Abstract
Rationale: Bronchial thermoplasty (BT) is a bronchoscopic procedure in which controlled thermal energy is applied to the airway wall to decrease smooth muscle.
Objectives: To evaluate the effectiveness and safety of BT versus a sham procedure in subjects with severe asthma who remain symptomatic despite treatment with high-dose inhaled corticosteroids and long-acting β2-agonists.
Methods: A total of 288 adult subjects (Intent-to-Treat [ITT]) randomized to BT or sham control underwent three bronchoscopy procedures. Primary outcome was the difference in Asthma Quality of Life Questionnaire (AQLQ) scores from baseline to average of 6, 9, and 12 months (integrated AQLQ). Adverse events and health care use were collected to assess safety. Statistical design and analysis of the primary endpoint was Bayesian. Target posterior probability of superiority (PPS) of BT over sham was 95%, except for the primary endpoint (96.4%).
Measurements and Main Results: The improvement from baseline in the integrated AQLQ score was superior in the BT group compared with sham (BT, 1.35 ± 1.10; sham, 1.16 ± 1.23 [PPS, 96.0% ITT and 97.9% per protocol]). Seventy-nine percent of BT and 64% of sham subjects achieved changes in AQLQ of 0.5 or greater (PPS, 99.6%). Six percent more BT subjects were hospitalized in the treatment period (up to 6 wk after BT). In the posttreatment period (6–52 wk after BT), the BT group experienced fewer severe exacerbations, emergency department (ED) visits, and days missed from work/school compared with the sham group (PPS, 95.5, 99.9, and 99.3%, respectively).
Conclusions: BT in subjects with severe asthma improves asthma-specific quality of life with a reduction in severe exacerbations and healthcare use in the posttreatment period.
Clinical trial registered with www.clinialtrials.gov (NCT00231114).
Keywords: asthma, Alair Bronchial Thermoplasty System, bronchial thermoplasty, bronchoscopic procedure, Asthma Quality of Life
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Bronchial thermoplasty is a novel intervention for asthma that delivers controlled thermal energy to the airway wall during a series of bronchoscopy procedures.
What This Study Adds to the Field
The current study reflects one of the largest sham-controlled trials in pulmonary medicine to test a novel device to perform bronchial thermoplasty, a procedure for the treatment of severe asthma. Bronchial thermoplasty improves asthma-specific quality of life and decreases severe exacerbations in patients with severe asthma.
Bronchial thermoplasty (BT) is a novel intervention for asthma that delivers controlled thermal energy to the airway wall during a series of bronchoscopy procedures, resulting in a prolonged reduction in airway smooth muscle (ASM) mass (1, 2). Increased mass and contractility of ASM augments asthma morbidity by causing greater bronchoconstriction and airflow obstruction (3). Decreasing the amount and/or contractility of ASM may provide a means to ameliorate the symptoms of asthma.
Previous clinical trials of BT were nonrandomized (4) or randomized to include a standard of care control group (5, 6). In these initial studies, BT was associated with a decrease in the long-term rate of mild asthma exacerbations, improvement in asthma control, and improvements in lung function (4–6). To mitigate an expected placebo effect stemming from an interventional procedure, a randomized, sham-controlled trial was conducted (Asthma Intervention Research [AIR2] Trial) that examined the effectiveness and safety of BT for the treatment of severe persistent asthma in adults. To our knowledge, this is the largest sham-controlled trial to test a new device for the treatment of severe asthma in adults. Data from this trial have been previously published in abstract form (7–10).
METHODS
Study Subjects
Eligible subjects were adults (18–65 years of age) diagnosed with asthma who required regular maintenance medications of inhaled corticosteroids (ICS >1,000 μg/d beclomethasone or equivalent) and a long-acting β2-agonist (LABA ≥100 μg/d salmeterol or equivalent). Other medications were allowed, including leukotriene modifiers, omalizumab (if used for at least 1 year prior), and oral corticosteroids (OCS) 10 mg/d or less. Key inclusion criteria were: subjects on stable maintenance asthma medications for at least 4 weeks before entry, baseline Asthma Quality of Life Questionnaire (AQLQ) score 6.25 or lower (a higher AQLQ score represents better quality of life) (11), prebronchodilator FEV1 ≥60% of predicted, airway hyperresponsiveness (methacholine PC20 <8 mg/ml), at least 2 days of asthma symptoms during the 4-week baseline period, and being a nonsmoker for at least 1 year with less than 10 pack-years smoking history. Key exclusion criteria were: life-threatening asthma; chronic sinus disease; respiratory diseases such as emphysema; use of immunosuppressants, β-adrenergic blocking agents, or anticoagulants; and history in the previous year of three or more hospitalizations for asthma, three or more lower respiratory tract infections, and four or more pulses of OCS use for asthma.
Study Design
This randomized, double-blind, sham-controlled, clinical trial was conducted at 30 investigational sites in six countries and was approved by the respective Ethics Committee at each site. All participating subjects provided written informed consent. Enrollment began in October 2005, and a 12-month follow-up of the last subject was completed in July 2008. An independent Data and Safety Monitoring Board oversaw the study.
An electronic diary (LogPad; PHT Corp., Charlestown, MA) was used to record daytime and nighttime asthma symptoms, peak expiratory flow (PEF), and rescue medication use. Subjects' ability to comply with the use of a peak flow meter and completion of the daily diary was assessed in the first week. Compliant subjects used the diary to collect baseline data over 4 weeks.
Randomization
Eligible subjects were randomized (2:1) to the BT or the sham group according to a computer-generated scheme stratified by baseline AQLQ, percentage of symptom-free days, and site, using the Minimum Dynamic Allocation method (12). The treatment assignment that yielded the least imbalance between groups was given a 90% probability of being chosen for each subject if groups had an imbalance or 50% probability if both groups were balanced at the time of randomization.
Treatment
All randomized subjects were scheduled to undergo three bronchoscopy procedures performed 3 weeks apart. The treatment was administered by an unblinded bronchoscopy team. All follow-up and assessment visits were conducted by a blinded assessment team. Thus, neither the subject nor the assessor was aware of the individual treatment assignment. BT was performed by delivering radiofrequency (RF) energy to the airway using the Alair Bronchial Thermoplasty System (Asthmatx Inc., Sunnyvale, CA) as previously described (4, 13). Control group subjects in this study underwent three sham bronchoscopy procedures, each separated by at least 3 weeks. The sham bronchoscopy procedures involved necessary medication for conscious sedation and bronchoscopy that mimicked the BT treatment. The Alair catheter was deployed into the airways through the bronchoscope, the electrode array expanded, and the sham RF controller activated. The sham RF controller produced audio and visual signals that were indistinguishable from the active RF controller, except that no RF energy was delivered. The duration of each bronchoscopy procedure and the number of “sham” activations were to match an active treatment procedure.
