Abstract
Rationale: Chronic rejection, manifested pathologically as airway fibrosis, is the major problem limiting long-term survival in lung transplant recipients. Airway hypoxia and ischemia, resulting from a failure to restore the bronchial artery (BA) circulation at the time of transplantation, may predispose patients to chronic rejection. To address this possibility, clinical information is needed describing the status of lung perfusion and airway oxygenation after transplantation.
Objectives: To determine the relative pulmonary arterial blood flow, airway tissue oxygenation and BA anatomy in the transplanted lung was compared with the contralateral native lung in lung allograft recipients.
Methods: Routine perfusion scans were evaluated at 3 and 12 months after transplantation in 15 single transplant recipients. Next, airway tissue oximetry was performed in 12 patients during surveillance bronchoscopies in the first year after transplant and in 4 control subjects. Finally, computed tomography (CT)-angiography studies on 11 recipients were reconstructed to evaluate the post-transplant anatomy of the BAs.
Measurements and Main Results: By 3 months after transplantation, deoxygenated pulmonary arterial blood is shunted away from the native lung to the transplanted lung. In the first year, healthy lung transplant recipients exhibit significant airway hypoxia distal to the graft anastomosis. CT-angiography studies demonstrate that BAs are abbreviated, generally stopping at or before the anastomosis, in transplant airways.
Conclusions: Despite pulmonary artery blood being shunted to transplanted lungs after transplantation, grafts are hypoxic compared with both native (diseased) and control airways. Airway hypoxia may be due to the lack of radiologically demonstrable BAs after lung transplantation.
Keywords: lung transplantation, graft rejection, ischemia, bronchial arteries, fibrosis
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
There is strong support for the concept that inflammation in hypoxic or ischemic tissue predisposes that tissue to fibrotic wound repair. Experimental and clinical evidence suggests that lung transplant airways are at risk for hypoxia and ischemia.
What This Study Adds to the Field
This study demonstrates that despite the majority of pulmonary blood being shunted to the transplanted lung within 3 months after surgery, transplant airways are relatively hypoxic compared with the contralateral native airways in diseased lungs and compared with normal lungs. Computed tomography–angiography studies reveal a failure of bronchial arteries to grow into transplanted lungs. Thus, chronic airway tissue hypoxia due to the lack of a viable bronchial artery circulation may put lung transplant patients at risk for developing the bronchiolitis obliterans syndrome.
Microvascular loss may be an unappreciated root cause of chronic rejection for solid organ transplants (1–5). For this reason, the tenuous nature of the vasculature in lung allografts may explain, in part, this transplant's proclivity for developing chronic rejection, otherwise known as the bronchiolitis obliterans syndrome (BOS) (6). BOS is a pathologically heterogeneous fibroobliterative terminal airway disease and is the histologic hallmark of chronic rejection. Despite identification of other risk factors for the development of BOS, such as acute rejection and cytomegalovirus infection, the etiology of the fibroproliferative changes associated with BOS remains unknown. Autopsy studies performed by Luckraz and colleagues at Papworth Hospital demonstrated a significant loss of vessels in otherwise normal airways neighboring BOS airways, a finding suggesting that airway ischemia precedes airway fibrosis (1, 2).
Lung transplants are the only solid organ allografts that do not routinely undergo direct systemic arterial reconnection at the time of surgery. Airways are normally supplied by a dual circulation derived from the bronchial arteries (BAs) and the pulmonary artery; the source of microvessels observed around airways after transplantation is not fully understood. Only the pulmonary artery circulation is surgically restored at the time of transplantation. Because the bronchial anastomosis heals well with the current surgical procedure, reconnecting the BAs has been deemed unnecessary (7). Preclinical canine studies suggested that the BA circulation can be restored de novo after transplantation through angiogenesis (8, 9), and heart-lung transplant recipients occasionally develop systemic collateral circulation to the airways from the coronary arteries (10, 11). However, the regrowth of BAs has not been assessed in lung transplantation. After lung transplantation, the low O2 pulmonary artery circulation is presumably the major source of blood and microvasculature for transplanted lungs.
In experimental airway transplantation, evidence suggests that the combination of hypoxia, alloimmune inflammation, and ultimately ischemia culminates in irreversible fibrosis (6). Given the aforementioned autopsy study suggesting that microvascular dropout precedes BOS development and given the modern surgical practice of not establishing a systemic arterial circulation at the time of transplantation surgery, there appear to be at least two reasons to better consider the baseline perfusion and oxygenation status of the normal allograft after transplantation. To our knowledge, no prior group has studied airway tissue oxygenation in humans (in any condition) or BA anatomy in nonrevascularized transplant recipients. Although a recent study quantitatively analyzed lung perfusion in single-lung transplants, it did not look at standard time intervals in serial evaluations within their patient group (12). In the current study, we demonstrate that despite pulmonary artery blood being preferentially shunted to transplanted lungs soon after transplantation, transplant airways remain relatively hypoxic compared with airways in native (diseased) lungs and airways in normal lungs. Because computed tomography (CT)-angiography studies failed to demonstrate an identifiable BA circulation beyond the anastomotic level within the transplanted lung, airway hypoxia may be due, in part, to the failure of BAs to regrow into the lung after transplantation. The resulting limited oxygenation and vascular supply of lung transplants may, consequently, be a predisposing factor for subsequent fibrotic remodeling.
