Abstract
Rationale: Bronchopulmonary dysplasia (BPD) and emphysema are characterized by arrested alveolar development or loss of alveoli; both are significant global health problems and currently lack effective therapy. Bone marrow–derived mesenchymal stem cells (BMSCs) prevent adult lung injury, but their therapeutic potential in neonatal lung disease is unknown.
Objectives: We hypothesized that intratracheal delivery of BMSCs would prevent alveolar destruction in experimental BPD.
Methods: In vitro, BMSC differentiation and migration were assessed using co-culture assays and a modified Boyden chamber. In vivo, the therapeutic potential of BMSCs was assessed in a chronic hyperoxia-induced model of BPD in newborn rats.
Measurements and Main Results: In vitro, BMSCs developed immunophenotypic and ultrastructural characteristics of type II alveolar epithelial cells (AEC2) (surfactant protein C expression and lamellar bodies) when co-cultured with lung tissue, but not with culture medium alone or liver. Migration assays revealed preferential attraction of BMSCs toward oxygen-damaged lung versus normal lung. In vivo, chronic hyperoxia in newborn rats led to air space enlargement and loss of lung capillaries, and this was associated with a decrease in circulating and resident lung BMSCs. Intratracheal delivery of BMSCs on Postnatal Day 4 improved survival and exercise tolerance while attenuating alveolar and lung vascular injury and pulmonary hypertension. Engrafted BMSCs coexpressed the AEC2-specific marker surfactant protein C. However, engraftment was disproportionately low for cell replacement to account for the therapeutic benefit, suggesting a paracrine-mediated mechanism. In vitro, BMSC-derived conditioned medium prevented O2-induced AEC2 apoptosis, accelerated AEC2 wound healing, and enhanced endothelial cord formation.
Conclusions: BMSCs prevent arrested alveolar and vascular growth in part through paracrine activity. Stem cell–based therapies may offer new therapeutic avenues for lung diseases that currently lack efficient treatments.
Keywords: stem cells, aging, lung, oxygen
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Effective therapies for arrested lung and vascular growth in chronic lung disease of prematurity are lacking. In some settings, mesenchymal stem cells (MSCs) show promise for organ repair, but whether MSC treatment can enhance lung structure after neonatal lung injury is unknown.
What This Study Adds to the Field
MSC treatment preserved alveolar structure in a model of chronic lung disease in newborn rats caused by severe hyperoxia, which may be due to a paracrine effect on lung cells. MSCs may have therapeutic potential for preventing neonatal lung diseases characterized by alveolar damage.
Acute and chronic lung diseases pose a growing socioeconomic burden (1). Preterm birth is on the rise and the leading cause of perinatal mortality and morbidity, accounting for more than 85% of all perinatal complications and death (2). Improvements in perinatal care have increased the survival of extremely premature newborns (born at less than 28 wk of gestation) (3). These infants, however, are at high risk for long-term injury to both lung and brain (3). Each year, 5,000–10,000 newborns suffer from bronchopulmonary dysplasia (BPD) (http://www.nhlbi.nih.gov/health/dci/Diseases/Bpd/Bpd_WhoIsAtRisk.html), a chronic lung disease that follows ventilator and oxygen therapy for acute respiratory failure after premature birth (4). BPD has long-term respiratory and neurodevelopmental consequences that extend beyond childhood and result in increased health care costs (3). Further advances are required to increase survival free of neonatal morbidity or neurodevelopmental impairment. Alarming reports suggest that BPD results in early-onset emphysema (5, 6). Emphysema, defined as airspace enlargement distal to terminal bronchioles, is a major component of chronic obstructive pulmonary disease, the fourth leading cause of death in the United States (1). BPD and emphysema are characterized by interrupted development and loss of alveolar structures, respectively, and therapy is palliative.
Increasing attention has been focused on the use of adult-derived stem cells to regenerate damaged organs. Systemically injected mouse bone marrow–derived mesenchymal stem cells (BMSCs) have been demonstrated to differentiate into parenchymal cells of various nonhematopoietic tissues including lung (7). These properties have already been harnessed to treat various diseases, including among others inborn errors of metabolism, neurodegenerative disorders, and cardiovascular diseases (8, 9). In contrast, relatively little information is available on the therapeutic potential of MSCs in lung diseases characterized by alveolar damage (reviewed in References 10–12). In the adult lung, studies have focused on proof of principle that BM-derived cells can home to the lung and adopt various lung cell phenotypes in various injury models. These findings have been called into question (13, 14). Reports now suggest that BM-derived stem cells can prevent adult lung injury (15–18). The therapeutic benefit of these cells in arrested alveolar development has not been explored.
Here, we provide evidence that BMSCs improve survival and exercise capacity and prevent alveolar growth arrest in a well-established, chronic O2-induced model of BPD characterized by otherwise irreversible alveolar and lung capillary rarefaction. We also provide direct in vitro evidence that the beneficial effects of MSCs might be mediated through paracrine activity.
METHODS
Expanded methods are available in the online supplement. All procedures and protocols were approved by the Animal Health Care Committee of the University of Alberta (Edmonton, AB, Canada).
MSC Harvest and Cell Culture
BM from adult Sprague-Dawley rats was plated into tissue culture flasks. Adhered cells were allowed to grow to about 75% confluency and then trypsinized and reseeded at a density of 105 cells/cm2. This procedure was performed for two passages. Pulmonary artery smooth muscle cells (PASMCs), used as control cells, were obtained from adult Sprague-Dawley rats as previously described (19).