Follow-up
Subjects were evaluated 6 weeks after the last procedure (at the end of the treatment period). The posttreatment period extended from 6 to 52 weeks after the last procedure, and assessments were completed at 3, 6, 9, and 12 months. Subjects completed their daily diary from baseline to 12 weeks after the last procedure and over 4-week periods preceding the 6- and 12-month follow-up visits. The following assessments were performed: AQLQ, Asthma Control Questionnaire (ACQ) (14), physical examination, review of asthma symptoms, exacerbations, asthma medications, and active solicitation of adverse events, including healthcare use.
Outcome Measures
The primary outcome was the difference between study groups in the AQLQ score change from baseline to the average of the 6-, 9-, and 12-month scores (integrated AQLQ). The proportion of subjects within each group that achieved an AQLQ score change of 0.5 or greater (i.e., minimal important difference) was analyzed (15).
Secondary outcomes included changes in: AQLQ (absolute and individual domains), ACQ scores, percentage of symptom-free days, symptom scores, morning PEF, rescue medication use, and FEV1. Additional outcomes included the numbers of severe asthma exacerbations (i.e., those requiring systemic corticosteroids or doubling of ICS dose) (16), the percentage of subjects experiencing severe exacerbations, respiratory-related unscheduled physician office visits, emergency department (ED) visits, hospitalizations, and days missed from work/school or other activities due to asthma.
Monitoring Adverse Events
Adverse events were actively solicited and recorded at each visit. Investigators reported severity for all events in the treatment and posttreatment periods.
Statistical Analyses
The target enrollment goal was a minimum of 225 evaluable subjects (150 in the BT group and 75 in the sham group). All endpoints were analyzed using Bayesian statistics. Bayesian statistics is an axiomatic approach that provides the probability of hypotheses conditional on observed data rather than the traditional approach of calculating the probability of data conditional on hypotheses. The posterior probability is a central measure of uncertainty within the Bayesian approach and is used to quantify the strength of the evidence regarding hypotheses, such as the probability of superiority, which is used in this study. The target posterior probability of superiority (PPS) of BT over sham was 95%, except for the primary AQLQ endpoint, where the target PPS was 96.4% (adjusted for two interim looks for early declaration of success). Bayesian imputation methods were used to handle missing data in the primary effectiveness analysis, and baseline AQLQ was used as a covariate. Missing data for secondary endpoints were imputed using the Last Observation Carried Forward method. Unless otherwise stated, results are reported as mean ± SD.
Effectiveness analyses were performed on the intent-to-treat (ITT: all randomized subjects who underwent at least one bronchoscopy) and prespecified per protocol (PP: all randomized subjects who completed all three bronchoscopy procedures, did not take interfering concomitant medications, or missed any follow-up visits at 6, 9, or 12 month) populations. Univariate logistic regression was used to investigate which baseline variables were statistically significant predictors of AQLQ response (responder/nonresponder) within the BT-treated group. The method of Bang and colleagues (17) was used to assess the success of blinding in each of the treatment groups (i.e., the ability of subjects in each group to identify their treatment assignment with greater accuracy than random guessing alone), with P < 0.05 indicating statistical significance.
Safety was assessed by reviewing all adverse events occurring during the treatment and posttreatment periods. Bayesian posterior probabilities of superiority were estimated for incidence rates of adverse events, and those events with superiority of greater than 95% in any group were reported. Event rates were analyzed using Poisson Regression in the Bayesian model.
RESULTS
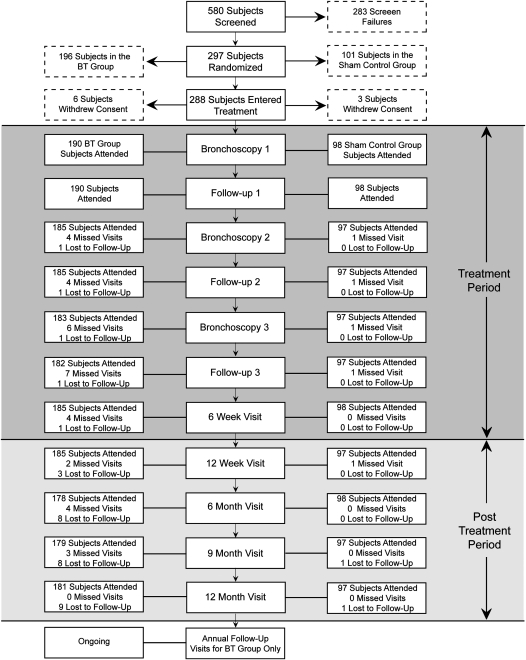
Of 580 subjects screened, 297 were randomized to the BT group (196 subjects) or the sham control group (101 subjects) (Figure 1). Of these, 190 subjects in the BT group and 98 subjects in the sham group underwent at least one bronchoscopy (ITT population). Baseline demographics and clinical characteristics were well matched between groups (Table 1). The subjects enrolled in this trial had severe and inadequately controlled asthma as evidenced by the requirement for high-dose ICS and LABA, a high ACQ score consistent with poorly controlled asthma (18), and a low AQLQ score and percentage of symptom-free days. Analysis of the characteristics of these subjects demonstrated that 86% of the BT group (163 subjects) and 88% of the sham control group (86 subjects) met American Thoracic Society criteria for severe refractory asthma (19). Ten subjects were lost to follow-up (nine Alair, one sham).
Figure 1.
Disposition of AIR2 subjects. Of the 288 subjects who underwent a bronchoscopy procedure, 190 were randomized to the bronchial thermoplasty (BT) group, and 98 were randomized to the sham control group. All 288 subjects qualified for the intent-to-treat and safety populations. Additionally, 268 subjects (173 in the BT group and 95 in the sham control group) qualified for inclusion in the per protocol population.
TABLE 1.