METHODS
Perfusion Scans
Routine V·/Q· scans are performed at 3 and 12 months after lung transplantation. To undertake a study of “normal” lung transplantation physiology, we chose to study uninfected, nonrejecting single-lung transplant recipients without airway or vascular stenoses and used the native (diseased) lung as the contralateral control. Thirty routine quantitative perfusion scans were performed at 3 and 12 months after transplantation and were evaluated in 15 single-lung transplant recipients. For these studies, 2 mCi (37–74 MBq) of Tc99m-macroaggregated albumin was injected, and images were obtained in the posterior, left posterior oblique, left lateral, anterior, right lateral and right posterior oblique projections. The posterior planar image was transferred from the γ-camera workstation to a personal computer. On the public domain, an image-analysis program (National Institutes of Health image program version 156b18) determined the area of pixels and was used to calculate at every 10% cutoff level the maximal pixel radioactivity, from 10% to 100%, a value for the perfused area (PAn) (as described in Reference 13). The threshold width number (n) was taken from 1 to 9 for every additional 10% threshold.
Airway Oximetry Study
To our knowledge, this is the first study that attempts to measure in vivo airway tissue O2 saturations. All studies were approved by the local institutional review board committee. We developed a protocol to assess airway O2 saturations using the T-Stat 303 Microvascular Tissue Oximeter (Spectros, Portola Valley, CA). This oximeter is approved by the Food and Drug Administration and monitors absolute local hemoglobin O2 saturation in tissue at the capillary level. Measurements are independent of pulse or blood pressure. The oximeter emits white light from a probe placed near the measured tissue and collects any light returning to the probe from the tissue. The collected light is separated by wavelength into 2,048 bins, which are measured simultaneously (14). The blue-to-yellow portion of the visible spectrum (476–584 nm) is used to solve for light scattering and for the concentration of each of the major forms of hemoglobin (deoxyhemoglobin, oxyhemoglobin, and optionally methemoglobin and carboxyhemoglobin). This process uses first differential spectroscopy and least-squares fitting to known hemoglobin spectra. Tissue hemoglobin is estimated as [deoxyhemoglobin + oxyhemoglobin] and the tissue hemoglobin O2 saturation is determined as [oxyhemoglobin]/[deoxyhemoglobin + oxyhemoglobin]. The oximetry measurements are continuous and typically require 5 to 50 milliseconds, depending on the intensity of the reflected light. In our study, 16 oximetry studies were performed in 12 lung transplant patients (i.e., 4 patients had two studies performed) and four “normal” control subjects without known lung disease. Transplant patients undergoing bronchoscopy were given 2 to 3 L/min nasal cannula O2 to target an oxygen saturation of 94 to 96% (by pulse oximetry). All lung transplant studies were performed on single-lung transplant recipients at the time of surveillance biopsies, which are performed at 1, 2, 3, 6, and 12 months at this Center; none of the patients had symptoms of rejection nor were they found to subsequently demonstrate evidence of graft rejection or infection. The oximetry studies in normal study subjects were performed immediately after patients were intubated for planned nonthoracic surgical procedures; these control patients were maintained on a FiO2 of 0.30 to achieve a pulse oximetry saturation value of 94 to 96%.
The T-Stat Endoscopy Sensor probe was threaded into the bronchoscope working channel and three measurements were taken at each anatomic site. Probes were sterile and disposed of after each procedure. The anatomic sites were as follows: midtrachea, main carina, 2°carina, carina to upper lobes, carina to middle lobes (meaning either the right middle lobe or lingula), and carina to lower lobes. Because the focus of this study was single-lung transplants, all the anastomoses were distal to the carina and proximal to the 2°carina. Therefore, all measures proximal to the anastomosis (i.e., main carina and midtrachea) were always native in origin.
CT-Angiography Studies
For all cases (n = 13), CT angiograms were obtained by using 64-channel multidetector row CT (MDCT) scanners (VCT; GE Medial Systems, Milwaukee, WI, and Siemens Sensation 64; Siemens Medical Systems, Erlangen, Germany). Specific scanning parameters included CT angiogram imaging protocols tailored for the detection of pulmonary embolisms or the visualization of the aorta and great thoracic vessels. Imaging parameters were as follows: 1- to 1.25-mm detector width, 1.375 to 1.5 pitch, 0.3- to 0.5-second gantry rotation, automated tube current modulation 120 kVp, 1.25 nominal reconstructed section thickness, 0.8- to 1-mm reconstruction interval. A total of 100 to 120 ml of nonionic contrast medium with an iodine content of 350 mg/ml (Omnipaque-350; GE Healthcare Biosciences, Princton, NJ) was injected at a 4 to 5 ml/second through a 20-gauge antecubital intravenous catheter. CT data were transferred to a server-based online three-dimensional workstation (Aquarius Net; TeraRecon, San Mateo, CA). Transverse CT sections, multiplanar reformations, and volume renderings were assessed interactively and in real time by two interpreting radiologists.
Statistical Methods
Statistical analyses were performed using PASW Statistics 18. Paired comparisons with normal group distributions used the paired t test. Nonpaired comparisons with normal group distributions used the unpaired t test. Finally, nonpaired nonparametric group comparisons were done using the Mann-Whitney test. For oximetry study, significant differences between transplant, native, and normal lungs were identified by one-way analysis of variance with post hoc analysis using least significant difference test. P value of less than 0.05 was taken as statistically significant. (In these studies, all comparison between pairs had normal distributions, whereas all unpaired analyses failed to meet normality and were analyzed with nonparametric testing.) These two types of comparisons were performed to evaluate whether individual recipients demonstrated a perfusion or BA length difference between their transplant and native lungs (i.e., paired comparisons) and whether there were meaningfully similar group values for perfusion or BA length in transplant and native lungs (i.e., unpaired comparisons).