Fluorescence-activated Cell Sorting of Cell Surface Markers
Phycoerythrin labeling for rat monoclonal antibodies against CD31, CD34, CD44, CD45, CD54, CD73, and CD90 (Santa Cruz Biotechnology, Santa Cruz, CA) was used according to the manufacturer's protocol and were selected in accordance with the position statement for the minimal criteria to define an MSC, from the International Society for Cellular Therapy (20). Cells were analyzed with a FACScan (Becton Dickinson, Franklin Lakes, NJ) and CellQuest software as described (21).
Stem Cell Lineage Differentiation Assay
The lineage differentiation assay was performed in accordance with the position statement for the minimal criteria to define an MSC, from the International Society for Cellular Therapy (20).
Adipogenic, osteogenic, and chondrogenic induction was performed on passage 2 BMSCs as previously described (21).
Reverse Transcription-Polymerase Chain Reaction Analysis for Lineage Conversion
Total RNA was extracted from undifferentiated (control) and differentiated BMSCs and analyzed by reverse transcription-polymerase chain reaction (RT-PCR) with primers specific for adipogenic (Pparg2, Lpl), osteogenic (Bglap, Runx2), and chondrogenic (Col2a1 and Col10a1) lineage conversion.
Stem Cell Migration Assay
BMSCs were seeded in the upper chamber of a modified Boyden chamber (Millipore, Temecula, CA) on an 8-μm mesh. Cells then were allowed to migrate for 6 hours in a 37°C incubator toward either Dulbecco's modified Eagle's medium (DMEM), DMEM plus normoxic lung tissue, or DMEM plus hyperoxic lung tissue in the lower chamber.
Co-Culture Assay
BMSCs were seeded in the bottom of a modified Boyden chamber (Corning Inc., Corning, NY) with a 0.4-μm mesh separating the upper and lower chambers and exposed to various culture conditions. Cells were stained for surfactant protein (SP)-C and 4′,6-diamidino-2-phenylindole and imaged with a confocal microscope. A TaqMan one-step RT-PCR master mix reagent kit (Applied Biosystems, Foster City, CA) was used to quantify the copy number of cDNA targets as described in Reference 22.
Animal Model
Experimental BPD was induced as previously described (22). Sprague-Dawley rats (Charles River, Saint Constant, QC, Canada) were exposed to normoxia (21% O2) or hyperoxia (95% O2, BPD model) from birth to Postnatal Day 14 (P14) in sealed Plexiglas chambers with continuous O2 monitoring (BioSpherix, Redfield, NY).
In Vivo Experimental Design
Newborn rat pups were randomized to four groups: (1) normoxia (21% O2, control group), (2) hyperoxia (95% O2, BPD group), (3) hyperoxia plus BMSCs, and (4) hyperoxia plus PASMCs. Cells were administered on P4 (prevention studies) or P14 (regeneration studies) via an intratracheal injection (1.0 × 105 cells per animal). Before administration, BMSCs were labeled with the intravital green fluorescent dye 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Sigma Canada, Oakville, ON, Canada) according to the manufacturer's protocol. Animals were harvested on P21 (prevention studies) or P45 (regeneration studies).
Exercise Capacity
Rats were run according to a predetermined protocol. Exhaustion was defined as the animal running exclusively on the lower third of the treadmill coupled with hitting of the shock panel twice within 30 seconds.
Lung Morphometry
Lungs were inflated and fixed via the trachea with a 4% formaldehyde solution at a constant pressure of 20 cm H2O (22). Lungs were paraffin embedded and cut into 4-μm-thick serial sections, and lung sections were stained with hematoxylin and eosin. Alveolar structures were quantified using the mean linear intercept as described (22).
Barium Angiogram
Barium was instilled into the pulmonary vasculature as previously described (22). The barium was imaged with a rodent SPECT-CT (FLEX Pre-clinical platform) using Amira software package (Gamma Medica, Northridge, CA).
Mercox Vascular Casting and Scanning Electron Microscopy
Mercox vascular casting were prepared and imaged as previously described (22).
Right Ventricular Hypertrophy
The right ventricle free wall was separated from the left ventricle and the septal wall. The tissue was dried overnight and weighed the next day (19).
Pulmonary Artery Acceleration Time
Pulmonary artery acceleration time (PAAT), a valid measure of mean pulmonary arterial pressure in rodents, was assessed by Doppler echocardiography as previously described (19, 23).
MSC Engraftment
Lungs from P14 and P21 animals (n = 3 per time point) were inflated with Tissue-Tek optimal cutting temperature (O.C.T.) medium (Ted Pella, Inc., Redding, CA) and subsequently frozen in a block of O.C.T. medium. Lungs were stained for the type 2 alveolar epithelial cell (AEC2)–specific marker SP-C, pro-SP-C, and cellular nuclei (4′,6-diamidino-2-phenylindole) and imaged with a confocal microscope. BMSCs labeled with the cytoplasmic dye CFSE were manually counted in 25 random fields throughout the lung for a total cell count of 10,624 cells on P14, and 8,224 cells on P21. In addition, we used deconvolution microscopy (Volocity restoration and classification modules; Improvision, Waltham, MA) to assess engraftment.
Generation of BMSC Conditioned Medium
Passage 2 BMSCs were grown to about 80% confluency. Medium was aspirated and cells were rinsed three times with phosphate-buffered saline. Cells were cultured in serum-free medium for 12 hours. Conditioned medium (CdM) was removed and filtered through a 50-μm mesh to remove cellular debris.
AEC2 Isolation
AEC2 were isolated from time-dated fetal day 19.5 rat lungs as described, using serial differential adhesions to plastic and low-speed centrifugations (24).