SUBJECT DEMOGRAPHICS AND BASELINE CHARACTERISTICS (ITT POPULATION)
| BT (n = 190)* | Sham (n = 98)* | |
|---|---|---|
| Age (years) | 40.7 ± 11.89 | 40.6 ± 11.85 |
| Sex, n (%) | ||
| Male | 81 (42.6) | 38 (38.8) |
| Female | 109 (57.4) | 60 (61.2) |
| Race/Ethnicity, n (%) | ||
| White | 151 (79.5) | 72 (73.5) |
| African American/Black | 19 (10.0) | 15 (15.3) |
| Other | 20 (10.5) | 11 (11.2) |
| Methacholine PC20 (mg/ml) | ||
| Geometric mean | 0.27 (n=178) | 0.31 (n=94) |
| 95% Confidence interval bounds | (0.22, 0.34) | (0.22, 0.43) |
| Prebronchodilator FEV1 (% predicted) | 77.8 ± 15.65 | 79.7 ± 15.14 |
| Inhaled corticosteroid dose† (μg/d), mean (median) | 1960.7 (2,000) | 1834.8 (2,000) |
| Long-acting β2-agonist dose‡ (μg/day) | 116.8 ± 34.39 (n=189) | 110.3 ± 26.70 (n=97) |
| AQLQ baseline score | 4.30 ± 1.17 | 4.32 ± 1.21 |
| Percent symptom-free days§ | 16.4 ± 24.04 | 16.8 ± 23.10 |
| Number and percentage of subjects on other asthma maintenance medications | ||
| Oral corticosteroids | 7 (3.7) | 1 (1.0) |
| Methylxanthines | 6 (3.2) | 5 (5.1) |
| Leukotriene modifiers | 47 (24.7) | 18 (18.4) |
| Omalizumab | 2 (1.1) | 3 (3.1) |
| Other | 15 (7.9) | 9 (9.2) |
| Any of the above maintenance medications | 59 (31.1) | 25 (25.5) |
| Oral corticosteroids dose (mg/d) | 6.4 ± 1.97 (n = 7) | 5.0 (n = 1) |
Definition of abbreviations: AQLQ = Asthma Quality of Life Questionnaire; BT = Bronchial thermoplasty; ITT = intent-to-treat.
Values are mean ± SD
Sample size for all variables unless otherwise stated.
Beclomethasone or equivalent
Salmeterol or equivalent
Percentage of symptom-free days indicates the percentage of days with no night awakenings and each individual symptom score was zero.
The performance of the bronchoscopic procedures did not allow for the subjects to be unblinded, as evidenced by the inability of the subjects in the BT or the sham group to correctly guess their treatment assignment after the first procedure (within-group comparison P values: BT, 0.135; sham, 0.128). During subsequent assessments, subjects in the sham group could not correctly guess their treatment assignment throughout the follow-up period. In the BT group, a larger proportion correctly guessed their treatment assignment after the first bronchoscopy (e.g., within-group comparison P values at second bronchoscopy: BT, 0.011; sham, 0.342).
Asthma Quality of Life
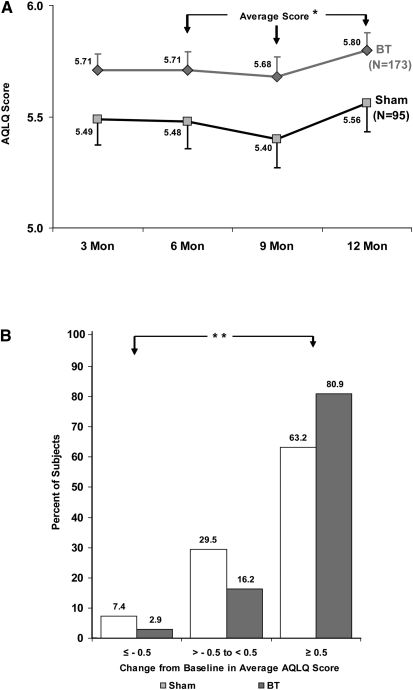
The mean change in integrated AQLQ score in the ITT population was greater in the BT group (1.35 ± 1.10) than in the sham group (1.16 ± 1.23; PPS, 96.0%; Table 2). The mean change in integrated AQLQ score in the PP population was 1.38 ± 1.10 in the BT group and 1.14 ± 1.24 in the sham group (PPS, 97.9%; Figure 2A). In the ITT population, a larger proportion of subjects in the BT group (79%) compared with the sham group (64%) had a clinically meaningful improvement in AQLQ score of 0.5 or greater (PPS, 99.6%). A smaller proportion of subjects in the BT group (3%) had a clinically meaningful deterioration in AQLQ of −0.5 or less compared with the sham group (7%). The net benefit in AQLQ in this study was 76% (79–3%) in the BT group versus 57% (64–7%) in the sham group (PPS, 100.0%). In the PP population, 81% of subjects in the BT group, compared with 63% subjects in the sham group, had a clinically meaningful improvement in AQLQ score of 0.5 or greater (PPS, 99.9%). A smaller proportion of subjects in the BT group (3%) had a clinically meaningful deterioration in AQLQ of −0.5 or less compared with the sham group (7%). The net benefit in AQLQ in this study was 78% (81–3%) in the BT group versus 56% (63–7%) in the sham group (PPS, 100.0%; Figure 2B).
TABLE 2.