RESULTS
Quantitative Perfusion Studies
Routine perfusion scans were evaluated in single-lung transplanted patients with idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD). The 3- and 12-month perfusion scan results are shown in Table 1. Pulmonary blood flow is preferentially shunted from the native to the transplanted lung by 3 months (73% [transplant] vs. 27% [native]), and this shunting is still evident by 12 months (78% [transplant] vs. 22% [native]). The difference between transplant blood flow and native lung blood flow is highly statistically different by 3 months by paired analyses (P < 0.0001), but there was no statistical difference between the 3-month and the 12-month groups regarding the distribution of pulmonary blood flow. Similarly, unpaired group comparisons between transplant sides and native sides demonstrate significant shunting to the transplanted lung by 3 months that persists at 12 months (73% [transplant] vs. 27% [native] at 3 mo and 78% [transplant] vs. 22% [native], P < 0.0001).
TABLE 1.
QUANTITATIVE LUNG PERFUSION STUDIES FROM SINGLE-LUNG TRANSPLANT RECIPIENTS
| 3 mo* |
12 mo† |
|||||
|---|---|---|---|---|---|---|
| Patient No. | Dx | Tp Side | Tp (%) | Native (%) | Tp (%) | Native (%) |
| 1 | IPF | L | 64 | 36 | 70 | 30 |
| 2 | IPF | L | 50 | 50 | 88 | 12 |
| 3 | IPF | L | 80 | 20 | 87 | 13 |
| 4 | IPF | R | 77 | 23 | 73 | 27 |
| 5 | IPF | R | 77 | 23 | 77 | 23 |
| 6 | IPF | R | 78 | 22 | 76 | 24 |
| 7 | IPF | R | 71 | 29 | 73 | 27 |
| 8 | IPF | R | 80 | 20 | 80 | 20 |
| 9 | IPF | R | 73 | 27 | 78 | 22 |
| 10 | IPF | R | 64 | 36 | 64 | 36 |
| 11 | COPD | L | 76 | 24 | 85 | 15 |
| 12 | COPD | L | 85 | 15 | 88 | 12 |
| 13 | COPD | R | 60 | 40 | 73 | 27 |
| 14 | COPD | R | 85 | 15 | 86 | 14 |
| 15 | COPD | R | 73 | 27 | 67 | 33 |
| Group Means ± SE | 73 ± 3 | 27 ± 3 | 78 ± 2 | 22 ± 2 | ||
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; Dx = diagnosis; IPF = idiopathic pulmonary fibrosis; L = left; R = right; Tp = transplant lung.
Transplanted lung perfusion vs. native lung perfusion at 3 mo after transplant; paired analyses, P < 0.0001 by paired t test; unpaired analyses, P < 0.0001 by unpaired t test.
Transplanted lung perfusion vs. native lung perfusion at 12 mo after transplant; paired analyses, P < 0.0001 by paired t test; unpaired analyses, P < 0.0001 by unpaired t test.
Airway Oximetry Studies
To assess whether increased pulmonary blood flow to the transplanted lung correlated with increased airway tissue O2 saturations, single-lung transplant patients undergoing routine surveillance biopsies after transplantation were consented for airway oximetry studies. The transplant patient characteristics were as follows: eight patients with IPF (five right and three left lung transplants) and four patients with COPD. Sixteen studies were performed in 12 patients in the first year after transplantation and were composed of the following complete airway oximetry studies: 10 patients with IPF (7 right and 3 left lung transplants) and 6 patients with COPD (3 right and 3 left lung transplants). Normal subjects ranged in age from 47 to 53 years and were scheduled to undergo the following procedures: mastectomy, prostatectomy, exploratory laparotomy, and upper extremity mass resection. Airway tissue saturations were significantly diminished in transplanted airway segments relative to native (diseased) airways analyzed distal to the 2°carina and relative to normal control airways with a mean O2 saturation difference of approximately 5% (Table 2).
TABLE 2.
AIRWAY OXIMETRY STUDY IN SINGLE-LUNG TRANSPLANT RECIPIENTS
| Transplant Recipients |
P Value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tp |
TpN |
Normal Controls |
Post Test |
|||||||||
| Airway Region | n* | Mean O2 Sat (%) | SD | Mean O2 Sat (%) | SD | n* | Mean O2 Sat (%) | SD | ANOVA | Tp vs. TpN | Tp vs. Normal | TpN vs. Normal |
| Midtrachea† | 63.3 | 5.4 | 65.5 | 1.9 | ||||||||
| Carina† | 61.1 | 4.4 | 66.2 | 1.5 | ||||||||
| 2° carina | 16 | 60.3 | 4.3 | 62.6 | 7.0 | 8 | 63 | 1.3 | 0.35 | 0.22 | 0.23 | 0.85 |
| UL | 16 | 59.8 | 4.0 | 66.1 | 5.1 | 8 | 63.6 | 1.2 | <0.001 | <0.001 | 0.04 | 0.17 |
| ML | 15 | 60.5 | 4.2 | 66.7 | 6.4 | 8 | 63.6 | 2.2 | 0.006 | 0.002 | 0.15 | 0.16 |
| LL | 16 | 59.2 | 3.9 | 65.4 | 6.2 | 8 | 64.7 | 2.3 | 0.002 | 0.002 | 0.028 | 0.94 |
| Mean | 59.9 | 4.0 | 65.2 | 6.3 | 63.7 | 1.8 | <0.01 | <0.01 | 0.03 | 0.36 | ||
Definition of abbreviations: ANOVA = analysis of variance; LL = lower lobe; ML = middle lobe; Sat = saturation; Tp = transplant lung; TpN = native lung; UL = upper lobe
n value refers to the number of complete airway studies performed for each anatomic location and represents the mean of three measurements at each site for each study.