O2-induced Injury of Fetal Rat AEC2
AEC2 (106 cells/ml) were seeded onto poly-l-lysine–coated glass coverslips in 24-well cell culture plates. Cells were cultured in DMEM or CdM and exposed to 21 or 85% O2 for 36 hours. DNA damage was assessed with a dual-color Apo-BRDU immunohistochemistry kit (Millipore, Temecula, CA) that labels DNA breaks and total cellular DNA. Ten random fields of view were taken for each group. To assess apoptosis we performed immunoblotting for cleaved caspase-3 (ab13847; Abcam, Cambridge, MA). The intensity of the bands was normalized to the intensity of a reporter protein (actin), using the Kodak Gel-doc system (Kodak, Rochester, NY).
Wound Healing Assay with Fetal Rat AEC2
AEC2 (106 cells/ml) were seeded into a plastic 24-well cell culture plate. At about 80 hours, the cell monolayer was scraped with a p200 pipette tip and medium was replaced with CdM or DMEM. The surface area of the wound was recorded over time with OpenLab (Quorum Technologies Inc, Guelph, ON, Canada).
Endothelial network formation assay.
The formation of cordlike structures by rat lung microvascular endothelial cells (RLMVECs; VEC Technologies Inc., Rensselaer, NY) was assessed by seeding RLMVECs (80,000 cells per well) into 24-well plates coated with Matrigel (BD Biosciences, Mississauga, ON, Canada) (22, 23), supplemented with DMEM or BMSC CdM and incubated at 37°C for 8–12 hours in 21 or 85% O2. Cordlike structures were observed with an inverted phase-contrast microscope (Olympus, Melville, NY) and quantified by measuring the number of intersects and the length of structures in random fields from each well.
Statistics
Values are expressed as means ± SEM. Intergroup differences were assessed by Student paired t test. Analysis of variance with post hoc analysis (Fisher's probable least significant difference test) (Statview 5.1; Abacus Concepts) was used to assess the differences between multiple groups. Survival curves were derived by the Kaplan-Meier method and differences were evaluated by log-rank tests. A value of P < 0.05 was considered statistically significant. All evaluations were done by investigators blinded to the experimental groups.
RESULTS
BMSCs Migrate toward Injured Lung and Adopt Features of AEC2 in Vitro
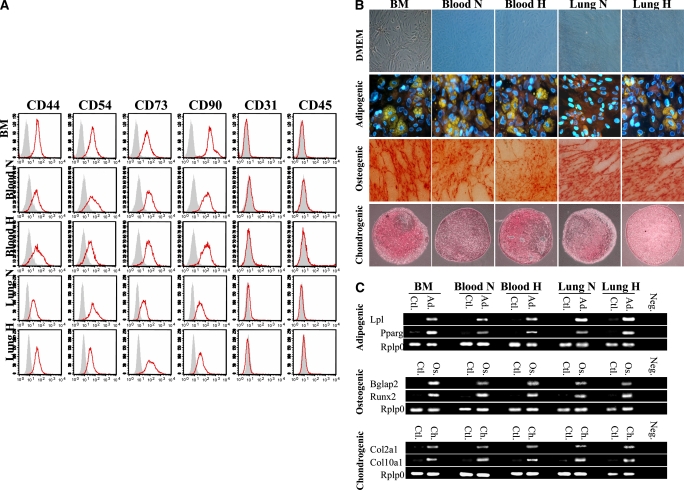
MSCs extracted from the BM formed a homogeneous population of cells by the second passage. The analysis of cell surface phenotype indicated that the MSC population was positive for CD44, CD54, CD73, and CD90 whereas negative for CD31 and CD45 (Figure 1A), consistent with the International Society for Cellular Therapy position statement for the minimal criteria to define an MSC (20). These MSCs differentiated into three mesenchymal lineages (adipo-, osteo-, and chondrocytes) when grown in specific medium for each lineage as shown by immunohistochemistry (Figure 1B) and RT-PCR (Figure 1C). These cells were also found in the circulating blood and the lung.
Figure 1.
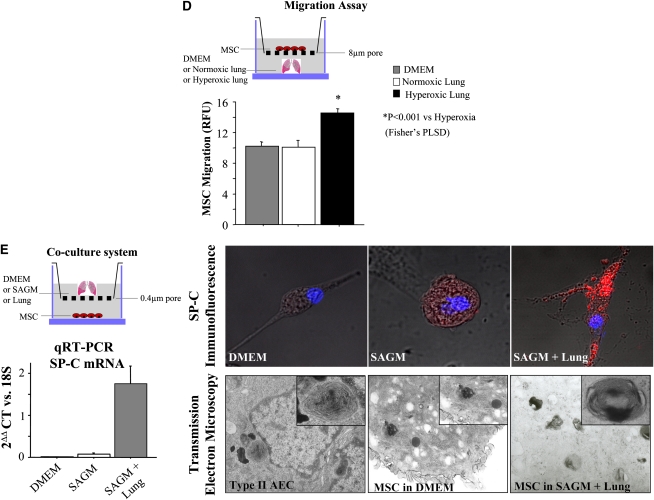
(A) Characterization of mesenchymal stem cell (MSC) immunophenotype. Shown are fluorescence intensity histograms with specific antibodies for membrane antigens (red line) and irrelevant isotypic-matched antibody as negative control (gray area). Experiments were performed in triplicate. MSC differentiation potential was assessed by (B) histochemistry and (C) mRNA expression of lineage-specific genes for adipogenic (Ad), osteogenic (Os), and chondrogenic (Ch) differentiation. Passage 2 bone marrow (BM)–derived adherent cells, after culture in differentiation medium (Ad, Os, or Ch), were stained for oil red O (Ad staining), alizarin red (Os staining), and safranin O (Ch staining). Ctl = control proliferation medium; Neg = polymerase chain reaction without cDNA. All experiments were performed in triplicate. DMEM = Dulbecco's modified Eagle's medium; H = hypoxic; N = normoxic. (D) Migration assay in Boyden chamber. In vitro, MSCs placed in the upper chamber migrate preferentially to lungs from O2-exposed rats than to culture medium only or lungs from rats housed in room air. PLSD = Fisher's probable least significant difference; RFU = relative fluorescence units. (E) In vitro, bone marrow–derived mesenchymal stem cells (BMSCs) differentiate into type II alveolar epithelial cells (AEC2). The lung microenvironment induces BMSCs to adopt an AEC2 phenotype. Co-culture experiments of BMSCs with O2-injured lung, but not with culture medium alone, show molecular (surfactant protein [SP]-C mRNA and protein expression) and ultrastructural (lamellar bodies in electron microscopy) features of AEC2. SAGM = small airway growth medium.
