Abstract
The pathologic classification of diffuse lung disease in children and adolescents has undergone revision in recent years in response to rapid developments and new discoveries in the field. A number of important advancements have been made in the last 10 years including the description of new genetic mutations causing severe lung disease in infants and children, as well as the description of new pathologic entities in infants. These recently described entities, including ABCA3 surfactant disorders, pulmonary interstitial glycogenosis, and neuroendocrine cell hyperplasia of infancy, are being recognized with increasing frequency. This review will include brief discussion of the etiology and pathogenesis of the major groups of diffuse lung disease in children. Histopathologic features are discussed for each of the major categories of diffuse lung disease in children, beginning with the genetic, developmental, and alveolar growth disorders common in infancy, followed by brief discussion of airway diseases, immunologic diseases, and pulmonary vascular diseases seen more commonly in older children. A protocol for handling pediatric wedge lung biopsies is also discussed, which optimizes the diagnostic yield of lung biopsies in this population.
Pediatric Lung Biopsy
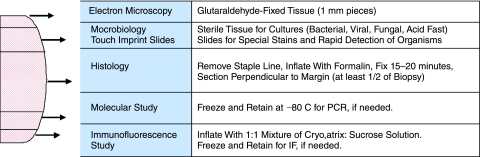
Accurate pathologic diagnosis of diffuse lung disease in children begins with attention to proper handling and distribution of tissue from the wedge lung biopsy. While histologic examination is paramount for characterizing patterns of injury, inflammation, and fibrosis, other ancillary studies are often necessary to determine specific etiology. In 2006, a protocol for tissue handling was recommended by the Children's Interstitial Lung Disease Network pathology working group (Fig. 1).1 This protocol acknowledges the importance of electron microscopy in the diagnosis of genetic disorders of surfactant metabolism and other forms of pediatric lung disease, including viral infection and pulmonary interstitial glycogenosis (PIG). Because embedding of lung tissue for electron microscopy requires low cost and only tiny pieces of tissue, collection of lung tissue in glutaraldehyde is recommended for all pediatric wedge lung biopsies. Frozen tissue for polymerase chain reaction (PCR)-based molecular studies is also recommended in all cases due to the higher incidence of genetic disease in children compared with adults. PCR detection of infectious agents is also used increasingly in pathology laboratories, and collection of frozen tissue enables this type of testing if indicated. For pulmonary hemorrhage syndromes and autoimmune diseases, a portion of lung tissue is prepared for immunofluorescence study by injection inflation with a modified cryomatrix compound, which is diluted in order to allow injection of the compound through a needle and syringe.2 This modified cryomatrix compound can be made easily in the histology laboratory by mixing standard cryomatrix gel with an equal amount of 0.5 M sucrose solution, and can be stored for prolonged periods in the refrigerator. Saline has also been used instead of sucrose solution, with satisfactory results.
FIG. 1.
Guidelines for handling the pediatric wedge lung biopsy. In order to increase the diagnostic yield of lung biopsy, a number of special studies are routinely performed. Histology with formalin inflation, electron microscopy, microbiology cultures, and frozen tissue for molecular studies should be collected in virtually all cases. Touch imprints may be useful for rapid diagnosis of opportunistic infection in immunocompromised patients. Any biopsy taken for evaluation of autoimmune disease or pulmonary hemorrhage syndrome should include collection of tissue for immunofluorescence study. The following protocol is recommended for any pediatric wedge lung biopsy, but can be modified based on clinical indications and amount of tissue provided. For maximal diagnostic yield, a wedge lung biopsy should be at least 1 cm deep and 3 cm wide.
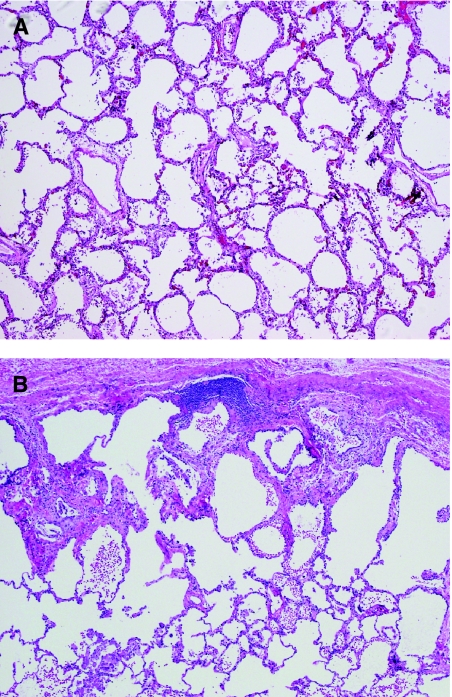
Following collection of frozen tissue for molecular studies and immunofluorescence, tissue for microbiology cultures, tissue for electron microscopy, and preparation of touch imprint slides (if indicated), the remaining central portion of the biopsy is prepared for histologic examination. Proper biopsy inflation is critical for recognition of disorders affecting alveolar growth and development, a common group of disorders in infants and young children. Injection of formalin into the lung biopsy allows the alveoli to re-expand and aids in assessment of both alveolar architecture and degree of interstitial widening (Fig. 2). While this protocol can be modified by the pathologist if the clinical indication for lung biopsy is known or if the lung biopsy is too small for all studies to be completed, it provides a useful construct to ensure that all potentially useful diagnostic studies can be performed if needed, particularly when the clinical questions and reason for biopsy are not known at the time the tissue is received in the laboratory.
