Abstract
Rationale: The impact of REM-predominant sleep-disordered breathing (SDB) on sleepiness, quality of life (QOL), and sleep maintenance is uncertain.
Objective: To evaluate the association of SDB during REM sleep with daytime sleepiness, health-related QOL, and difficulty maintaining sleep, in comparison to their association with SDB during non-REM sleep in a community-based cohort.
Methods: Cross-sectional analysis of 5,649 Sleep Heart Health Study participants (mean age 62.5 [SD = 10.9], 52.6% women, 22.6% ethnic minorities). SDB during REM and non-REM sleep was quantified using polysomnographically derived apnea-hypopnea index in REM (AHIREM) and non-REM (AHINREM) sleep. Sleepiness, sleep maintenance, and QOL were respectively quantified using the Epworth Sleepiness Scale (ESS), the Sleep Heart Health Study Sleep Habit Questionnaire, and the physical and mental composites scales of the Medical Outcomes Study Short Form (SF)-36.
Measurements and Main Results: AHIREM was not associated with the ESS scores or the physical and mental components scales scores of the SF-36 after adjusting for demographics, body mass index, and AHINREM. AHIREM was not associated with frequent difficulty maintaining sleep or early awakening from sleep. AHINREM was associated with the ESS score (β = 0.25; 95% confidence interval [CI], 0.16 to 0.34) and the physical (β = −0.12; 95% CI, −0.42 to −0.01) and mental (β = −0.20; 95% CI, −0.20 to −0.01) components scores of the SF-36 adjusting for demographics, body mass index, and AHIREM.
Conclusions: In a community-based sample of middle-aged and older adults, REM-predominant SDB is not independently associated with daytime sleepiness, impaired health-related QOL, or self-reported sleep disruption.
Keywords: epidemiology; sleep apnea syndromes; sleep, REM; hypersomnia
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The impact of REM-predominant sleep-disordered breathing (SDB) on sleep and health-related quality of life (HR-QOL) is unknown. The findings from prior studies on the association of REM-predominant SDB and daytime sleepiness have been inconsistent.
What This Study Adds to the Field
This study evaluates the association of REM-predominant SDB and HR-QOL and sleep disruption and the association of REM-predominant SDB and daytime sleepiness in a large community-based patient sample. The findings suggest that in the absence of non-REM SDB, REM SDB is not associated with sleepiness, impaired QOL, or difficulty maintaining sleep.
Sleep-disordered breathing (SDB) has been associated with excessive daytime sleepiness (1–4), insomnia (1, 5), and decreased vitality (6). SDB events may occur during both REM and non-REM sleep. In patients with mild and moderately severe SDB, events often predominate during REM sleep resulting in selective fragmentation or deprivation of REM sleep (7). The role of REM sleep and the impact of REM sleep deprivation are controversial (8). Experimental restriction of REM sleep by awakening study subjects at the onset of REM has been associated with daytime sleepiness in one study (9). Another study, however, concluded that experimental REM sleep deprivation had an alerting effect (10). Furthermore, the effects of selective REM sleep fragmentation are unknown and SDB events during REM sleep are associated with greater degrees of hypoxemia compared with events in non-REM sleep. It is therefore possible that SDB in REM sleep exhibits a different clinical presentation from non-REM SDB.
In a clinic-based study of patients with excessive daytime sleepiness and apnea-hypopnea index (AHI) of 5 to 126, no differences were found in the Epworth Sleepiness Scale (ESS) scores or mean sleep latency measured by maintenance of wakefulness test between subjects with AHI in REM sleep (AHIREM)/AHI in non-REM sleep (AHINREM) greater than 2 compared with subjects with AHIREM/AHINREM less than or equal to 2 (7). Another clinic-based study of patients with excessive daytime sleepiness and an AHI less than 10, AHIREM correlated with daytime sleepiness explaining 35% of the variance in sleep latency (P = 0.001) on the multiple sleep latency test (MSLT) (11). An AHIREM greater than 15 events/hour was predictive of reduced sleep latency on MSLT (11). These findings could not be replicated in a larger clinical sample, however, wherein AHIREM explained only 0.2% of the variance in MSLT among subjects with AHI less than 10 (12). Another clinic-based study found that neither AHIREM nor other indices of SDB during REM were associated with reduced sleep latency regardless of the AHINREM (13). The lack of consistency between the results of these studies may be due to inconsistency in definition of REM SDB, overadjustment or underadjustment for non-REM AHI, and difference in sample size.