In migration experiments (Figure 1D), BMSCs were preferentially attracted to O2-damaged lung placed in the bottom of a modified Boyden chamber as compared with culture medium or noninjured lung, suggesting that the injured lung recruits MSCs to modulate lung injury.
MSCs co-cultured in vitro with normoxic (data not shown) or hyperoxic lung tissue, but not with culture medium alone or liver (data not shown), adopted immunophenotypic and ultrastructural characteristics of AEC2. MSCs co-cultured with lung expressed SP-C mRNA (Figure 1E) and showed the organized and granular distribution of SP-C protein expression (Figure 1E). Transmission electron microscopy visualized lamellar bodies (a characteristic structure found only in AEC2) in MSCs co-cultured with lung tissue (Figure 1E). Freshly isolated fetal AEC2 provided a reference for the structure and distribution of lamellar bodies.
Irreversible O2-induced Lung Injury Is Associated with a Reduction of Lung Resident MSCs
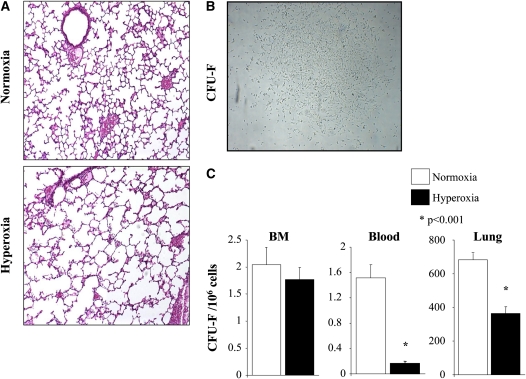
After 2 weeks of exposure to hyperoxia, the lung had a consistent, irreversible histological pattern of alveolar simplification (e.g., larger but fewer alveoli; Figure 2A). This was associated with a reduced number of circulating and resident MSCs (expressed as colony-forming unit-fibroblasts [CFU-F], shown in Figure 2B) in the lung without a reduction in the BM population of MSCs (Figure 2C).
Figure 2.
(A) Exposure of rat pups to hyperoxia during the critical period of alveolar development results in arrested alveolar growth. Representative hematoxylin and eosin–stained lung sections show larger and fewer alveoli in hyperoxia-exposed lungs as compared with room air–housed control animals. (B) Representative figure of a colony-forming unit-fibroblast (CFU-F). (C) CFU-F frequency. Shown is the number of CFU-F (mean ± SEM) per 106 cells from bone marrow (BM), blood, and lung in normoxia or hypoxia. *P < 0.001.
Intrapulmonary Delivery of BM-derived MSCs Improves Survival and Exercise Capacity
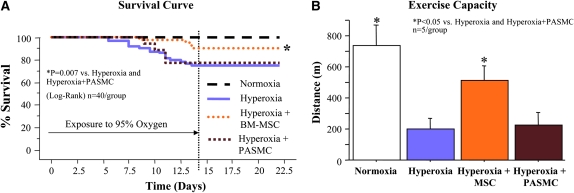
These data formed the rationale to test the therapeutic potential of MSCs in this model. Kaplan-Meier analysis demonstrated that intratracheal delivery of MSCs given on Postnatal Day 4 significantly improved survival as compared with both the hyperoxic group and the hyperoxic plus PASMC group (Figure 3A).
Figure 3.
Intratracheal bone marrow–derived mesenchymal stem cell (BM-MSC) administration on Postnatal Day 4 improves survival and exercise capacity. (A) Kaplan-Meier survival curve showing significant improvement in survival of MSC-treated animals as compared with untreated or pulmonary artery smooth muscle cell (PASMC)-treated animals. (B) Exercise capacity assessed according to a predetermined protocol shows that MSC-treated animals run for longer distances as compared with untreated or PASMC-treated animals.
Exercise capacity, using a graduated treadmill exercise protocol by a blinded observer, was significantly decreased in untreated O2-exposed rat pups (Figure 3B). MSC treatment on Postnatal Day 4 (prevention) or Day 14 (regeneration; see Figure E1 in the online supplement) significantly increased the total distance ran. PASMCs had no effect.
Intrapulmonary Delivery of BM-derived MSCs Preserves Alveolar and Vascular Development in Irreversible O2-induced BPD
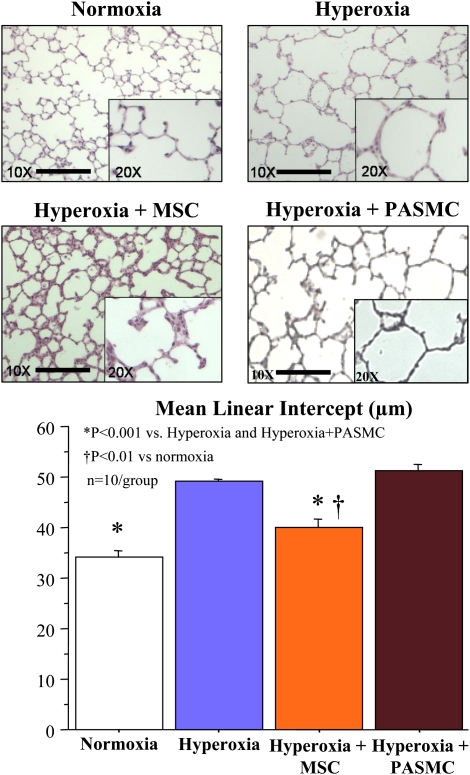
Hyperoxia induced a histological pattern reminiscent of human BPD, characterized by airspace enlargement with simplified and fewer alveolar structures (Figure 4). Intratracheal administration of MSCs on Day 4 significantly improved alveolarization as quantified by the mean linear intercept. Conversely, PASMCs had no protective effect on lung architecture. Intratracheal MSC administration on Day 14 did not significantly improve lung architecture (see Figure E2 in the online supplement).