EFFECTIVENESS OUTCOMES
| Baseline |
12 Month |
Posterior Probability of Superiority | |||
|---|---|---|---|---|---|
| BT (n = 190) | Sham (n = 98) | BT (n = 190) | Sham (n = 98) | ||
| Primary effectiveness endpoint | |||||
| AQLQ | 4.30 ± 1.17 | 4.32 ± 1.21 | 5.66 ± 1.06* | 5.48 + 1.15* | |
| Change from baseline | — | — | 1.35 ± 1.10 | 1.16 ± 1.23 | 0.960 |
| AQLQ responder analysis | |||||
| Percent of subjects with AQLQ change ≥0.5 | — | — | 78.9% | 64.3% | 0.996 |
| Secondary effectiveness endpoints | |||||
| AQLQ symptoms domain | 4.38 ± 1.20 | 4.39 ± 1.29 | 5.64 ± 1.04* | 5.49 ± 1.11* | 0.863 |
| AQLQ activity limitations domain | 4.54 ± 1.18 | 4.53 ± 1.21 | 5.79 ± 1.08* | 5.60 ± 1.21* | 0.900 |
| AQLQ emotional functions domain | 3.89 ± 1.51 | 3.99 ± 1.71 | 5.59 ± 1.28* | 5.38 ± 1.48* | 0.950 |
| AQLQ environmental stimuli domain | 3.94 ± 1.52 | 3.95 ± 1.64 | 5.41 ± 1.33* | 5.24 ± 1.42* | 0.856 |
| ACQ | 2.13 ± 0.87 | 2.09 ± 0.90 | 1.31 ± 0.94 | 1.32 ± 0.91 | |
| Change from baseline | — | — | −0.82 ± 0.95 | −0.77 ± 1.08 | 0.638 |
| FEV1 Pre-BD, % predicted | 77.8 ± 15.65 | 79.7 ± 15.14 | 76.6 ± 17.74 | 79.1 ± 15.98 | 0.241 |
| FEV1 Post-BD, % predicted | 86.1 ± 15.76 | 87.4 ± 13.18 | 83.4 ± 16.36 | 85.2 ± 14.13 | 0.371 |
| amPEF (L/min) | 383.8 ± 104.32 | 386.3 ± 112.59 | 411.6 ± 110.45 | 408.7 ± 117.56 | 0.806 |
| Total symptom score† | 3.8 ± 2.34 | 3.9 ± 2.53 | 2.1 ± 2.22 | 2.3 ± 2.17 | 0.637 |
| Percent symptom-free days‡ | 16.4 ± 24.04 | 16.8 ± 23.10 | 40.8 ± 38.22 | 37.9 ± 36.95 | 0.776 |
| Rescue medication use (puffs/7 days) | 13.4 ± 19.17 | 11.8 ± 11.24 | 7.4 ± 15.01 | 7.5 ± 12.60 | 0.813 |
| % Days rescue medication used | 52.1 ± 36.48 | 51.8 ± 35.41 | 28.0 ± 36.09 | 29.8 ± 34.96 | 0.680 |
| Severe exacerbations§ (exacerbations/subject/year) | 0.48 ± 0.067 | 0.70 ± 0.122 | 0.955 | ||
| Days lost from work/school/other activities due to asthma | 1.315 ± 0.361 | 3.915 ± 1.553 | 0.993 | ||
Definition of abbreviations: ACQ = Asthma Control Questionnaire; AQLQ = Asthma Quality of Life Questionnaire, BD = bronchodilator, BT = bronchial thermoplasty, amPEF = morning peak expiratory flow.
Values reported as mean ± SD.
Average of the value at 6-, 9-, and 12-months.
The total symptom score comprises the sum of these six asthma symptom measurements recorded in the daily diary: wheeze during the night, cough during the night, wheeze during the day, cough during the day, breathlessness during the day, and sputum production during the day. Each of the symptoms is scored on a scale of 0 to 30 each day by the subject. The sum of the scores for these 6 symptoms comprises the total symptom score, which measures overall asthma symptoms. The maximum score possible is 18. A lower total symptom score represents better asthma control.
% symptom free days was the percent of days in which there were no night awakenings and each individual symptom score was 0.
Severe exacerbation defined as exacerbation requiring treatment with systemic corticosteroids or doubling of the ICS dose.
Figure 2.
Change in asthma quality of life by treatment group. (A) Change in Asthma Quality of Life Questionnaire (AQLQ) score over 12 months after treatment with bronchial thermoplasty (BT) (diamonds) or sham control (squares) in the per protocol population. *Posterior probability of superiority = 97.9%. (B) Percentage of subjects achieving an AQLQ score change of 0.5 or greater (the minimal important difference), −0.05 to less than 0.5, and −0.5 after treatment with BT (blue) or sham control (gray) in the per protocol population. **Posterior probability of superiority = 100.0% for “Net” benefit ([proportion improving–proportion deteriorating in the BT group] – [proportion improving–proportion deteriorating in the sham group]).
Analysis of BT subjects suggested that responders, as defined by AQLQ score change of 0.5 or greater, had lower baseline AQLQ scores (responders: 4.1 ± 1.1 [n = 150] vs. nonresponders: 5.1 ± 1.1 [n = 40]; P < 0.001) and higher ACQ scores (responders: 2.2 ± 0.9 [n = 150] vs. nonresponders: 1.9 ± 0.8 [n = 40]; P = 0.041).
Other Asthma Measures (Using ITT Population)
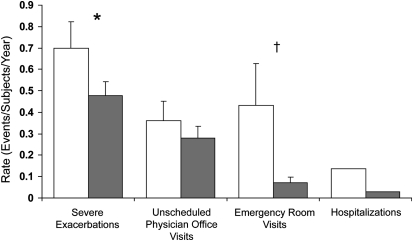
During the posttreatment period, there was a 32% reduction in the rate of severe exacerbations in the BT group (94% OCS, 6% double ICS use) compared with the sham group (92% OCS, 8% double ICS use) (0.48 vs. 0.70 exacerbations/subject/yr, respectively; PPS, 95.5%; Figure 3). Of the BT subjects, 26.3% (50/190) experienced severe exacerbations, compared with 39.8% (39/98) of sham subjects (PPS 99.0%). In the posttreatment period, subjects in the BT group reported fewer days lost from work/school or other activities due to asthma (1.32 ± 0.36 d/yr) compared with sham (3.92 ± 1.55 d/yr; PPS, 99.3%).
Figure 3.
Healthcare utilization events during the posttreatment period. Severe exacerbations (exacerbation requiring treatment with systemic corticosteroids or doubling of the inhaled corticosteroids dose), emergency department visits, and hospitalizations occurring in the posttreatment period. Open bars, sham; shaded bars, bronchial thermoplasty. All values are means ± SEM. *Posterior probability of superiority = 95.5%. †Posterior probability of superiority = 99.9%.
Secondary endpoint measures of morning PEF, symptom-free days, symptom score, ACQ, and rescue medication use (Table 2) showed an improvement over baseline in the BT and sham groups, although the differences between the groups were not statistically significant (PPS, ≤95.0%). Each of the four individual domains of AQLQ showed improvement in the BT group compared with sham (Table 2), although statistical significance was reached only for the emotional function domain.