In general, clinic-based studies are prone to referral bias and tend to select symptomatic patients or patients with comorbidities. REM SDB has not been previously evaluated in a community-based sample. In this study we explore the association of daytime sleepiness, health-related quality of life (HR-QOL), and self-reported difficulty maintaining sleep with SDB during REM sleep in comparison to their association with SDB during non-REM sleep in the Sleep Heart Health Study (SHHS), a large community-based cohort selected independently of the presence of symptoms or comorbidities.
METHODS
Study Sample
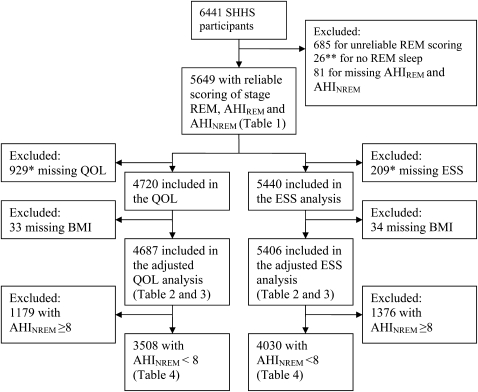
All subjects are participants in the SHHS, a multicenter study of the cardiovascular effects of SDB. The design of the SHHS has been described (14). Subjects chosen for this analysis included SHHS participants who had polysomnography (PSG) with adequate scoring of REM sleep and who completed the ESS or the Medical Outcomes Study 36-item short form (SF-36) HR-QOL survey. Figure 1 provides a description of the study sample.
Figure 1.
Description of the study sample. *38 subjects missing both QOL and ESS. **34 additional subjects with REM time less than 10 minutes were excluded in a secondary analysis. AHI = apnea-hypopnea index; BMI = body mass index; ESS = Epworth Sleepiness Scale; NREM = non-REM; QOL = quality of life; SHHS = Sleep Heart Health Study.
Measurements
SHHS participants underwent PSG using previously published methods, scoring guidelines, and quality-assurance procedures (14–16). The high reliability of the AHI and sleep stage scoring in the SHHS has been reported (16, 17). The AHIREM and the AHINREM were computed as the number of apneas plus hypopneas per hour of REM and non-REM sleep, respectively. Analyses were repeated with AHIREM and AHINREM defined as the number of apneas and hypopneas in REM or non-REM, respectively, per hour of total sleep time.
The ESS was used to measure daytime sleepiness (18, 19), and the physical (PCS) and mental composite scales (MCS) of the SF-36 were used to assess HR-QOL (20–23). Usual sleep duration and sleep-related symptoms were assessed using the SHHS sleep habits questionnaire. Details are provided in the online supplement.
Statistics
The primary independent variables for this analysis were AHIREM and AHINREM. Because the distributions of AHIREM and AHINREM were skewed, both these variables were log transformed using the formula AHI = log(AHI + 0.1) in analyses that treated SDB as a continuous variable. The primary dependent variable was the ESS. Secondary dependent variables included two health-related QOL summary measures (PCS and MCS) and two subjective measures of sleep disruption (trouble staying asleep and early morning awakening with inability to return to asleep).
We analyzed the association of REM SDB with sleepiness and HR-QOL using multiple linear regression (proc REG in SAS 9.1) with REM SDB defined as a continuous variable. The main model adjusted for age, race, sex, and body mass index (BMI). Models further adjusted for self-reported sleep time and history of cardiovascular disease, respiratory disease, and diabetes. To adjust for a possible confounding effect of AHINREM and explicitly evaluate the association of REM-predominant SDB with sleepiness and HR-QOL measures, analyses that included both AHIREM and AHINREM in the same model were also performed. Because AHIREM and AHINREM show a considerable degree of correlation we also performed analyses that excluded subjects in the highest quartile of AHINREM distribution (AHINREM ≥8) rather than including both AHIREM and AHINREM in the same model. Because of differences in the prevalence of REM SDB and sometimes SDB-related symptoms between men and women (24) analyses were also stratified by sex.
The analyses were repeated in the subset with AHINREM less than 8 using analysis of covariance (proc GLM) with REM SDB defined as a categorical variable following quantiles of AHIREM (defined using quartiles of AHIREM with the highest quartile split in half), which allowed evaluation of the association of REM SDB and sleepiness and QOL in the group with the most severe REM SDB. In a secondary analysis logistic regression (proc logistic) was used to evaluate the association of REM SDB and difficulty staying asleep and early awakening treated as binary variables.