Figure 4.
Intratracheal bone marrow–derived mesenchymal stem cell (MSC) administration on Postnatal Day 4 improves lung structure. O2-exposed lungs display the characteristic features of alveolar simplification that are unchanged with pulmonary artery smooth muscle cells (PASMCs). Conversely, lungs treated with intratracheal bone marrow–derived mesenchymal stem cells (BMSCs) have smaller and more numerous alveoli. Quantification of alveolar structures, using the mean linear intercept (Lm), confirms improved alveolarization in MSC-treated animals as compared with the other O2-exposed groups.
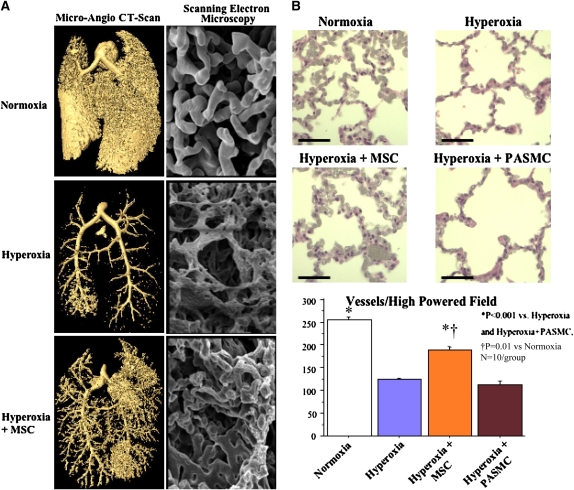
Capillary rarefaction is another hallmark of BPD and emphysema. Barium angiograms using computed tomographic scanning showed a severe rarefaction of pulmonary capillaries in chronic hyperoxia. Likewise, scanning electron microscopy revealed a dense vascular network with relatively large, rounded, smooth, and well-organized vessels in control lungs (Figure 5A). Hyperoxic exposure caused severe capillary rarefaction, thinning and scarring of the vasculature. The pulmonary vasculature from MSC-treated lungs demonstrated greater organization, as well as larger and rounder vessels, resembling more the normal lung, although some scarring was still present.
Figure 5.
Intratracheal bone marrow–derived mesenchymal stem cells restore the hyperoxia-induced decrease in lung capillary density. (A) Microangiograph computed tomography (CT) scan and Mercox casts examined by scanning electron microscopy of the lung capillary bed show that intratracheal mesenchymal stem cells (MSCs) promote lung angiogenesis and restore a denser capillary network in the lungs of O2-exposed rats. (B) Representative hematoxylin and eosin–stained barium angiograms and mean capillary count show decreased capillary density in O2-exposed lungs. Intratracheal MSCs, but not pulmonary artery smooth muscle cells (PASMCs), restore arterial density to control values.
Quantification of barium–gelatin-injected lungs confirmed the severe decrease in pulmonary vascular density in the hyperoxic group (Figure 5B). MSCs, but not PASMCs, significantly increased pulmonary vascular density.
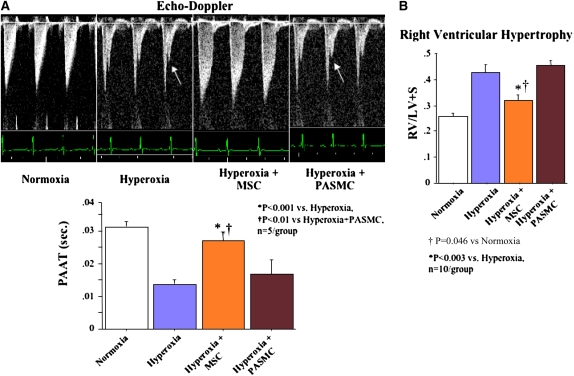
Intrapulmonary Delivery of BM-derived MSCs Reduces Pulmonary Hypertension
Pulmonary hypertension is one of the most important comorbidities of severe BPD and recapitulated in the chronic hyperoxia model as assessed by echocardiographic (PAAT; Figure 6A) and morphological (right ventricular hypertrophy [RVH]; Figure 6B) features. MSC therapy normalized the PAAT to near control levels and reduced RVH; PASMCs had no effect.
Figure 6.
Intratracheal bone marrow–derived mesenchymal stem cell (BMSC) administration prevents pulmonary hypertension associated with O2-induced lung injury. (A) Pulmonary arterial acceleration time (PAAT). PAAT was significantly decreased in chronic hyperoxia–induced lung injury and showed a characteristic notch indicating pulmonary hypertension (arrows). Intratracheal BMSCs, but not pulmonary artery smooth muscle cells (PASMCs), restored the PAAT almost to control levels. (B) Right ventricular hypertrophy (RVH). Hyperoxic-exposed rats had significant RVH as indicated by the increase in RV/LV+S (right ventricle/left ventricle plus septum) ratio compared with normoxic controls. Intratracheal BMSCs, but not PASMCs, reduced RVH.
