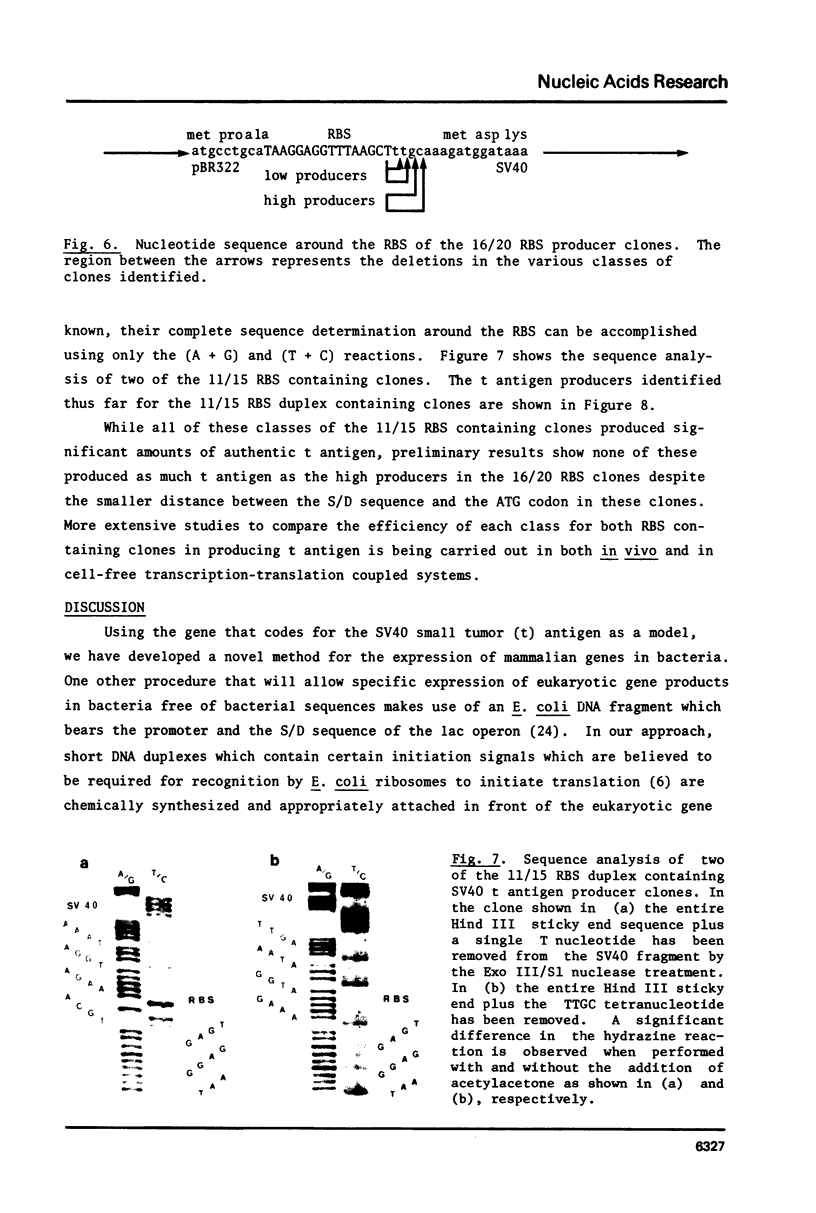
Abstract
Mammalian genes, when inserted into bacterial plasmid or phage DNAs, will not be expressed into the corresponding specific proteins in E. coli unless proper initiations signals required for recognition by E. coli ribosomes are provided. We have studied these signals and chemically synthesized two DNA duplexes each containing different initiation signals. These have been inserted in front of the Simian virus 40 (SV40) small tumor antigen gene (SV40 t gene) at varying distances from the ATG initiation codon prior to its cloning into pBR322 plasmid DNA. Plasmid containing clones carrying either of these two synthetic ribosome binding sites (RBS) at varying distances from the SV40 t gene all produced a 17K protein identical to authentic t antigen by immunologic, electrophoretic and proteolytic digestion analyses. This provides a novel method to ensure the specific expression of any contiguous mammalian gene to be cloned to bacteria, and also a unique in vivo method for studying the structure-function (efficiency) relationship of RBS with specific base changes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baralle F. E., Brownlee G. G. AUG is the only recognisable signal sequence in the 5' non-coding regions of eukaryotic mRNA. Nature. 1978 Jul 6;274(5666):84–87. doi: 10.1038/274084a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M., Gros F. Initiation mechanisms of protein syntehesis. Prog Nucleic Acid Res Mol Biol. 1977;20:209–284. doi: 10.1016/s0079-6603(08)60474-2. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Narang S. A., Bahl C. P., Marians K. J., Wu R. Chemical synthesis and sequence studies of deoxyribooligonucleotides which constitute the duplex sequence of the lactose operator of Escherichia coli. J Biol Chem. 1975 Jun 25;250(12):4592–4600. [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Seth A. K., Jay G. Specific binding of a chemically synthesized prokaryotic ribosome recognition site. Prospect for molecular cloning and expression of eukaryotic genes. J Biol Chem. 1980 May 10;255(9):3809–3812. [PubMed] [Google Scholar]
- Jay G., Khoury G., Seth A. K., Jay E. Construction of a general vector for efficient expression of mammalian proteins in bacteria: use of a synthetic ribosome binding site. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5543–5548. doi: 10.1073/pnas.78.9.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Narang S. A., Brousseau R., Hsiung H. M., Michniewicz J. J. Chemical synthesis of deoxyoligonucleotides by the modified triester method. Methods Enzymol. 1980;65(1):610–620. doi: 10.1016/s0076-6879(80)65063-0. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Determination of a nucleotide sequence in bacteriophage f1 DNA by primed synthesis with DNA polymerase. J Mol Biol. 1974 Dec 5;90(2):315–333. doi: 10.1016/0022-2836(74)90376-3. [DOI] [PubMed] [Google Scholar]
- Scherer G. F., Walkinshaw M. D., Arnott S., Morré D. J. The ribosome binding sites recognized by E. coli ribosomes have regions with signal character in both the leader and protein coding segments. Nucleic Acids Res. 1980 Sep 11;8(17):3895–3907. doi: 10.1093/nar/8.17.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A. K., Jay E. A study of the efficiency and the problem of sulfonation of several condensing reagents and their mechanisms for the chemical synthesis of deoxyoligoribonucleotides. Nucleic Acids Res. 1980 Nov 25;8(22):5445–5459. doi: 10.1093/nar/8.22.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Guarente L., Roberts T. M., Kimelman D., Douhan J., 3rd, Ptashne M. Expression of the human fibroblast interferon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5230–5233. doi: 10.1073/pnas.77.9.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]