RESULTS
The characteristics of the study subjects with AHINREM greater than or equal to 8 and those with AHINREM less than 8 further stratified by quartiles of AHIREM are presented in Table 1. Subjects with more severe REM-predominant SDB (AHINREM <8) were on average older and had a higher BMI. They were also more likely to be men, American Indian, and to have diabetes, cardiovascular disease, or respiratory disease. Participants excluded from the sleepiness analysis were on average older (64.9 vs. 62.5 yr), more likely to be women (54.6 vs. 52.5%), less likely to be white (70 vs. 78%), and more likely to have diabetes (16 vs. 10%) and cardiovascular (17 vs. 13%) or respiratory diseases (17 vs. 14%). Participants excluded from the QOL analysis were more likely to be American Indian (34% vs. 0), have diabetes (20 vs. 7%), and cardiovascular (17 vs. 12%) or respiratory diseases (16 vs. 13%). There was a moderate correlation between AHIREM and AHINREM (correlation coefficient 0.6; P < 0.001)
TABLE 1.
CHARACTERISTICS OF SUBJECTS STRATIFIED BY APNEA-HYPOPNEA INDEX
| AHINREM <8 |
||||||
|---|---|---|---|---|---|---|
| Characteristic | AHIREM <1.25 | AHIREM 1.25–4.96 | AHIREM 4.97–12.9 | AHIREM ≥13 | AHINREM ≥8 | P Value* |
| N | 1,052 | 1,060 | 1,055 | 1,056 | 1,426 | |
| Mean age, yr (SD) | 59.6 (11.3) | 60.8 (10.8) | 62.7 (10.6) | 63.2 (10.6) | 65.1 (10.4) | <0.0001 |
| Females, % | 66 | 59 | 57 | 57 | 31.3 | <0.0001 |
| Race, % | ||||||
| White | 78 | 79 | 77 | 74 | 78 | 0.046 |
| Black | 8 | 8 | 8 | 7 | 8 | |
| Native American | 5 | 7 | 8 | 11 | 9 | |
| Hispanic | 6 | 4 | 5 | 5 | 4 | |
| Asian/Pacific Islander | 3 | 2 | 1 | 2 | 1 | |
| Mean BMI, kg/m2 (SD) | 26.8 (4.2) | 26.8 (4.2) | 28.1 (4.5) | 30.7 (5.7) | 30.2 (5.6) | <0.0001 |
| Mean usual sleep time, h (SD) | 7.1 (1.1) | 7.1 (1.2) | 7.0 (1.2) | 7.0 (1.2) | 7.1 (1.3) | 0.093 |
| Cardiovascular disease, % | 8.3 | 9.3 | 11.3 | 13.8 | 19.1 | <0.0001 |
| Respiratory disease, % | 12.3 | 13.3 | 15.5 | 15.0 | 12.9 | 0.03 |
| Diabetes, % | 5.8 | 8.0 | 8.5 | 11.9 | 14.6 | <0.0001 |
| Median AHI, events/h (IQR) | 0.4 (0.1–2.4) | 1.3 (0.8–2.2) | 3.3 (2.3–4.8) | 7.5 (5.5–9.9) | 18.9 (13.3–29.1) | <0.0001 |
| Median AHIREM, events/h (IQR) | 0 (0–0.7) | 2.7 (1.9–3.7) | 8.3 (6.5–10.6) | 22.7 (17.3–32.1) | 26.1 (12.7–42.7) | <0.0001 |
| Median AHINREM, events/hr (IQR) | 0.4 (0–1.1) | 0.8 (0.2–2.0) | 1.8 (0.7–3.7) | 3.2 (1.7–5.3) | 16.6 (11.3–27.2) | <0.0001 |
| Median AHIREM/AHINREM (IQR) | 0 (0–0.9) | 2.7 (1.1–5.8) | 4.4 (2.3–9.5) | 7.9 (5.0–14.0) | 1.2 (0.7–2.2) | <0.0001 |
| REM sleep, % total sleep time (SD) | 20.7 (6.4) | 21.4 (5.8) | 20.7 (5.7) | 20.0 (5.9) | 18.2 (6.0) | <0.0008 |
| REM time, min (SD) | 76.6 (28.5) | 80.1 (26.8) | 76.2 (26.2) | 71.8 (26.2) | 64.2 (25.5) | <0.0001 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; ESS = Epworth sleepiness scale; IQR = interquartile range; NREM = non-REM.
P value testing for linear trend across groups defined by AHIREM quartiles.