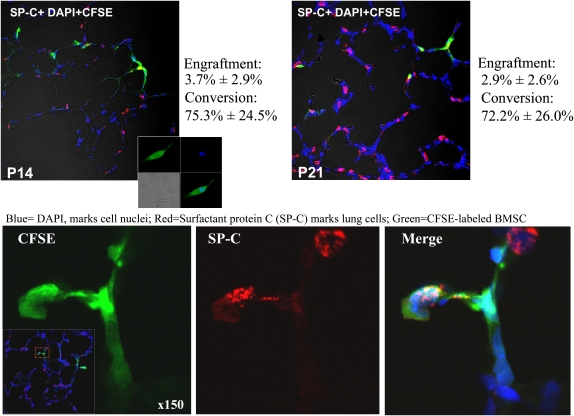
Engrafted MSCs Adopt a Distal Lung Cell Phenotype
CFSE-labeled MSCs accounted for 3.7 ± 2.9% of cells counted on P14 and 2.9 ± 2.6% of cells counted on P21 in hyperoxic animals (Figure 7). Engraftment in room air–housed animals was only 0.1 ± 0.06%.
Figure 7.
Bone marrow–derived mesenchymal stem cells (BMSCs) administered intratracheally engraft and adopt a type II alveolar epithelial cell (AEC2) phenotype. BMSCs were labeled with the green fluorescent marker 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (inset) and injected intratracheally into 4-day-old rat pups. Immunofluorescence of frozen lung sections examined by confocal microscopy reveals nuclear staining (4′,6-diamidino-2-phenylindole [DAPI], blue) and colocalization of BMSCs (green) and the distal AEC2 marker surfactant protein (SP)-C (red).
To determine whether MSCs adopt the phenotype of the host organ, we costained lung sections for SP-C, a specific marker for AEC2. Confocal microscopy revealed SP-C colocalization with CFSE-labeled MSCs (Figure 7). Furthermore, 75.3 ± 24.5% of MSCs on P14 converted to the AEC2 phenotype (coexpression of SP-C based on immunofluorescence) and 72.2 ± 28.0% on P21.
Deconvolution microscopy (see video in the online supplement) provided additional evidence that CFSE-labeled cell coexpress pro-SP-C.
Protective Effects of BM-derived MSC CdM in Vitro
Because of the discrepancy between the therapeutic benefit and the low rate of MSC engraftment and accumulating evidence in the literature suggesting a paracrine activity of MSCs, we explored the potential protective effect of BMSC CdM in three in vitro assays.
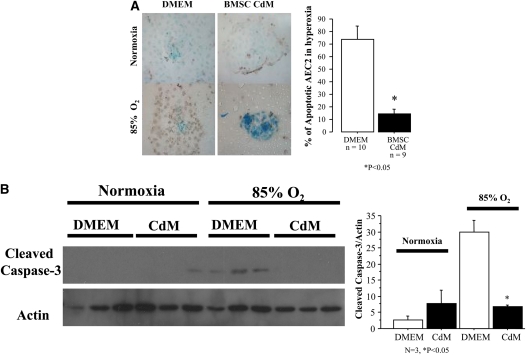
First, AEC2 were cultured with either DMEM or CdM and exposed to either 21 or 85% O2. CdM prevented O2-induced AEC2 DNA damage (Figure 8A) and apoptosis (Figure 8B) compared with AEC2 cultured in DMEM.
Figure 8.
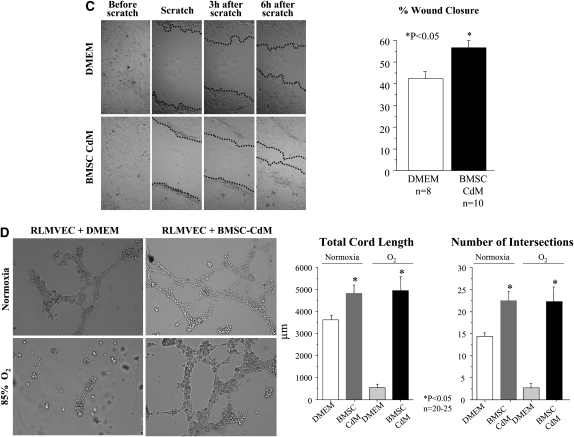
Protective effect of bone marrow–derived mesenchymal stem cell (BMSC) conditioned medium (CdM) in vitro. (A) BMSC CdM protects type II alveolar epithelial cells (AEC2) from O2-induced DNA damage. AEC2 undergo DNA damage (brown) when exposed to hyperoxia. BMSC CdM prevented O2-induced AEC2 DNA damage as compared with control medium. (B) BMSC CdM protects AEC2 from O2-induced apoptosis. AEC2 exposed to hyperoxia expressed cleaved caspase-3. BMSC CdM prevented O2-induced AEC2 apoptosis as compared with control medium. (C) BMSC CdM accelerates AEC2 wound closure. Confluent monolayers of AEC2 were damaged with a pipette tip, washed to remove damaged cells, and incubated in Dulbecco's modified Eagle's medium (DMEM) or BMSC CdM. CdM accelerated AEC2 wound closure as compared with DMEM. (D) BMSC CdM promotes endothelial network formation. Quantitative assessment of cordlike structure formation shows a significant decrease in the number of intersects and the total length of cordlike structures in hyperoxia. BMSC CdM preserved the number of intersects and total cord-structure length. RLMVEC = rat lung microvascular endothelial cells.
Second, AEC2 underwent a wound scratch assay (Figure 8C). After 6 hours, AEC2 wound closure was significantly higher with CdM compared with DMEM (52 vs. 37%; P < 0.05).
Third, RLMVECs suspended in DMEM or CdM were exposed to room air or 85% O2 in serum-free Matrigel and assessed for the formation of vessel-like networks (Figure 8D). Hyperoxia significantly decreased endothelial cordlike structure formation when cultured in DMEM whereas BMSC CdM significantly counteracted the effect of O2 and promoted endothelial network formation.