Adverse Events
During the treatment period, both groups experienced an increase in respiratory adverse events, with more events reported in the BT (85% of subjects; 1.0 events/bronchoscopy) than in the sham group (76% of subjects; 0.7 events/bronchoscopy). The severity of respiratory adverse events for the BT and sham groups was as follows: mild, 43.6 versus 58.7%; moderate, 53.2 versus 39.8%; and severe, 3.1 versus 1.5%, respectively. The most common events were typical of airway irritation, including worsening asthma symptoms (wheezing, chest discomfort, cough, and chest pain), and upper respiratory tract infections. The majority of respiratory adverse events occurred within 1 day of the bronchoscopy and resolved within 7 days. During the treatment period, 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms (worsening of asthma, 12 in 10 subjects; segmental atelectasis, 3 in 2 subjects; lower respiratory tract infection, one subject; low FEV1, one subject; hemoptysis, one subject; and aspirated prosthetic tooth; one subject) compared with two subjects (2.0%) in the sham group requiring two hospitalizations (both worsening of asthma). Ten of the 19 hospitalizations in the BT group occurred on the day of the procedure. All these events resolved with standard therapy, including the hemoptysis, which was managed with bronchial artery embolization.
During the posttreatment period, fewer adverse respiratory events were reported in the BT group (70% of subjects vs. 80% in the sham group). There was a 36% risk reduction in the proportion of subjects reporting worsening of asthma (multiple symptoms) in the BT group than in the sham group (27.3 vs. 42.9%, respectively; PPS 99.7%). Consistent with this improvement in asthma, there was an 84% risk reduction in ED visits for respiratory symptoms in the BT group compared with sham group (0.07 vs. 0.43 visits/subject/yr; PPS 99.9%; Figure 3). Five subjects (2.6%) in the BT group had a total of six hospitalizations for respiratory symptoms (one subject had two hospitalizations), compared with 12 hospitalizations in four subjects (4.1%) in the sham group (one subject had nine hospitalizations).
The rate of upper and lower respiratory tract infections requiring antibiotics was 0.007 ± 0.014 events/subject/wk (24.1% of subjects) in the BT group and 0.006 ± 0.012 events/subject/wk (24.5% of subjects) in the sham group. The adverse event profile of the 10 subjects lost to follow-up was not remarkable during the period of the trial for which their data were available (data not shown).
Over the entire study period (from the day of first bronchoscopy to the 12-month follow-up), the number of severe exacerbations per subject in the BT group was 1.02 (53.6% of subjects) and in the sham group was 0.91 (45.9% of subjects) (pp superiority sham >BT = 25.8%); the number of ED visits for respiratory symptoms per subject in the BT group was 0.13 (8.4% of subjects) and in the sham group was 0.45 (15.3% of subjects) (pp superiority sham >BT = 99.7%); and the number of respiratory-related hospitalizations per subject in the BT group was 0.13 (10.5% of subjects) and in the sham group was 0.14 (5.1% of subjects) (pp superiority sham >BT = 57.2%).
DISCUSSION
Patients with severe asthma suffer significant morbidity and disability despite the use of multiple medications (20). The Asthma Intervention Research (AIR2) Trial is the largest sham-controlled trial to test a new device for the treatment of severe asthma in adults. This study evaluated the effectiveness and safety of BT in subjects with severe asthma who were symptomatic despite treatment with high doses of ICS and LABA, the current standard of care (18). These results validate the findings of two previous randomized, controlled studies that compared BT with usual care without a sham control (5, 6).
The use of a patient-centered subjective endpoint, AQLQ, required that the study be sham-controlled and that subjects remain blinded throughout the study to adequately assess the added benefit of BT beyond the current standard of care. The use of sham control subjects for interventional procedures poses risks to research subjects without the prospect of direct benefit from participation in trials (21). However, as previously demonstrated (22, 23), a well-conducted sham-controlled study, in which subjects have been adequately informed, is justified (24). Proper execution of this double-blind, sham-controlled study required investigational sites to have separate teams to deliver the treatment and perform follow-up assessments. The sham procedure successfully duplicated the BT procedure except for delivery of RF energy. Analysis of blinding assessments indicated that during the posttreatment follow-up period, Assessment physicians were unaware of treatment assignments, and subject beliefs, specifically in the BT group, were unlikely to affect outcome assessments (data not shown).
An important goal of asthma management strategies is to improve health-related quality of life (25, 26). The AQLQ is a validated tool for assessing the impact of asthma and evaluating outcomes of various therapies (27). This study demonstrates a clear effect of BT on improving the asthma-specific quality of life over 1 year despite a larger-than-expected improvement in the sham group. The improvement in the AQLQ score of 1.35 ± 1.10 in the BT group is consistent with changes that were previously observed after BT in patients with moderate to severe asthma (5) and in patients with severe-persistent asthma (6). This improvement occurred in subjects who were already taking high doses of ICS and LABA and yet is of similar magnitude to that seen in previous asthma studies where subjects were taking less medication (28, 29).
We observed a substantial mean improvement of 1.16 in AQLQ in the sham group despite the a priori expectation of approximately 0.5. This expectation was based on literature reports of typical placebo responses of 40 to 60% (30) compared with AQLQ changes after BT in prior clinical trials (5, 6). Some improvement in a control group of patients with asthma can be expected from participation in a clinical trial (31), most likely due to the regression effect. We believe that in the present study, the preconceived expectations about this promising therapy, together with the care and attention provided by the study staff, contributed to the substantial sham effect. Furthermore, the anticipation of an upcoming study visit may have heightened expectations in both groups related to the electronic diary data collected for 1 month before the 6- and 12-month study visits. The augmentation of the placebo effect in this study is consistent with the findings of another recent study showing that an optimistic presentation of a drug (or device in this case) can enhance the placebo effect for patient-centered outcomes, such as questionnaire scores (32). However, a larger proportion of BT subjects compared with sham group subjects experienced a clinically meaningful within-subject improvement in AQLQ score of 0.5 or greater. This improvement in the quality of life demonstrates the superiority of BT over sham treatment.
Consistent with an improved quality of life, subjects in the BT group had significantly fewer severe exacerbations and emergent use of healthcare. The improvement in quality of life after BT in these patients with severe asthma is associated with a subsequent reduction in ED visits, as has been demonstrated in other asthma studies (33–35). Individuals with severe, uncontrolled asthma account for a large proportion of health care use and costs (34, 36). Asthma is also the fourth leading cause of work absenteeism for adults, resulting in nearly 15 million missed or “less productive” workdays each year (37). In the current study, the improved quality of life and reduced exacerbations in BT-treated subjects likely led to a decrease in lost work/school days, consistent with these measures being interrelated.
BT-treated subjects had a substantial decrease in severe exacerbations and ED visits, whereas the BT and sham groups had similar respiratory tract infection rates during the posttreatment period. These findings suggest that treatment with BT may result in less bronchoconstriction in the setting of a known trigger of asthma exacerbation. The mechanisms underlying the modified host response to respiratory tract infections after BT could be an important area of future investigation.