Midtrachea and carina measurements were all native airway tissues because all single-lung transplant anastomoses were distal to the carina.
CT-Angiography Studies
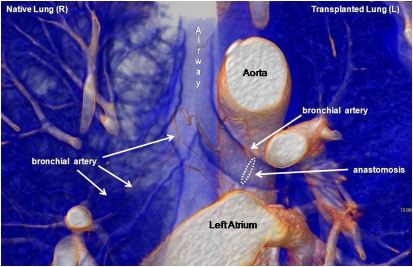
To determine whether the hypoxia observed in transplanted airways was due to a persistent absence of BA circulation, chest CT angiography studies on single-lung transplant recipients (as usually performed to rule out pulmonary emboli) were used to evaluate the BA anatomy after lung transplantation. Twelve studies from 11 patients at Stanford were analyzed for radiologically detectable BAs. Studies were performed as early as 26 days after transplant and as late as 612 days after transplant (median = 49 d). The vasculature was evaluated for BA length, which was defined as the distance beyond the carina the vessel was noted to traverse the airway (Table 3). In all but one case, the BA was not noted to cross the anastomosis line on the transplanted side but was detected more distally in the airways of the native lung (P < 0.0001). The single exception was detected in a CT evaluation performed 612 days after transplantation where the BA crossed over the anastomosis. The mean BA length measured from the carina distally down the airway was 4.7 ± 0.55 cm on the native lung side versus 2.0 ± 0.36 cm on the transplanted lung side. When all transplant BA lengths were compared with all native airway BA lengths (i.e., every BA measured counted individually), the values were similarly skewed: (5.1 ± 0.59 cm [native] vs. 2.2 ± 0.34 cm [transplant]). As Table 4 demonstrates, with the exception of the above-noted late study, the BA was not noted to extend beyond the anastomosis, so BA blood flow could not be conclusively demonstrated in transplanted lungs. There was no significant difference in the diameter of the BAs as they emerged from their originating vessel (no difference between left vs. right lung or native vs. transplant lung). Figure 1 and the online supplemental movie illustrate the anatomy of the normal BA traversing the right native lung bronchus and the abbreviated transplant BA, which ends at or near the anastomosis.
TABLE 3.
LUNG COMPUTED TOMOGRAPHY–DETERMINED BRONCHIAL ARTERY LENGTHS IN SINGLE-LUNG TRANSPLANT RECIPIENTS
| Patient No. | Disease | Tp Side | Radiographic Length of BA(s) on Tp Side* (cm) | Radiographic Length of BA(s) on Native Side* (cm) | Average Tp BA Length† (cm) | Average Native BA Length (cm) |
|---|---|---|---|---|---|---|
| 1 | IPF | L | 3.3 | 7.5 | 3.3 | 7.5 |
| 2 | IPF | L | 2.2 | 5.9 | 2.2 | 5.9 |
| 3 | IPF | L | 3.6 | 4.6 | 3.6 | 4.6 |
| 4‡ | IPF | R | 1.3, 1.4, 1.9 | 2.1, 6.9, 2.5, 6.5 | 1.5 | 4.5 |
| 5 | IPF | R | 0.24 | 4 | 0.24 | 4 |
| 6 | IPF | R | 5.3, 1.3 | 9.2, 4.8, 8.5 | 3.3 | 7.5 |
| 7 | COPD | R | 2.2, 2.2 | 2.1 | 2.2 | 2.1 |
| 8 | COPD | L | 3.8 | 7.3 | 3.8 | 7.3 |
| 9 | COPD | R | 1.4 | 2.6 | 1.4 | 2.6 |
| 10 | COPD | L | 2 | 3.5 | 2 | 3.5 |
| 11 | COPD | R | 0.78 | 3.3 | 0.78 | 3.3 |
Definition of abbreviations: BA = bronchial artery; COPD = chronic obstructive pulmonary disease; CT = computed tomography; IPF = idiopathic pulmonary fibrosis; L = left; R = right; Tp = transplant lung.
Represents all measurements of all BAs (e.g., one length means one measurable BA was observed assessed). Length measurement began at the carina and followed down the bronchus as far as could be radiologically discerned.
Average Tp BA length vs average native BA length, P < 0.0001 by paired t test; and all measured Tp BAs vs. all measure native BA, P = 0.0005 by Mann-Whitney test.
Patient had two serial CT scans and BA measurements.
TABLE 4.