DISCUSSION
Our findings demonstrate that arrested alveolar development is associated with depletion of MSCs in the developing lung and show therapeutic benefit of intrapulmonary BM-derived MSCs to prevent irreversible chronic O2–induced alveolar damage in newborn rats. We also provide direct in vitro evidence that the beneficial effects of MSCs might be mediated through paracrine activity.
Resident Stem/Progenitor Cells in Lung Development, Injury, and Repair
AEC2 are the putative distal lung progenitor cells that contribute to alveolar homeostasis after injury (25). Progress in stem cell biology suggests that the distal lung harbors various other cells with progenitor properties, including endothelial progenitor cells (EPCs), side-population (SP) cells, and bronchoalveolar stem cells (26). The role of these cells during lung development is unknown. EPCs and SP cells seem to contribute to lung repair, because their depletion in the developing lung is associated with arrested lung development (27, 28). In lung diseases such as BPD, emphysema, or acute respiratory distress syndrome, the exploitation of putative local progenitor cells to protect or regenerate the alveolar space may be clinically relevant. The role of resident MSCs during lung development and in response to injury is unknown. One report identified cells in tracheal aspirates from ventilated human preterm infants that expressed MSC markers and correlated with prolonged ventilator dependence and O2 requirement (29). Our data demonstrate that MSCs migrate preferentially toward injured lung in vitro, suggesting that MSCs contribute to attenuate lung injury. Interestingly, however, after 2 weeks of hyperoxia the circulating and lung resident MSC population was reduced. Our interpretation is that chronic hyperoxia overwhelms the repair capacity of the developing lung, causing a depletion of resident progenitor cells that results in the irreversible arrest of alveolar development, features described in all experimental BPD models (30) and more recently in humans (5, 6). Given some uncertainty about the specificity of the molecular markers, it is possible that BM and lung MSCs display intrinsic differences in their properties. Endogenous fibroblastic progenitor cells have been isolated in the adult mouse lung (31). These cells are highly enriched in the sca-1–positive cell fraction and were predominantly representative of mesenchymal cell lineages. This work emphasizes the importance of the identification of alternative markers and robust functional assays for the identification and characterization of epithelial and fibroblastic stem and progenitor cell populations in the adult lung. Our findings formed the rationale for exogenous MSC administration to prevent alveolar injury.
Therapeutic Benefit of Intrapulmonary Delivery of BM-derived MSCs
Because of the lung's unique accessibility via the airways, we used the intratracheal route of delivery. A strength of the intratracheal approach is that it mimics the clinical setting and could theoretically be used (if safe and effective) to treat premature infants who are likely to develop BPD, concomitantly with routine surfactant administration. Another advantage is the local administration, enhancing the number of cells that reach the target site. The prevention approach is also clinically relevant as one can predict which premature infants are at high risk for developing BPD. Intratracheal administration of MSCs on Day 4 significantly improved not only lung architecture but also functional outcomes such as survival and exercise capacity. Intratracheal administration of MSCs at 14 days significantly improved exercise capacity, but there was only a trend toward improved lung architecture.
Lung Engraftment, Transdifferentiation, and Paracrine Activity of MSCs
Intratracheal administration resulted in a 4% engraftment rate within the lung. There are conflicting reports regarding the engraftment and transdifferentiation potential of MSCs within the lung. Intravenous administration resulted in various engraftment rates ranging from 20% (32), 14% (33), and 4% (34), to 0% (13, 35). These discrepancies may be partly explained by numerous experimental variables including the various putative stem cells used, routes of administration, injury models, and methods of analysis to assess engraftment. Data in the developing lung are lacking.
We also demonstrate that the engrafted MSCs adopt an AEC2 phenotype in vivo. This in vivo plasticity is further supported by our in vitro co-culture experiments showing that MSCs express SP-C and produce lamellar bodies when exposed to a lung microenvironment. This is consistent with previous in vitro demonstration of embryonic stem cells and MSCs (36–40) transdifferentiating into AEC2 and suggests that soluble factors play a role in instructing MSCs to adopt the phenotype of the homing organ. One mechanism we did not address for the in vivo plasticity of the MSCs is cellular fusion. There are numerous studies indicating the presence of cellular fusion in the reconstitution of epithelial tissues (7). However, marrow cell conversion to epithelial cells within the liver, skin, and lung may occur without cellular fusion (41). At present, the mechanism of MSC transdifferentiation is unclear, but the presence or absence of cellular fusion does not detract from the demonstrated therapeutic benefit.
Finally, it is intriguing that despite a low engraftment rate, MSCs provide therapeutic benefit in various lung injury models (15–17). The low numbers of engrafted stem cells seem insufficient to account for the therapeutic response, suggesting that these cells may have other local effects. There is increasing evidence that the therapeutic benefit of stem cells is mediated through paracrine activity. For example, stem cell–derived neurotrophic factors, more than cell replacement, promote repair after spinal cord injury (42). Similar observations have been made in the heart (43). Genes encoding several potential protective factors are significantly up-regulated in MSCs (44). Additional evidence for the paracrine hypothesis in lung diseases is provided by Ortiz and colleagues (45) and Xu and colleagues (46). CdM obtained from MSCs blocked the proliferation of an IL-1α–dependent T cell line and inhibited production of tumor necrosis factor-α by activated macrophages in vitro (45). In endotoxin-induced systemic inflammation, BMSCs decreased both the systemic and local inflammatory responses (46), independent of lung engraftment or stem cell differentiation, suggesting that humoral and physical interactions between stem cells and lung cells account for the beneficial effect. Our data corroborate these observations by providing direct in vitro evidence for the protective effect of BMSC CdM. BMSC-derived CdM prevented O2-induced AEC2 apoptosis and enhanced AEC2 wound healing and endothelial network formation. Thus, beyond cell replacement, soluble factors secreted by BMSCs prevented arrested alveolar and vascular growth in this model by protecting AEC2—the putative distal lung progenitor cells that contribute to alveolar homeostasis after injury (25)—and preserving angiogenesis, crucial for alveolar development and repair (47).