Bronchoscopy in asthma is known to worsen symptoms and potentially induce complications, even more so in severe asthma (38). Data from this trial suggest that treatment with BT may further aggravate the airways in the short term. The adverse events after BT in this study were short in duration, as in previous trials (5, 6), and patients responded well to therapy. Although there was an increase in respiratory adverse events in the BT group compared with the sham group in the treatment period, fewer subjects in the BT group reported respiratory adverse events in the posttreatment period. Furthermore, subjects in the BT group reported fewer exacerbations and better quality of life scores in this posttreatment period.
In summary, this study demonstrates that BT provides clinically meaningful improvements in severe exacerbations requiring corticosteroids, ED visits, and time lost from work/school during the posttreatment period in patients with severe and inadequately controlled asthma, together with improvements in quality of life. We conclude that the increased risk of adverse events in the short-term after BT is outweighed by the benefit of BT that persists for at least 1 year. BT offers clinicians a novel, procedure-based, add-on therapy beyond the current use of high-dose ICS and LABA to decrease the morbidity of severe asthma.
Acknowledgments
The authors thank Alan Leff, M.D. (University of Chicago, Chicago, IL) and Elizabeth Juniper, M.C.S.P., M.Sc. (McMaster University, Hamilton, ON, Canada), for their valuable contributions to the design and interpretation of this study and Michael Laufer, M.D., who conceived of bronchial thermoplasty for the treatment of asthma.
Supported by Asthmatx, Inc.
A list of the members of the AIR2 Trial Study Group can be found at the end of this article.
Originally Published in Press as DOI: 10.1164/rccm.200903-0354OC on October 8, 2009
Conflict of Interest Statement: M.C. received $10,001–$50,000 from Asthmatx, $1,001–$5,000 from Schering, $1,001–$5,000 from Electrocore, and $1,001–$5,000 from BMS in consultancy fees; $5,001–$10,000 from Genentech in advisory board fees; $50,001–$100,000 from AstraZeneca, $10,001–$50,000 from Boehringer Ingelheim, $10,001–$50,000 from Pfizer, $5,001–$10,000 from Genentech, and $5,001–$10,000 from Merck in lecture fees; more than $100,001 from Asthmatx, more than $100,001 from Amgen, more than $100,001 from Centocor, more than $100,001 from Ception, more than $100,001 from Genentech, more than $100,001 from Med Immune, more than $100,001 from Merck, and more than $100,001 from GlaxoSmithKline in industry-sponsored grants; and $1,001–$5,000 from Elsevier in royalties. A.S.R. received $1,001–$5,000 from Boehringer Ingelheim in advisory board fees, $1,001–$5,000 from AstraZeneca and $1,001–$5,000 from Novartis in lecture fees, and more than $100,001 from Asthmatx, Inc. in institutional industry-sponsored grants. M.L. received $1,001–$5,000 from Merck Frosst, up to $1,000 from Asthmatx, and $1,001–$5,000 from Nycomed in lecture fees for CMA and more than $100,001 from Asthmatx, more than $100,001 from Merck Frosst, $50,001–$100,001 from MedImmune, $50,001–$100,000 from Broncus, and more than $100,001 from GlaxoSmithKline in industry-sponsored grants for clinical trials. J.F. received up to $1,000 from AstraZeneca, up to $1,000 from Novartis, and up to $1,000 from GlaxoSmithKline for a lecture on asthma; $10,001–$50,000 from Novartis, $10,001–$50,000 from Cerexa, $10,001–$50,000 from Altana Pharma, and more than $100,001 from Asthmax Inc. in grants for research; and $1,001–$5,000 from GlaxoSmithKline in grants for funding International Congress. M.D.A.L. received $1,001–$5,000 from Novartis, $1,001–$5,000 from Merck, $1,001–$5,000 from GlaxoSmithKline, and $1,001–$5,000 from Boheringer in lecture fees (promotional) and $1,001–$5,000 from Novartis, more than $100,001 from Asthmatx, $50,001–$100,000 from Broncus, $10,001–$50,000 from Gilead, and $1,001–$5,000 from Actelion in institutional grants. P.L.S. received more than $100,001 from Asthmatx in industry-sponsored grants for institutional reimbursement to Chelsea & Westminster Hospital for clinical trial expenses. E.F. received $1,001–$5,000 from Wyeth in advisory board fees, more than $100,001 from Asthmatx for clinical trial-institutional, $10,001–$50,000 from Pfizer, $10,00–$50,000 from AstraZeneca, and $5,001–$10,000 from GlaxoSmithKline in industry-sponsored grants for clinical trials. R.O. received more than $100,001 from Asthmatx in industry-sponsored grants. N.C.T. received $1,001–$5,000 from Wyeth, $1,001–$5,000 from Merck, and $1,001–$5,000 from Novartis in advisory board fees; $1,001–$5,000 from Merck, $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Novartis, and up to $1,000 from AstraZeneca in lecture fees; and $10,001–$50,000 from Novartis, more than $100,001 from GlaxoSmithKline, $10,001–$50,000 from Centocor, and more than $100,001 from Asthmatx in institutional grants. R.M.N. received more than $100,001 from Asthamax, Inc. in institutional grants; and $1,001–$5,000 each from Novartis, AstraZeneca, and GlaxoSmithKline for lecture fees or advisory boards. I.D.P. received $1,001–$5,000 from MSD in advisory board fees, $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from AstraZeneca in honoraria, more than $100,001 for a study of severe asthma from GlaxoSmithKline and more than $100,001 from Asthmatx for AIR1, RISA, and two studies. M.S. served as a consultant for the Olympus Corporation but received no financial remuneration, served as an expert witness for the Olympus Corporation, with travel expenses paid for to and from CMS in Baltimore, MD (coach flight, hotel for one night), more than $100,001 from Asthmatx, and $50,001–$100,000 from Broncus; Henry Ford Health System and Hospital received research funding as per contracted relationship. D.R.D. received more than $100,001 from Asthmatx Inc. for industry-sponsored clinical trial support. C.M. received $1,001–$5,000 from Asthmatx in institutional consultancy fees, up to $1,000 from Pfizer and $1,001–$5,000 from Boehringer Ingelheim in lecture fees, more than $100,001 from Asthmatx, $10,001–$50,000 from Pfizer, more than $100,001 from Spiration, and $5,001–$10,000 in industry-sponsored grants (Institutional, PI). R.B. received more than $100,001 from Asthmatx, Inc. as a principal