LUNG COMPUTED TOMOGRAPHY BRONCHIAL ARTERY ANATOMIC STUDY OF SINGLE-LUNG TRANSPLANT RECIPIENTS
| Patient No.* | Tp Side | Days after Tp | No. of Tp-Side BA(s) | Located End of Tp BA | Origin of Tp BA | BA Diameter at Takeoff (mm) | No. of Native-Side BA(s) | Located End of Native BA | Origin of Native BA | BA Diameter at Takeoff (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L | 26 | 1 | A | Aorta, Common Origin with R BA | 1.4 | 2 | Too small to follow | R Subclavian | N/A |
| 4th | Aorta, Common Origin with L BA | 3.0 | ||||||||
| 2 | L | 31 | 1 | A | Aortic Arch | 1.3 | 1 | 3rd | R Subclavian | 2.1 |
| 3 | L | 50 | 1 | A | Aortic Arch | 1.5 | 1 | 2nd–3rd | Aortic Arch | 1.5 |
| 4† | R | 55, 69 | 1 | A | Intercostal | 1.8 | 2 | LM | Aorta | 1.1 |
| A | R Subclavian | 1.6 | 2nd–3rd | Aorta | 1.8 | |||||
| 5 | R | 65 | 1 | Pre-A | R Subclavian | 1.5 | 1 | Distal LM | Aorta | 2 |
| 6‡ | R | 612 | 2 | A | Intercostal | 2.1 | 3 | 4th | Aorta | 2.8 |
| LLL | Aorta | 1.9 | ||||||||
| Pre-3rd | R Subclavian | 1.8 | 3rd | Aorta | 1.7 | |||||
| 7 | R | 29 | 2 | A | Intercostal | 1.5 | 1 | LM | Aorta | 0.8 |
| A | R Subclavian | 1.0 | ||||||||
| 8 | L | 40 | 1 | Distal LM | Aorta | 1.5 | 2 | Too small to follow | R Subclavian | 1.3 |
| 3rd | Intercostal | 2.4 | ||||||||
| 9 | R | 40 | 1 | A | Intercostal | 1.6 | 1 | Distal LM | Aorta | N/A |
| 10 | L | 69 | 1 | Pre-A | Aorta | 1.3 | 1 | 2nd | Intercostal | 2.9 |
| 11 | R | 274 | 1 | Pre-A | R Subclavian | 1.6 | 1 | Mid LM | Aorta | 1.2 |
Definition of abbreviations: A = anastomosis; BA = bronchial artery; CT = computed tomography; L = left; LL = lower lobe; M = main bronchus; N/A = not available; Pre-A = before anastomosis; Pre-3rd = before third bifurcation; R = right; Tp = transplant lung; 2nd = second airway bifurcation; 3rd = third airway bifurcation; 4th = fourth airway bifurcation.
Same patients listed in Table 3.
Patient had two serial CT scans and BA measurements; BA diameter represents averages for two studies.
Patient #6 had a BA that arose from the right subclavian artery and crossed anastomosis to 3rd bifurcation of airway. This was the only case in which the BA was detected beyond the anastomosis.
Figure 1.
Reconstructed computed tomography–angiogram in a single-lung transplant recipient 3 months after left lung transplantation. Although the high O2 bronchial artery (BA) circulation is preserved to the native right lung, the BA stops near the anastomosis site of the left lung. This image is further represented in an online supplemental movie, which provides a three dimensional perspective of the anatomy.
DISCUSSION
In this study, we have shown that deoxygenated pulmonary artery blood is shunted to transplanted lungs soon after transplantation. We show for the first time that the relative percentage of blood flow to the transplanted lung is relatively constant in the first year after transplantation. The values noted for relative perfusion are similar to values presented in a prior study demonstrating 83% of perfusion to the transplant in COPD lungs and 69% of perfusion to the transplant in IPF lungs (12). Reconstructed CT-angiography studies suggest that transplant airway hypoxia may be due, in part, to the lack of a radiologically demonstrable BA circulation in the lung after transplantation. The differences in BA lengths between transplant and native lungs were highly significant; a finding illustrating that the BA was uniformly abbreviated in length on the transplant side within individual patients and that, collectively, transplant BAs were significantly shorter than native airway BAs. Interestingly, the one exception to this was observed in the latest patient to be evaluated (612 d after transplant). It is possible that given time, a systemic artery may regrow into the graft. Indeed, this form of collateralization has been observed in several cases of heart-lung transplant recipients (10, 11), wherein the coronary arteries serve as a source of vessels to the presumably hypoxic airways. However, to date, this kind of collateralization has not been observed in single- or double-lung transplants.
To our knowledge, the current study is the first to attempt to measure airway tissue O2 saturations in humans. We found that transplant airway tissue remains relatively hypoxic compared with airways in native and normal lungs. The fact that the 2°carina, which are just distal to the anastomosis, were not significantly hypoxic compared with the contralateral native airway, suggests the possibility that the systemic circulation may be growing slowly (possibly due to hypoxia-driven angiogenesis) from the recipient into the donor at the microvascular level. It should be noted that parenchymal tissue O2 saturations are always significantly lower than blood O2 saturations, with hemoglobin O2 saturation of blood in the microvascular tissue spaces typically running closer to venous saturation than to arterial saturation. Although reference ranges for tissue O2 saturations have not been established in health and disease, measured values of O2 saturation for many tissues are typically 71 ± 3%, or a 95% confidence interval of 65 to 77% (R. Kum; Spectros; personal communication). In the current study, the only clear and relevant difference between the higher O2 (64–65%) saturation groups (native and normal airways) and the lower O2 saturation (60%) group (transplant airways) was the presence of a BA in the higher O2 saturation group. The tissue oximeter used in this study is a Food and Drug Administration–approved device routinely deployed through a colonoscope to assess intestinal oxygenation during vascular surgery (14, 15). Future studies are anticipated to further validate this new approach by expanding its use in lung transplantation and other pulmonary diseases.
Although the results demonstrate that transplant airways are relatively hypoxic, a 5% O2 saturation difference is of unclear biological relevance. There are a number of factors to consider with the results of the current study. First, the T-Stat oximeter assesses tissue oxygenation rather than arterial O2 saturation. As such, these are more mixed venous oxygen (capillary) saturations, which likely underestimate the true local arteriolar O2 saturation difference and rather represent tissue oxygen deficit. Furthermore, we currently do not have the technology to directly assess exertional airway tissue oxygenation, which would likely reveal greater tissue hypoxia in transplanted airways with exercise as there would be no source, to our knowledge, of highly oxygenated blood to supply airways during increased metabolic demand. Additionally, our animal studies demonstrate that inflammation itself greatly exacerbates airway tissue hypoxia and could potentially unmask a larger difference during rejection than the differences currently noted (6). In our group's animal model, which assesses the tissue Po2 (rather than O2 saturation) of functional tracheal transplants, the tissue Po2 can go as low as 7 mm Hg (from a baseline in the low 30s) with unmitigated rejection (data not shown). Finally, the tissue oxygen saturation values reported in the current study were obtained in healthy, noninfected, nonrejecting, and non-BOS patients, and the observed differences simply indicate that the baseline quiescent healthy lung transplant is relatively hypoxic. It is clearly possible that without other concomitant risks, such as infection and rejection, this relative hypoxia is, in itself, an insufficient cause for BOS development.