Collectively, we have shown that one of the underlying mechanisms of BPD could be decreased repair capacity because of destruction of resident MSCs and/or reduced homing of MSCs to the lung after chronic hyperoxic exposure during the crucial period of alveolar development. Our results also provide evidence for the therapeutic potential of BMSCs in preventing arrested alveolar growth in experimental BPD and further support the notion that MSCs exert their therapeutic benefit through paracrine activity. Identification of the active compound(s) in MSC CdM may lead to new treatment strategies for lung diseases. Regeneration (i.e., repair of established injury as opposed to injury prevention) through cell-based therapies might be more difficult to achieve, as the loss of the supportive extracellular matrix that provides the lung scaffold may preclude restoration of normal alveolar structure; additional strategies (48) may be necessary to achieve regeneration of new alveoli.
Acknowledgments
The authors thank Dr. Thierry Lacaze-Masmonteil for technical assistance in the isolation of AEC2.
Supported by the Canada Foundation for Innovation, the Alberta Heritage Foundation for Medical Research (AHFMR), the Canadian Institutes for Health Research (CIHR), the Canadian Stem Cell Network, and the Stollery Children's Hospital Foundation and a Canada Research Chair (B.T.); Tomorrow's Research Cardiovascular Health Professionals (TORCH) and the Maternal Fetal Neonatal Health Training Program sponsored by CIHR-IHDCYH (T.v.H.); and NIH-RO1-HL071115 (S.L.A.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200902-0179OC on August 27, 2009
Conflict of Interest Statement: T.v.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.Y.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A. holds a patent with B.T. Pat. App. No: 60/745,848: Stem Cells for Treating Lung Disease. M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.R.-P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.L.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.T. holds a patent with J.A. Pat. App. No: 60/745,848: Stem Cells for Treating Lung Disease.
References
- 1.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:502–506. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–269. [DOI] [PubMed] [Google Scholar]
- 4.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368. [DOI] [PubMed] [Google Scholar]
- 5.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med 2008;358:743–745, author reply 745–746. [DOI] [PubMed] [Google Scholar]
- 6.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 2008;32:321–328. [DOI] [PubMed] [Google Scholar]
- 7.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 2007;25:2896–2902. [DOI] [PubMed] [Google Scholar]
- 8.Stewart DJ, Zhao YD, Courtman DW. Cell therapy for pulmonary hypertension: what is the true potential of endothelial progenitor cells? Circulation 2004;109:e172– e173; author reply e172– e173. [DOI] [PubMed] [Google Scholar]
- 9.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 10.Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med 2007;58:285–298. [DOI] [PubMed] [Google Scholar]
- 11.van Haaften T, Thebaud B. Adult bone marrow–derived stem cells for the lung: implications for pediatric lung diseases. Pediatr Res 2006;59:94R–99R. [DOI] [PubMed] [Google Scholar]
- 12.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow–derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow–derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855–1863. [DOI] [PubMed] [Google Scholar]
- 16.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow–derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, et al. An abnormal mitochondrial-hypoxia inducible factor-1α–Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 2006;113:2630–2641. [DOI] [PubMed] [Google Scholar]
- 20.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 21.Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 2006;24:2202–2208. [DOI] [PubMed] [Google Scholar]
- 22.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 23.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005;172:750–756. [DOI] [PubMed] [Google Scholar]
- 24.Raoul W, Chailley-Heu B, Barlier-Mur AM, Delacourt D, Maître B, Bourbon JR. Effects of vascular endothelial growth factor on isolated fetal alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L1293–L1301. [DOI] [PubMed] [Google Scholar]
- 25.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465. [DOI] [PubMed] [Google Scholar]
- 26.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292:L1073–L1084. [DOI] [PubMed] [Google Scholar]
- 28.Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, et al. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol 2007;293:L941–L951. [DOI] [PubMed] [Google Scholar]
- 29.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 2007;175:1158–1164. [DOI] [PubMed] [Google Scholar]
- 30.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 2006;30:179–184. [DOI] [PubMed] [Google Scholar]
- 31.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 2009;27:623–633. [DOI] [PubMed] [Google Scholar]
- 32.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow–derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 33.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–1338. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
- 35.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denham M, Cole TJ, Mollard R. Embryonic stem cells form glandular structures and express surfactant protein-C following culture with dissociated fetal respiratory tissue. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1210– L1215. [DOI] [PubMed] [Google Scholar]
- 37.Popov BV, Serikov VB, Petrov NS, Izusova TV, Gupta N, Matthay MA. Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng 2007;13:2441–2450. [DOI] [PubMed] [Google Scholar]
- 38.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood–derived mesenchymal stem cells. Am J Respir Crit Care Med 2008;177:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM, Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng 2005;11:1177–1187. [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 2005;102:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow–derived epithelia. Science 2004;305:90–93. [DOI] [PubMed] [Google Scholar]
- 42.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 2003;181:115–129. [DOI] [PubMed] [Google Scholar]
- 43.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 44.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell–mediated cardiac protection and functional improvement. FASEB J 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 2007;104:11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow–derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 2007;293:L131–L141. [DOI] [PubMed] [Google Scholar]
- 47.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuang PP, Lucey E, Rishikof DC, Humphries DE, Bronsnick D, Goldstein RH. Engraftment of neonatal lung fibroblasts into the normal and elastase-injured lung. Am J Respir Cell Mol Biol 2005;33:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]