investigator for an institutional grant. N.H.T.T.H. received more than $100,001 for payment participation in an Alair study from Asthmatx. M.E.W. received $10,001–$50,000 as a consultant for Asthamatx and more than $100,001 from Asthmax in grants as an investigator in the trial. M.E.W also received $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Medimmune, $1,001–$5,000 from Medicinova, $1,001–$5,000 from AstraZeneca, $1,001–$5,000 from Genentech, up to $1,000 from NKT Therapeutics, $10,001–$50,000 from Merck, $10,001–$50,000 from Novartis, for medical advisory board, consulting or speaker services. M.H. received up to $1,000 from AstraZeneca, $1,001–$5,000 from Boehringer Ingelheim, and $1,001–$5,000 from Pfizer in lecture fees; $10,001–$50,000 from Turner Freeman Lawyers, and $10,001–$50,000 from Slater and Gordon Lawyers for serving as an expert witness; and more than $100,001 from Asthmatx, $50,001–$100,000 from AstraZeneca, $50,001–$100,000 from GlaxoSmithKline, and $50,001–$100,000 from Boehringer Ingelheim in industry-sponsored grants for contracted research. M.J.P. received up to $1,000 from AstraZeneca in advisory board fees and more than $100,001 from Asthmatx for payment of costs involved in research study. S.E. received more than $100,001 from Asthmatx in grants. W.L.'s institution, Baylor College of Medicine, received more than $100,001 from Asthmatx in industry-sponsored grants. E.I. received $1,001–$5,000 from Amgen, $1,001–$5,000 from Astellas Pharma US, Inc., $10,001–$50,000 from Asthmatx, $10,001–$50,000 from Genentech, $5,001–$10,000 from Icagen, Inc., $10,001–$50,000 from MedImmune, $10,001–$50,000 from Merck & Co, Inc., $10,001–$50,000 from Novartis, $10,001–$50,000 from PDL BioPharma, $5,001–$10,000 from Pfizer, $10,001–$50,000 from Schering Plough, $1,001–$5,000 from Sepracor, and $10,001–$50,000 from Teva Specialty Pharmaceuticals in consultancy fees; $10,001–$50,000 from Genentech, $10,001–$50,000 from Merck & Co., Inc., and $10,001–$50,000 from Novartis in lecture fees; $5,001–$10,000 from Ficksman & Conley, LLP and $1,001–$5,000 Prince, Lobel, Glovsky & Tye, LLP for serving as an expert witness; and $50,001–$100,000 from Aerovance, $50,001–$100,000 from Asthmatx, $50,001–$100,000 from Boehringer Ingelheim, $50,001–$100,000 from Centocor, $50,001–$100,000 from Ception Therapeutics, $50,001–$100,000 from Genentech, $50,001–$100,000 from Icagen, Inc., $50,001–$100,000 from Johnson & Johnson, $50,001–$100,000 from MedImmune, $50,001–$100,000 from Merck & Co., Inc., $50,001–$100,000 from PDL BioPharma, and $50,001–$100,000 from Schering Plough in industry-sponsored grants. N.J. received $10,001–$50,000 from Asthmatx for review of CT scans, concept discussions, and advisory group meetings; $1,001–$5,000 from GlaxoSmithKline and $1,001–$5,000 from Genentech for advisory board fees; $1,001–$5,000 from Merck and Co. in lecture fees; and more than $100,001 from GlaxoSmithKline and more than $100,001 from Genentech in industry-sponsored grants. M.K. received $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Sepracor, and $1,001–$5,000 from Merck in advisory board fees; $5,001–$10,000 from Merck and $5,001–$10,000 from GlaxoSmithKline in lecture fees; more than $100,001 for investigator initiated research from GlaxoSmithKline; $50,001–$100,000 as site PI from Asthmatx; $10,001–$50,000 as site PI from Biomarck; $10,001–$50,000 as co-PI from Broncus; more than $100,001 for a Phase I/II study from GE Healthcare; $1,001–$5,000 for the EXCELS clinical trial from Genentech; and more than $100,001 for the IL-13 Phase II trial from Novartis. S.M.B. received more than $100,001 from Asthmatx in consultancy fees. J.Q. received more than $100,001 from Asthmatx in consultancy fees and $10,001–$50,000 from US Smokeless Tobacco Manufacturing Company for Consulting with respect to Study Design 2007–2008. N.S.S. is a full time employee of Asthmatx, Inc. and holds stock ownership or options with Asthmatx (stock options are not currently tradable). G.C.'s spouse/life partner a clinical scientist for and full-time employee of Boehringer Ingelheim, received $1,001–$5,000 from Asthmatx in consultancy fees, $1,001–$5,000 from Boehringer Ingelheim as an advisory board member; $1,001–$5,000 from GlaxoSmithKline, $1,001–$5,000 from Actelion, and $1,001–$5,000 from Novartis in lecture fees; and more than $100,001 from Asthmatx in industry-sponsored grants for clinical trial operating expenses.
References
- 1.Cox P, Miller J, Mitzner W, Leff A. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004;24:659–663. [DOI] [PubMed] [Google Scholar]
- 2.Danek C, Lombard C, Dungworth D, Cox P, Miller J, Biggs M, Keast T, Loomas B, Wizeman W, Hogg J, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004;97:1946–1953. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: updated 2007. Bethesda, MD: National Institutes of Health; 2007.
- 4.Cox G, Miller J, McWilliams A, Fitzgerald J, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006;173:965–969. [DOI] [PubMed] [Google Scholar]
- 5.Cox G, Thomson N, Rubin A, Niven R, Corris P, Siersted H, Olivenstein R, Pavord I, McCormack D, Chaudhuri R, et al., for the AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327–1337. [DOI] [PubMed] [Google Scholar]
- 6.Pavord I, Cox G, Thomson N, Rubin A, Corris P, Niven R, Chung K, Laviolette M, for the RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176:1185–1191. [DOI] [PubMed] [Google Scholar]
- 7.Castro M, Rubin A, Laviolette M, Thomson N. Efficacy of bronchial thermoplasty (BT) in patients with severe asthma: the AIR2 trial [abstract]. Am J Respir Crit Care Med 2009;179:A3644. [Google Scholar]
- 8.Cox G, Israel E, Jarjour N, Kraft M. AIR2 trial: execution of a sham-controlled double-blind study of a novel device for the treatment of severe asthma [abstract]. Am J Respir Crit Care Med 2009;179:A2787. [Google Scholar]
- 9.Shah P, Fiterman J, McEvoy C, Erzurum S. Safety of bronchial thermoplasty (BT) in patients with severe, symptomatic asthma: positive safety profile in the AIR2 trial [abstract]. Am J Respir Crit Care Med 2009;179:A2814. [Google Scholar]
- 10.Wechsler M, Olivenstein R, Niven R, Pavord I. Asthma-related ER visits and hospitalizations following bronchial thermoplasty (BT) in patients with severe, symptomatic asthma [abstract]. Am J Respir Crit Care Med i;179:A2779.