When all subjects were considered, in analyses that treated AHIREM and AHINREM as continuous variables and adjusted for demographics (age, sex, race) and BMI, both AHIREM and AHINREM were associated with daytime sleepiness and poorer physical HR-QOL, but not mental health (Table 2). With further adjustment for sleep time and comorbidities, the association of AHIREM and physical HR-QOL became nonsignificant (results not shown). When both AHIREM and AHINREM were included in the same model, the association of AHIREM with daytime sleepiness and with physical HR-QOL became nonsignificant; however, AHINREM was associated with daytime sleepiness as well as poorer physical and mental HR-QOL in the main model (Table 3). The associations between AHINREM with daytime sleepiness and physical HR-QOL persisted after further adjustment for sleep time and comorbidities (results not shown). The interaction term for AHIREM and AHINREM was nonsignificant in the adjusted models for ESS and HR-QOL measures. There were no meaningful differences in the findings when the analyses were repeated with AHIREM defined as the number of apneas and hypopneas in REM per hour of total sleep time (results not shown), or when 34 subjects with REM time less than 10 minutes were excluded from the analyses.
TABLE 2.
ASSOCIATION OF SLEEP-DISORDERED BREATHING WITH SLEEPINESS AND HEALTH-RELATED QUALITY OF LIFE FOR OVERALL APNEA-HYPOPNEA INDEX AND REM AND NON-REM APNEA-HYPOPNEA INDEX
| Log(AHI + 0.1) |
Log(AHINREM + 0.1) |
Log(AHIREM + 0.1) |
|||||
|---|---|---|---|---|---|---|---|
| N | Adjusted* β-Coefficient (95% CI) | P Value | Adjusted* β-Coefficient (95% CI) | P Value | Adjusted* β-Coefficient (95% CI) | P Value | |
| Epworth Sleepiness Scale | 5,406 | 0.28 (0.19, 0.37) | <0.0001 | 0.25 (0.17, 0.33) | <0.0001 | 0.11 (0.04, 0.18) | 0.001 |
| Physical Composite Scale | 4,687 | −0.36 (−0.57, −0.16) | 0.0004 | −0.28 (−0.46, −0.10) | 0.002 | −0.20 (−0.36, −0.05) | 0.009 |
| Mental Composite Scale | 4,687 | −0.12 (−0.30, 0.06) | 0.2 | −0.14 (−0.30, 0.02) | 0.08 | 0.01 (−0.13, 0.15) | 0.89 |
Definition of abbreviations: AHI = apnea hypopnea index; BMI = body mass index; CI = confidence interval; NREM = non-REM.
Models adjusted for: age, sex race, BMI.
TABLE 3.
ASSOCIATION OF REM SLEEP-DISORDERED BREATHING WITH SLEEPINESS AND HEALTH-RELATED QUALITY OF LIFE ADJUSTING FOR NREM SLEEP-DISORDERED BREATHING
| Log(AHINREM + 0.1) |
Log(AHIREM + 0.1) |
||||
|---|---|---|---|---|---|
| N | Adjusted* β-Coefficient (95% CI) | P Value | Adjusted† β-Coefficient (95% CI) | P Value | |
| Epworth Sleepiness Scale | 5,406 | 0.26 (0.16, 0.34) | <0.0001 | −0.01 (−0.16, 0.08) | 0.97 |
| Physical Composite Scale | 4,687 | −0.21 (−0.42, −0.01) | 0.04 | −0.11 (−0.29, 0.07) | 0.23 |
| Mental Composite Scale | 4,687 | −0.20 (−0.38, −0.01) | 0.03 | 0.10 (−0.06, 0.26) | 0.24 |
For definition of abbreviations, see Table 2.
AHIREM and AHINREM are included in the same model.
Models adjusted for: age, sex, race, BMI, and AHIREM.
Models adjusted for: age, sex, race, BMI, and AHINREM.
As expected, in the sample with AHINREM less than 8, the association of AHINREM with daytime sleepiness and physical and mental HR-QOL measures was diminished, although it remained significant for daytime sleepiness in demographic- and BMI-adjusted models (Table 4). There were, however, no associations between AHIREM and daytime sleepiness or physical and mental HR-QOL measures in models adjusted for demographics and BMI (Table 4) or in sex-stratified analyses (Table 5).
TABLE 4.