The mechanisms by which hypoxia and ischemia contribute to postinflammatory fibrosis are not established but the clustering of hypoxia, ischemia, inflammation, and fibrosis is routinely observed in several clinical situations, such as normal skin wound healing (16) and chronic kidney diseases (17). In pulmonary fibrosis in mice and in humans, microarray data sets reveal hypoxic signaling among the most statistically important dysregulated pathways (18–20). In vitro studies have demonstrated profibrotic phenotypic change of fibroblasts in response to hypoxia (21, 22). Epithelial and endothelial cells can undergo mesenchymal transition under ischemia to become another source of activated fibroblasts (23). Hypoxia likely directly contributes to the progression of fibrosis by increasing the release of major extracellular matrix proteins (24). Transforming growth factor-β2–induced fibrosis is associated with intense vasoconstriction and tissue hypoxia (25). Which of the above phenomena (i.e., activated fibroblasts, mesenchymal transition, release of matrix proteins) contributes the most to airway fibrosis is not currently known, but it is clear that inflamed tissue subject to low Po2 and ischemia is at considerable risk for fibrotic remodeling.
It has been estimated in a canine study that about 50% of blood flow to the main bronchi normally comes from the BAs and 50% from the poorly oxygenated pulmonary artery circulation (26). Although there has been some debate about the existence of a functional bronchopulmonary vascular anastomosis in normal lungs, most evidence suggests that these connections likely exist in normal lungs with networks forming at the precapillary level (27, 28). The BA circulation is highly conserved through evolution (29), and the ramifications of living without this circulation are not known. In the absence of a BA revascularization step at the time of transplantation, the bronchi are presumably 100% supplied by the pulmonary circulation, which would result, as illustrated by this study, in the airways being more hypoxic. Preclinical and preliminary clinical studies demonstrate that BA revascularization improves tissue perfusion with more highly oxygenated blood (30, 31), is durable (32), is associated with less epithelial metaplasia (33), and is protective of pulmonary endothelium and type II pneumocytes (34). With modern surgical techniques, bronchial anastomoses healed well without BA reconnection, and for largely this reason, the highly oxygenated BA circulation is now sacrificed in all lung transplant recipients. Lung transplants now rely on the persistence of a microcirculation presumably arising from the deoxygenated pulmonary circulation to provide perfusion to the airways.
The Copenhagen group, led by Gosta Pettersson, demonstrated that BA revascularization is durable with vessels remaining fully patent for 2 years (32). A follow-up study showed that BA revascularization (BAR) may also postpone the development of BOS and improve patient survival (35) and that reanastomosed vessels remain patent for at least 2 years (32). It is not clear if the BOS that was ultimately seen in some of the patients within this BAR cohort was attributable to a late failure (beyond 2 years) of the BA grafts; if so, such a late failure could have occurred on the basis of rejection of the vasculature alone. If restoring a functional BA circulation is protective against the development of BOS, simply performing BAR itself is no guarantee of long-term airway perfusion because the donor-derived vasculature will remain a target for alloimmune injury. At a minimum, the BAR experience in Copenhagen suggests that a large patent vascular system from the outset is protective. Just as prevention of early graft injury likely has long-term beneficial effects (36), additional steps to preserving early airway microvascular integrity may similarly prove critical for BOS prevention (6, 37).
There are other sequelae, beyond hypoxia, that may occur after the loss of the BA circulation and could contribute to airway disease. These include the loss of airway nutrition, altered lymphatic flow, decreased airway lining fluid, attenuated innate immune defenses, diminished clearance of small particles, and reduced control of airway temperature and humidity (38, 39). The bronchial circulation is responsible for the formation of the epithelial lining fluid, which plays a role in the local defenses against inhaled irritants and foreign substances. In contrast to the pulmonary circulation, BA vascular transudates appear to contribute to lymphatic flow (40). What happens to lymphatic flow in the absence of this BA contribution is not known. A functional BA circulation is required for the maintenance of normal mucociliary transport (41). Interrupting the BA likely leads to interference with absorbing and clearing airway particles (40). Finally, the airway mucosa responds to the cooling of airways after the inhalation of cold air by increasing bronchial blood flow, and by doing this improves heat and water transfer from the air. This same circulation is capable of conserving moisture in dry environments such that only one-tenth of the normal humidity is exhaled (41). Therefore, the loss of a bronchial circulation could negatively impact the regulation of airway temperature and humidity. In summary, although exaggerated tissue hypoxia with inflammation (beyond what was measured here in quiescent transplants) could occur in the absence of BA circulation, there are several other functions normally performed by the BA circulation and not assessed in the current study that could also contribute to tissue fibrosis.