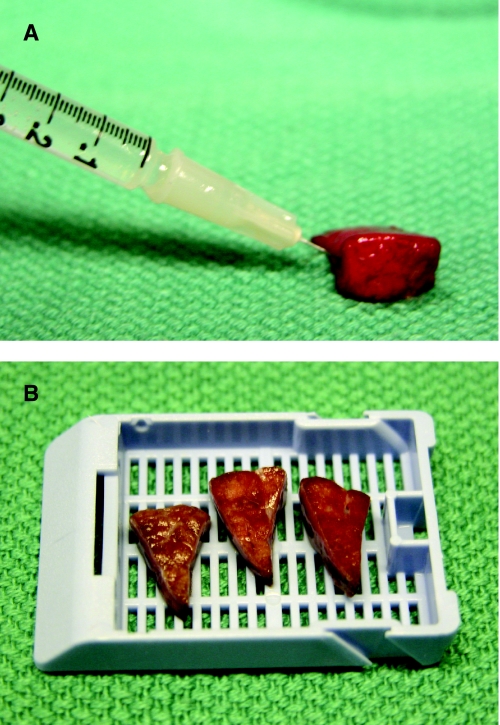
FIG. 2.
Inflation and fixation of the pediatric wedge lung biopsy. The midportion of the lung biopsy (∼50% volume) should be reserved for histologic examination. Inflation of the biopsy with formalin replicates in vivo expansion of the lung and is critical for assessment of alveolar growth and development in children. (A) Gentle inflation using a tuberculin syringe or other fine needle is recommended in order to minimize artifactual expansion of the interlobular septa and pleura. Once fully expanded, the lung biopsy is allowed to fix for 15–20 minutes before sectioning perpendicular to the surgical margin. (B) Each section should be triangular and includes both central parenchyma with small airways and peripheral parenchyma with pleura.
In the following sections, integrated histopathologic and ancillary diagnostic features are presented for some of the major diagnostic categories of diffuse lung disease in children. A classification of pediatric diffuse lung disease is summarized in Table 1, modified from a classification described by the Children's Interstitial Lung Disease group.3,4 A number of excellent clinical reviews are available on the topic of diffuse lung disease in children,5–13 and therefore diagnostic histopathologic criteria are emphasized in this review.
Table 1.
Major Causes of Diffuse Lung Disease in Infants, Children, and Adolescents
| Genetic and metabolic diseases |
| Cystic fibrosis |
| Primary ciliary dyskinesia |
| Surfactant dysfunction disorders |
| SP-B, SP-C, ABCA3 mutations, GMCSFR mutations, TTF mutations, other |
| Tuberous sclerosis |
| Lymphangioleiomyomatosis |
| Alveolar capillary dysplasia with misalignment of pulmonary veins |
| FOXO1A mutations/deletions, other |
| Metabolic/storage diseases |
| Niemann–Pick disease, mucopolysaccharidosis, glycogen storage disease, other |
| Other |
| Primary developmental disorders |
| Acinar dysgenesis |
| Congenital alveolar dysplasia |
| Alveolar growth abnormalities |
| Prematurity |
| Chronic neonatal lung disease |
| Bronchopulmonary dysplasia |
| Pulmonary hypoplasia |
| Chromosomal disorders (Down syndrome, other trisomies) |
| Congenital heart disease, with or without vasculopathy |
| Pulmonary interstitial glycogenosis |
| Associated with alveolar growth abnormalities, pulmonary vascular disease, congenital cysts, meconium aspiration, other forms of infant lung injury |
| Neuroendocrine cell hyperplasia of infancy |
| Infectious disease |
| Acute injury |
| Bronchiolitis/interstitial pneumonitis (viral) |
| Pneumonia (bacterial, Pneumocystis jiroveci) |
| Granulomatous pneumonitis (fungal, mycobacterial) |