THE ASSOCIATION OF SLEEP-DISORDERED BREATHING WITH SLEEPINESS AND HEALTH-RELATED QUALITY OF LIFE FOR SUBJECTS WITH NON-REM APNEA-HYPOPNEA SCORES LESS THAN 8 EVENTS PER HOUR
| Log(AHINREM + 0.1) |
Log(AHIREM + 0.1) |
||||
|---|---|---|---|---|---|
| N | Adjusted* β-Coefficient (95% CI) | P Value | Adjusted* β-Coefficient (95% CI) | P Value | |
| Epworth Sleepiness Scale | 4,030 | 0.18 (0.06, 0.29) | 0.002 | 0.03 (−0.04, 0.11) | 0.4 |
| Physical Composite Scale | 3,508 | −0.19 (−0.43, 0.06) | 0.14 | −0.14 (−0.32, 0.03) | 0.11 |
| Mental Composite Scale | 3,508 | −0.09 (−0.31, 0.13) | 0.44 | 0.08 (−0.08, 0.24) | 0.33 |
For definition of abbreviations, see Table 2.
All subjects had AHINREM <8, with separate models for AHIREM and AHINREM.
Models adjusted for: age, race, sex, BMI.
TABLE 5.
SEX-STRATIFIED ASSOCIATION OF REM SLEEP-DISORDERED BREATHING WITH SLEEPINESS AND HEALTH-RELATED QUALITY OF LIFE MEASURES
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Log(AHIREM + 0.1) |
Log(AHIREM + 0.1) |
|||||
| N | Adjusted* β-Coefficient (95% CI) | P Value | N | Adjusted* β-Coefficient (95% CI) | P Value | |
| Epworth Sleepiness Scale | 2,408 | 0.03 (−0.07, 0.12) | 0.59 | 1,622 | 0.05 (−0.08, 0.18) | 0.45 |
| Physical Composite Scale | 2,106 | −0.21 (−0.44, 0.02) | 0.08 | 1,402 | −0.01 (−0.28, 0.25) | 0.91 |
| Mental Composite Scale | 2,106 | −0.04 (−0.25, 0.17) | 0.70 | 1,402 | 0.24 (0.00, 0.48) | 0.052 |
For definition of abbreviations, see Table 2.
All subjects had AHINREM <8.
Models adjusted for: age, race, BMI.
In analyses treating AHIREM as a categorical variable, AHIREM was not associated with daytime sleepiness or QOL measures in demographics and BMI-adjusted models (Table 6) as well as in sex-stratified analysis (results not shown). Finally, AHIREM did not predict frequent difficulty maintaining sleep (OR = 1 [95% CI, 1–1.01]) or frequent early morning awakening (OR = 1 [95% CI, 0.99–10.1]) in models adjusted for demographics and BMI.
TABLE 6.
MEAN EPWORTH SLEEPINESS SCALE AND MEDICAL OUTCOMES STUDY SHORT FORM-36 HEALTH-RELATED QUALITY OF LIFE SCORES
| Unadjusted |
Model 1* |
|||
|---|---|---|---|---|
| AHIREM | N | Mean Epworth Sleepiness Score (95% CI) | N | Mean Epworth Sleepiness Score (95% CI) |
| <1.25 | 1,000 | 7.22 (7.95, 7.48) | 993 | 7.36 (7.08, 7.63) |
| 1.25–4.96 | 1,018 | 7.32 (7.06, 7.58) | 1,014 | 7.34 (7.08, 7.61) |
| 4.97–12.9 | 1,019 | 7.44 (7.18, 7.70) | 1,007 | 7.42 (7.16, 7.68) |
| 13.0–22.9 | 522 | 7.44 (7.21, 7.94) | 520 | 7.44 (7.08, 7.81) |
| ≥23.0 | 500 | 7.91 (7.53, 8.28) | 496 | 7.73 (7.35, 8.13) |
| P value | 0.04 | 0.58 | ||
| AHIREM | N | Mean Physical Composite Score (95% CI) | N | Mean Physical Composite Score (95% CI) |
| <1.25 | 900 | 49.7 (49.1, 50.3) | 892 | 48.6 (48.0, 49.2) |
| 1.25–4.96 | 890 | 49.0 (48.4, 49.6) | 885 | 48.4 (47.8, 49.0) |
| 4.97–12.9 | 871 | 47.9 (47.3, 47.3) | 859 | 48.4 (47.8, 49.0) |
| 13.0–22.9 | 462 | 46.4 (45.6, 47.3) | 459 | 46.4 (46.5, 48.2) |
| ≥23.0 | 415 | 45.7 (44.8, 46.6) | 413 | 47.5 (46.6, 48.4) |
| P value | <0.001 | 0.08 | ||
| AHIREM | N | Mean Mental Composite Score (95% CI) | N | Mean Mental Composite Score (95% CI) |
| <1.25 | 900 | 52.5 (52.0, 53.1) | 892 | 52.9 (52.4, 53.5) |
| 1.25–4.96 | 890 | 53.7 (53.2, 54.2) | 885 | 53.8 (53.3, 54.3) |
| 4.97–12.9 | 871 | 53.4 (52.9, 54.0) | 859 | 53.3 (52.8, 53.8) |
| 13.0–22.9 | 462 | 53.6 (52.8, 54.3) | 459 | 53.4 (52.6, 54.1) |
| ≥23.0 | 415 | 53.6 (52.8, 54.4) | 413 | 53.3 (52.5, 54.1) |
| P value | 0.03 | 0.25 | ||
For definition of abbreviations, see Table 2.