- 11.Juniper E, Guhatt G, Epstein R, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax 1992;47:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEntegart D. The pursuit of balance using stratified and dynamic randomization techniques: an overview. Drug Inf J 2003;37:293–308. [Google Scholar]
- 13.Mayse M, Laviolette M, Rubin A, Lampron N, Simoff M, Duhamel D, Musani A, Yung R, Mehta A. Clinical pearls for bronchial thermoplasty. J Bronchol Interventional Pulmonol 2007;14:115–123. [Google Scholar]
- 14.Juniper E, O'Byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–907. [DOI] [PubMed] [Google Scholar]
- 15.Juniper E, Guyatt G, Willan A, Griffith L. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol 1994;47:81–87. [DOI] [PubMed] [Google Scholar]
- 16.Busse W, Corren J, Lanier B, McAlary M, Fowler-Taylor A, Cioppa G, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108:184–190. [DOI] [PubMed] [Google Scholar]
- 17.Bang H, Liyun N, Davis C. Assessment of blinding in clinical trials. Control Clin Trials 2004;25:143–156. [DOI] [PubMed] [Google Scholar]
- 18.National Asthma Education and Prevention Program. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute; 2007.
- 19.American Thoracic Society Workshop. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 20.Slavin R, Haselkorn T, Lee J, Zheng B, Deniz Y, Wenzel S. Asthma in older adults: observations from the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) Study. Ann Allergy Asthma Immunol 2006;96:406–414. [DOI] [PubMed] [Google Scholar]
- 21.Hornig S, Miller F. Is placebo surgery unethical? N Engl J Med 2002;347:137–139. [DOI] [PubMed] [Google Scholar]
- 22.Connolly S, Sheldon R, Thorpe K, Roberts R, Ellenbogen K, Wilkoff B, Morillo C, Gent M, and VPS II. Investigators. Pacemaker therapy for prevention of syncope in patients with recurrent severe vasovagal syncope: second vasovagal pacemaker study (VPS II): a randomized trial. JAMA 2003;289:2224–2229. [DOI] [PubMed] [Google Scholar]
- 23.Moseley J, O'Malley K, Petersen N, Menke T, Brody B, Kuykendall D, Hollingsworth J, Ashton C, Wray N. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347:81–88. [DOI] [PubMed] [Google Scholar]
- 24.Miller F, Brody H. What makes placebo-controlled trials unethical? Am J Bioeth 2002;2:41–48. [DOI] [PubMed] [Google Scholar]
- 25.Juniper E. The impact of patient compliance on effective asthma management. Curr Opin Pulm Med 2003;9:S8–S10. [DOI] [PubMed] [Google Scholar]
- 26.Siroux V, Boudier A, Anto J, Cazzoletti L, Accordini S, Alonso J, Cerveri I, Corsico A, Gulsvik A, Jarvis D, et al. Quality-of-life and asthma-severity in general population asthmatics: results of the ECRHS II study. Allergy 2008;63:547–554. [DOI] [PubMed] [Google Scholar]
- 27.Baiardini I, Braido F, Brandi S, Tarantini F, Bonini S, Bousquet P, Zuberbier T, Demoly P, Canonica G. The impact of GINA suggested drugs for the treatment of asthma on health-related quality of life: a GA(2)LEN review. Allergy 2008;63:1015–1030. [DOI] [PubMed] [Google Scholar]
- 28.FDA New Drug Application (NDA) no. 21-077 for Advair Diskus, manufactured by GlaxoSmithKline, Inc., approved August 2000. Rockville, MD: Food and Drug Administration: 2000.
- 29.Buhl R, Hanf G, Solèr M, Bensch G, Wolfe J, Everhard F, Champain K, Fox H, Thirlwell J. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J 2002;20:1088–1094. [DOI] [PubMed] [Google Scholar]
- 30.Kaptchuk T, Goldman P, Stone D, Stason W. Do medical devices have enhanced placebo effects? J Clin Epidemiol 2000;53:786–792. [DOI] [PubMed] [Google Scholar]
- 31.Szefler S, Mitchell H, Sorkness C, Gergen P, O'Connor G, Morgan W, Kattan M, Pongracic J, Teach S, Bloomberg G, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomized controlled trial. Lancet 2008;372:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise R, Anthonisen N, Castro M, Holbrook J, Irvin CSL, or the American Lung Association Asthma Clinical Research Centers (ALA-ACRC). The placebo effect in asthma-effect of drug presentation on clinical outcomes. J Allergy Clin Immunol 2009;124:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisner M, Ackerson L, Chi F, Kalkbrenner A, Buchner D, Mendoza G, Lieu T. Health-related quality of life and future health care utilization for asthma. Ann Allergy Asthma Immunol 2002;89:46–55. [DOI] [PubMed] [Google Scholar]
- 34.Magid D, Houry D, Ellis J, Lyons E, Rumsfeld J. Health-related quality of life predicts emergency department utilization for patients with asthma. Ann Emerg Med 2004;43:551–557. [DOI] [PubMed] [Google Scholar]
- 35.Schatz M, Zeiger R, Mosen D, Vollmer W. Asthma-specific quality of life and subsequent asthma emergency hospital care. Am J Manag Care 2008;14:206–211. [PubMed] [Google Scholar]
- 36.Holgate S, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006;368:780–793. [DOI] [PubMed] [Google Scholar]
- 37.Morbidity and mortality weekly report, surveillance for asthma. Atlanta, GA; Centers for Disease Control and Prevention: 2002.
- 38.Moore W, Murphy J, Calhoun W, Castro M, Chung F, Erzurum S, Jarjour N, Wenzel S, Peters S, Bleecker E, and NHLBI Severe Asthma Research Program (SARP). Safety of investigative bronchoscopy in severe asthma. ATS 2006. Annual Meeting, San Diego, CA; 2006.