Although the Papworth Hospital autopsy studies suggested that microvascular dropout occurs before the development of BOS, perhaps through alloimmune-mediated damage of the donor vasculature, they also demonstrated a robust increase in the microvasculature in established BOS (1, 2). Based on experimental orthotopic tracheal transplant studies (6), we have previously argued that the increased angiogenesis observed in airway fibrosis may simply be a response to airway tissue hypoxia and ischemia in rejection. Hypoxia-induced angiogenesis in airways may be primarily attributed to the actions of hypoxia-inducible factor-1α (HIF-1α). HIF-1α is a transcriptional regulator that is responsive to a reduction of Po2 in tissues and actively promotes hypoxia-associated angiogenesis and vasculogenesis (42). HIF-1α–mediated vessel growth is mediated through the production of multiple angiogenic growth factors and by its differentiating effects on endothelial cells and bone marrow–derived endothelial progenitor cells. Accordingly, pharmacological or molecular approaches that engage hypoxic adaptation at the point of a major sensor like HIF-1α could conceivably lead to a profound sparing of hypoxic tissue and enhanced recovery of function after alloimmune inflammation. Therapy that protects vascular endothelial cells from injury or optimizes their recovery may limit chronic rejection.
As recently noted by Contreras and Briscoe (37), “a robust vasculature appears to be the ‘silver lining’ that is necessary to sustain long-term allograft function.” The lung transplant may be an especially vulnerable allograft given the relatively hypoxic starting point of its airways. Table E1 in the online supplement summarizes preclinical and clinical studies germane to this issue and suggests how BAR may result in a healthier organ after transplantation beyond simply improving the healing of the airway anastomosis. Viewed through the new perspective of a lung transplant as an oxygen-poor organ threatened by frank ischemia with rejection of its vascular supply, new therapeutic approaches focusing on surgical BAR at the time of transplantation and on stabilization of microvascular integrity have strong potential for limiting the development of chronic rejection.
Acknowledgments
The authors Thank Mr. Robert Kum of the Spectros Corporation for his assistance with the Spectros T-Stat device. They also thank Dr. Norbert Voelkel for critical reading of this manuscript.
Supported by Stanford University Startup Funds (M.R.N.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200910-1573OC on March 25, 2010
Conflict of Interest Statement: G.S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.Z. received $10,001–$50,000 from CSL Behring as a consultant/speakers bureau member, $1,001–$5,000 from CSL Behring in advisory board fees, $1,001–$5,000 from Roche, $5,001–$10,000 from CSL Behring in lecture fees, and more than $100,001 from XDX in industry-sponsored grants. J.E.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.V.D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.W. received more than $10,000 from Asbestos Defendants for serving as an expert witness. M.R.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Luckraz H, Goddard M, McNeil K, Atkinson C, Charman SC, Stewart S, Wallwork J. Microvascular changes in small airways predispose to obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant 2004;23:527–531. [DOI] [PubMed] [Google Scholar]
- 2.Luckraz H, Goddard M, McNeil K, Atkinson C, Sharples LD, Wallwork J. Is obliterative bronchiolitis in lung transplantation associated with microvascular damage to small airways? Ann Thorac Surg 2006;82:1212–1218. [DOI] [PubMed] [Google Scholar]
- 3.Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int 2005;67:321–332. [DOI] [PubMed] [Google Scholar]
- 4.Ozdemir BH, Demirhan B, Ozdemir FN, Dalgic A, Haberal M. The role of microvascular injury on steroid and OKT3 response in renal allograft rejection. Transplantation 2004;78:734–740. [DOI] [PubMed] [Google Scholar]
- 5.Labarrere CA, Nelson DR, Park JW. Pathologic markers of allograft arteriopathy: insight into the pathophysiology of cardiac allograft chronic rejection. Curr Opin Cardiol 2001;16:110–117. [DOI] [PubMed] [Google Scholar]
- 6.Babu AN, Murakawa T, Thurman JM, Miller EJ, Henson PM, Zamora MR, Voelkel NF, Nicolls MR. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest 2007;117:3774–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson GA. Airway revascularization: is it necessary? Ann Thorac Surg 1993;56:807–808. [DOI] [PubMed] [Google Scholar]
- 8.Pearson FG, Goldberg M, Stone RM, Colapinto RF. Bronchial arterial circulation restored after reimplantation of canine lung. Can J Surg 1970;13:243–250. [PubMed] [Google Scholar]
- 9.Siegelman SS, Hagstrom JW, Koerner SK, Veith FJ. Restoration of bronchial artery circulation after canine lung allotransplantation. J Thorac Cardiovasc Surg 1977;73:792–795. [PubMed] [Google Scholar]
- 10.Guthaner DF, Wexler L, Sadeghi AM, Blank NE, Reitz BA. Revascularization of tracheal anastomosis following heart-lung transplantation. Invest Radiol 1983;18:500–503. [DOI] [PubMed] [Google Scholar]
- 11.Singh SP, Nath H, McGiffin D, Kirklin J. Coronary tracheal collaterals after heart-lung transplant. Am J Cardiol 2003;92:1490–1492. [DOI] [PubMed] [Google Scholar]
- 12.Starobin D, Shitrit D, Steinmetz A, Fink G, Hardoff R, Kramer MR. Quantitative lung perfusion following single lung transplantation. Thorac Cardiovasc Surg 2007;55:48–52. [DOI] [PubMed] [Google Scholar]