Scores given across quantiles of AHIREM in subjects with AHINREM <8.
Model 1 adjusted for: age, race, sex, and BMI.
DISCUSSION
In a large, community-based sample of middle-aged and older adults, REM SDB was not associated with excessive daytime sleepiness as measured by the ESS or HR-QOL as assessed by the SF-36 PCS and MCS scores after adjusting for age, sex, race, BMI, and AHINREM. REM SDB was not associated with frequent subjective complaints of sleep disruption either. On the other hand, non-REM SDB was associated with excessive daytime sleepiness and with poorer physical and mental health after adjusting for demographics and BMI, and after further adjustment for AHIREM.
To our knowledge, this is the first study to evaluate the association of REM SDB with indices of HR-QOL, and the first study to examine the association of REM SDB with daytime sleepiness in a large ethnically diverse community-based sample selected independent of the presence of daytime sleepiness. Our results are consistent with the findings from two prior clinic-based studies, which showed that REM SDB was not associated with sleepiness as measured by MSLT (12, 13). The MSLT quantifies the actual time required by the subject to fall asleep. We used the well-validated ESS, an instrument that assesses the subjective perception of sleepiness. Clinical decisions in the management of patients with SDB are generally based in part on the patients' subjective report of sleepiness rather than the MSLT.
In contrast to our findings, a previous clinic-based study that reported an association between REM SDB and sleepiness as measured by MSLT examined a small sample of patients and did not adjust for confounding variables (11). A second clinic-based study that found no difference in daytime sleepiness measured by ESS between subjects with REM SDB versus those with non-REM SDB (7) also did not adjust for confounding variables. Clinic-based studies are generally subject to referral bias given that patients without clinical sleepiness are less likely to be referred for clinical testing. Furthermore, the use of an arbitrary definition of REM SDB (AHIREM/AHINREM >2) in the later study (11) could have resulted in misclassification as the REM SDB group had a mean AHINREM of 9.6 (SD = 7.9).
The lack of association between REM SDB and sleepiness is also consistent with experimental evidence. REM sleep restriction to 4% of sleep time in one study was not associated with excessive daytime sleepiness despite a concomitant reduction in total sleep time (10). This led the authors to conclude that REM restriction may have an alerting effect. In contrast, REM sleep restriction was associated with excessive daytime sleepiness to a level similar to non-REM sleep restriction in another study (9). In the latter study; however, REM sleep was only reduced to 9% of sleep time, and subjects were exposed to more frequent awakenings (12–18 vs. 9–11 in the former study) suggesting that sleep deprivation may have been related to repeated awakening rather than REM deprivation.
REM sleep usually accounts for less than 30% of total sleep time and non-REM sleep for more than 70% of sleep time. Thus, the severity of REM SDB as measured by AHIREM corresponds to fewer disruptions to total sleep than the same level of severity of non-REM SDB sleep as measured by AHINREM. Furthermore, the traditional definition of AHIREM could overestimate the severity of REM SDB in individuals with short REM duration compared with individuals who experience more SDB with longer REM duration. Yet, there was no meaningful difference in the findings when analyses were repeated with AHIREM defined as the number of apneas and hypopneas in REM/total sleep time or when 34 subjects with REM time less than 10 minutes were excluded from the analysis. Thus the lack of association between REM SDB and daytime sleepiness or HR-QOL does not reflect misspecification of the severity of REM SDB by the traditional definition of AHIREM.