- 13.Fukuchi K, Hayashida K, Nakanishi N, Inubushi M, Kyotani S, Nagaya N, Ishida Y. Quantitative analysis of lung perfusion in patients with primary pulmonary hypertension. J Nucl Med 2002;43:757–761. [PubMed] [Google Scholar]
- 14.Benaron DA, Parachikov IH, Friedland S, Soetikno R, Brock-Utne J, van der Starre PJ, Nezhat C, Terris MK, Maxim PG, Carson JJ, et al. Continuous, noninvasive, and localized microvascular tissue oximetry using visible light spectroscopy. Anesthesiology 2004;100:1469–1475. [DOI] [PubMed] [Google Scholar]
- 15.Friedland S, Soetikno R, Benaron D. Reflectance spectrophotometry for the assessment of mucosal perfusion in the gastrointestinal tract. Gastrointest Endosc Clin N Am 2004;14:539–553, ix–x. [DOI] [PubMed] [Google Scholar]
- 16.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321. [DOI] [PubMed] [Google Scholar]
- 17.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int 2008;74:867–872. [DOI] [PubMed] [Google Scholar]
- 18.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, Schwarz MI, Cool CD, Worthen GS. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med 2004;170:242–251. [DOI] [PubMed] [Google Scholar]
- 19.Kaminski N, Rosas IO. Gene expression profiling as a window into idiopathic pulmonary fibrosis pathogenesis: can we identify the right target genes? Proc Am Thorac Soc 2006;3:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 2006;174:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karakiulakis G, Papakonstantinou E, Aletras AJ, Tamm M, Roth M. Cell type-specific effect of hypoxia and platelet-derived growth factor-BB on extracellular matrix turnover and its consequences for lung remodeling. J Biol Chem 2007;282:908–915. [DOI] [PubMed] [Google Scholar]
- 23.Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, Kurokawa K, Fujita T, Ingelfinger JR, Nangaku M. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 2004;65:871–880. [DOI] [PubMed] [Google Scholar]
- 24.Distler JH, Jungel A, Pileckyte M, Zwerina J, Michel BA, Gay RE, Kowal-Bielecka O, Matucci-Cerinic M, Schett G, Marti HH, et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum 2007;56:4203–4215. [DOI] [PubMed] [Google Scholar]
- 25.Ledbetter S, Kurtzberg L, Doyle S, Pratt BM. Renal fibrosis in mice treated with human recombinant transforming growth factor-beta2. Kidney Int 2000;58:2367–2376. [DOI] [PubMed] [Google Scholar]
- 26.Barman SA, Ardell JL, Parker JC, Perry ML, Taylor AE. Pulmonary and systemic blood flow contributions to upper airways in canine lung. Am J Physiol 1988;255:H1130–H1135. [DOI] [PubMed] [Google Scholar]
- 27.Wagner EM, Mitzner W, Brown RH. Site of functional bronchopulmonary anastomoses in sheep. Anat Rec 1999;254:360–366. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa I, Kobayashi K, Kohda E, Hiramatsu K. Bronchopulmonary arterial anastomosis at the precapillary level in human lung. Visualization using CT angiography compared with microangiography of autopsied lung. Acta Radiol 1999;40:578–584. [DOI] [PubMed] [Google Scholar]
- 29.Bernard SL, Luchtel DL, Glenny RW, Lakshminarayan S. Bronchial circulation in the marsupial opossum, Didelphis marsupialis. Respir Physiol 1996;105:77–83. [DOI] [PubMed] [Google Scholar]
- 30.Sundset A, Tadjkarimi S, Khaghani A, Kvernebo K, Yacoub MH. Human en bloc double-lung transplantation: bronchial artery revascularization improves airway perfusion. Ann Thorac Surg 1997;63:790–795. [DOI] [PubMed] [Google Scholar]
- 31.Kamler M, Nowak K, Bock M, Herold U, Motsch J, Hagl S, Gebhard MM, Jakob H. Bronchial artery revascularization restores peribronchial tissue oxygenation after lung transplantation. J Heart Lung Transplant 2004;23:763–766. [DOI] [PubMed] [Google Scholar]
- 32.Norgaard MA, Efsen F, Andersen CB, Svendsen UG, Pettersson G. Medium-term patency and anatomic changes after direct bronchial artery revascularization in lung and heart-lung transplantation with the internal thoracic artery conduit. J Thorac Cardiovasc Surg 1997;114:326–331. [DOI] [PubMed] [Google Scholar]
- 33.Norgaard MA, Andersen CB, Pettersson G. Airway epithelium of transplanted lungs with and without direct bronchial artery revascularization. Eur J Cardiothorac Surg 1999;15:37–44. [DOI] [PubMed] [Google Scholar]
- 34.Nowak K, Kamler M, Bock M, Motsch J, Hagl S, Jakob H, Gebhard MM. Bronchial artery revascularization affects graft recovery after lung transplantation. Am J Respir Crit Care Med 2002;165:216–220. [DOI] [PubMed] [Google Scholar]
- 35.Norgaard MA, Andersen CB, Pettersson G. Does bronchial artery revascularization influence results concerning bronchiolitis obliterans syndrome and/or obliterative bronchiolitis after lung transplantation? Eur J Cardiothorac Surg 1998;14:311–318. [DOI] [PubMed] [Google Scholar]
- 36.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, Trulock EP, Hachem RR. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant 2008;8:2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contreras AG, Briscoe DM. Every allograft needs a silver lining. J Clin Invest 2007;117:3645–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner EM, Blosser S, Mitzner W. Bronchial vascular contribution to lung lymph flow. J Appl Physiol 1998;85:2190–2195. [DOI] [PubMed] [Google Scholar]
- 39.Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax 2009;64:444–450. [DOI] [PubMed] [Google Scholar]
- 40.Wagner EM, Foster WM. Importance of airway blood flow on particle clearance from the lung. J Appl Physiol 1996;81:1878–1883. [DOI] [PubMed] [Google Scholar]
- 41.Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463–481. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Regulation of tissue perfusion in mammals by hypoxia-inducible factor 1. Exp Physiol 2007;92:988–991. [DOI] [PubMed] [Google Scholar]