This study has several limitations. Although suggestive of a lack of association between REM SDB and daytime sleepiness and HR-QOL, confounding by unmeasured covariates cannot be excluded in an observational study. A randomized controlled trial assessing the effect of treating REM SDB on sleepiness and HR-QOL would provide superior evidence. Sixteen percent of SHHS participants (1,035 subjects) were excluded from the sleepiness analysis and 27% (1,754 subjects) from the HR-QOL analysis because of unreliable scoring of REM sleep or missing variables of interest. Bias is a potential limitation if the association of interest was different in subjects who were excluded; however, subjects excluded from the sleepiness or HR-QOL analyses were similar to included subjects in BMI, AHI, and where available ESS and AHIREM.
The significant degree of correlation between AHIREM and AHINREM raises concerns about collinearity in the models that include both variables; however, formal collinearity diagnostics using variance inflation measures and condition indices suggest that the regression estimates are not meaningfully affected by collinearity. Moreover, adjustment for the confounding effect of non-REM SDB by excluding subjects with the highest quartile of AHINREM led to the same conclusions.
The method used in this study (thermocouple plus inductance plethysmography) is less sensitive for detection of subtle airflow limitation than the currently recommended combination of thermocouple plus nasal cannula-pressure transducers systems. Yet, the use of a requirement for a 4% oxyhemoglobin desaturation in identifying hypopneas, as recommended in the current American Academy of Sleep Medicine guidelines, greatly mitigates this concern as few hypopneas will be missed. It is possible, however, that events associated with arousal but not hypoxemia could contribute to sleepiness, and would not be addressed in this study or in any study using the current standard desaturation criterion to identify hypopneas. Finally, the most appropriate tool to evaluate sleepiness remains controversial (25, 26). In this study the ESS, a self-completion questionnaire that measures the subjective perception of sleepiness on a 0 to 24-point scale, was used to quantify sleepiness. The MSLT, which measures the actual time required by the subject to fall asleep, is considered the gold standard for measuring sleepiness by some; however, others consider that there is no gold standard for measuring sleepiness. Furthermore, the clinical usefulness of the MSLT in patients with SDB is unknown. The ESS, on the other hand, is the most widely used tool for evaluating daytime sleepiness in patients with SDB. The ESS is reliable and well validated (19); it correlates inversely with sleep latency on the MSLT (3, 27) and positively with the AHI in patients with SDB (3, 4, 28) and with the likelihood of falling asleep while driving (29). The ESS was also shown to decrease with treatment for SDB (30). Balancing the limitations are several strengths to this work, including the large ethnically diverse community-based sample selected irrespective of daytime sleepiness and the standardized PSG measures obtained according to strict protocols and rigorous quality control measures.
Although a randomized controlled trial of therapy for REM SDB is needed to definitively assess the clinical significance of this condition, findings from this study suggest that in the absence of non-REM SDB, REM SDB is not associated with daytime sleepiness, impaired QOL, or sleep disruption in the general adult population. Thus, distinctions should be made between REM SDB and non-REM SDB in the management of patients with daytime sleepiness. Other causes of daytime sleepiness should be sought when SDB is present during REM sleep only. As the effects of REM SDB on cardiovascular outcome, metabolic function, and other neurocognitive functions, such as memory, are unknown, whether REM SDB should be treated in the absence of non-REM SDB remains to be determined.
Supplementary Material
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200908-1304OC on January 28, 2010
Conflict of Interest Statement: H.A.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R. received $5,001–$10,000 from Mannkind for DSMB for Inhaled Insulin Study and $5,001–$10,000 from Apnex Medical for serving on an advisory board for Phrenic Nerve Pacemaker Development for treatment of OSA, $10,001–$50,000 from Respironics as an expert witness in a patent infringement suit (2005–2008), $10,001–$50,000 from Watermark (Advanced Brain Monitoring) for NIH SBIR contract for evaluation of ambulatory sleep monitoring, more than $100,001 from Fisher & Paykel Healthcare for R&D contract for development of novel CPAP, $50,001–$100,000 from Korosensor for NIH SBIR contract for evaluation of novel effort belts for sleep studies, $50,001–$100,000 from Medtronic (Restore Medical) for central reading for sleep studies used in evaluation of Pillar implant for OSA, and more than $100,001 from Ventus Medical for evaluation of mechanism of Provent for OSA. He has patents from Covidian for CPAP and diagnostic technology (through NYU), patents from Fisher & Paykel Healthcare for CPAP technology, and a patent from Biologics for automated sleep scoring, and more than $100,001 from Covidian in royalties for patents for CPAP and $50,001–$100,000 from Fisher & Paykel in royalties for patents for CPAP. N.P. received $1,001–$5,000 from ResMed and $1,001–$5,000 from Respironics in lecture fees and $50,001–$100,000 from ResMed in industry-sponsored grants for a multicenter clinical trial on CPAP in diabetics. D.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Klink ME, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest 1987;91:540–546. [DOI] [PubMed] [Google Scholar]
- 2.Roehrs T, Zorick F, Wittig R, Conway W, Roth T. Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest 1989;95:1202–1206. [DOI] [PubMed] [Google Scholar]
- 3.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993;103:30–36. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, Nieto FJ, Rosenberg CE. Relation of sleepiness to respiratory disturbance index. Am J Respir Crit Care Med 1999;159:502–507. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994;149:722–726. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin CM, Griffith KA, Nieto J, O'Connor GT, Walsleben JA, Redline S. The association of SDB and sleep symptoms with quality of life measures in the sleep heart health study. Sleep 2001;24:96–105. [DOI] [PubMed] [Google Scholar]
- 7.Haba-Rubio J, Janssens J, Rochat T, Sforza E. Rapid eye movements-related disordered breathing clinical and polysomnographic features. Chest 2005;128:3350–3357. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH. Acute sleep deprivation. In Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine, 4th ed. Philadelphia: Elsevier Inc.; 2005, pp. 51–66.
- 9.Glovinsky P, Glovinsky PB, Spielman AJ, Carroll P, Weinstein L, Ellman SJ. Sleepiness and REM sleep recurrence: the effects of stage 2 and REM sleep awakenings. Psychophysiology 1990;27:552–559. [DOI] [PubMed] [Google Scholar]
- 10.Nykamp K, Rosenthal L, Folkerts M, Roehrs T, Guido P, Roth T. The effect of REM sleep deprivation on the level of sleepiness/alertness. Sleep 1998;21:609–614. [DOI] [PubMed] [Google Scholar]
- 11.Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med 1996;154:167–169. [DOI] [PubMed] [Google Scholar]
- 12.Cheverin R, Aldritch MS. The relation between multiple sleep latency test findings and the frequency of apneic events in REM and NREM sleep. Chest 1998;113:980–984. [DOI] [PubMed] [Google Scholar]
- 13.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep 2002;25:307–314. [PubMed] [Google Scholar]
- 14.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale and methods. Sleep 1997;20:1077–1085. [PubMed] [Google Scholar]
- 15.Redline S, Sander MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep 1998;21:759–767. [PubMed] [Google Scholar]
- 16.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 1998;21:749–757. [DOI] [PubMed] [Google Scholar]
- 17.Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, Redline S, Sanders M, Young T; Sleep Heart Health Study (SHHS) Research Group. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnogaphy—The Sleep Heart Health Study. Sleep 2002;25:843–849. [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376–381. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: reliability and validity in a patient population. Med Care 1988;26:724–735. [DOI] [PubMed] [Google Scholar]
- 22.Stewart AL, Greenfield S, Hays RD, Wels K, Rogers WH, Berry SD, McGlynn EA, Ware JE Jr. Functional status and well-being of patients with chronic conditions: results from the medical outcomes study. JAMA 1989;262:907–913. [PubMed] [Google Scholar]
- 23.Tarlov AR, Ware JE, Greenfield S, Nelson EC, Perrin E, Zubkoff M. The medical outcomes study: an application of methods for monitoring the results of medical care. JAMA 1989;262:925–930. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor C, Thornley K, Hanley P. Gender differences in the polysomnogrphic features of patients with sleep apnea. Am J Respir Crit Care Med 2000;161:1465–1472. [DOI] [PubMed] [Google Scholar]
- 25.Carskadon MA. Evaluation of excessive daytime sleepiness. Neurophysiol Clin 1993;23:91–100. [DOI] [PubMed] [Google Scholar]
- 26.Johns M. Rethinking the assessment of sleepiness. Sleep Med Rev 1998;2:3–15. [DOI] [PubMed] [Google Scholar]
- 27.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res 1997;42:145–155. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 1994;17:703–710. [DOI] [PubMed] [Google Scholar]
- 29.Maycock G. Sleepiness and driving: the experience of UK car drivers. Accid Anal Prev 1997;29:453–462. [DOI] [PubMed] [Google Scholar]
- 30.Engleman HM, Kingshott RN, Wraith PK, MacKay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:461–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.