| Diffuse alveolar damage, acute |
| Resolving/remote injury |
| Obliterative/constrictive bronchiolitis |
| Diffuse alveolar damage, organizing |
| Aspiration injury |
| Associated with gastroesophageal reflux, oropharyngeal/laryngotracheal abnormalities |
| Associated with meconium aspiration (neonates) |
| Immunologic reactions |
| Hypersensitivity pneumonitis |
| Drug/medication reactions |
| Eosinophilic pneumonia |
| Other |
| Systemic diseases |
| Autoimmune disease |
| Rheumatologic disorders; anti-neutrophil cytoplasmic antibodies (ANCA)-associated capillaritis; anti-granulocyte macrophage colony-stimulating factor (GMCSF)-associated alveolar proteinosis |
| Primary and acquired immunodeficiency |
| Lymphoid hyperplasia and lymphoproliferative processes |
| Opportunistic infection |
| Transplant-related disease |
| Graft-versus-host disease (bone marrow transplant) |
| Allograft rejection (lung transplant) |
| Posttransplant lymphoproliferative disorders |
| Opportunistic infection |
| Hematolymphoid diseases |
| Leukemia/lymphoma, Langerhans cell histiocytosis, other |
| Endocrine diseases |
| Congenital hypothyroidism (myxedema) |
| Vascular disorders |
| Pulmonary arteriopathy |
| Associated with congenital heart disease, other secondary causes |
| Chronic congestive vasculopathy |
| Associated with congenital heart disease, other secondary causes |
| Lymphatic disorders |
| Primary or secondary lymphangiectasia |
| Lymphangiomatosis |
| Pulmonary hemorrhage syndromes |
| Pulmonary capillaritis/other vasculitis |
| Associated with chronic vasculopathy |
| Idiopathic pulmonary hemosiderosis |
| Other |
Genetic Disorders of Surfactant Metabolism
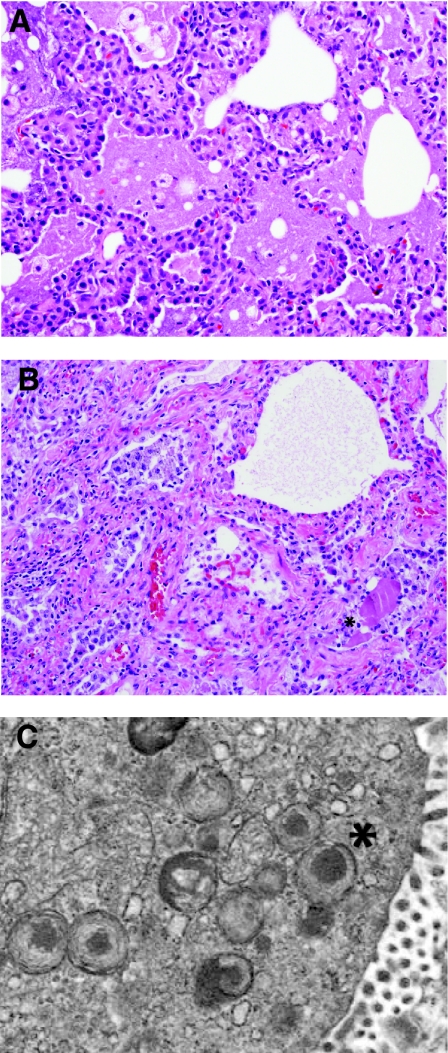
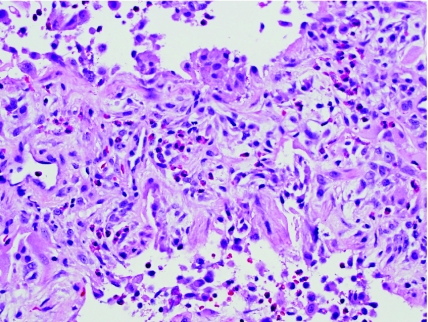
Inherited disorders of surfactant metabolism comprise an interesting and evolving group of chronic lung diseases in children. In 1993, genetic defects in the surfactant protein B gene (SFTPB) were recognized as a cause of fatal lung disease in neonates.14–16 These children typically present in the first hours, days, or weeks of life with acute respiratory distress and imaging findings that mimic hyaline membrane disease with diffuse “whiteout” of bilateral lung fields. A “crazy-paving” pattern of mosaic ground-glass opacities may be recognized on chest computed tomography (CT) imaging. Unlike neonatal respiratory distress syndrome, there is no evidence of hyaline membrane formation histologically. Instead, the lung histopathology is characterized by a variably prominent increase in intra-alveolar proteinosis material, which may take a granular or globular appearance (Fig. 3A). Periodic acid–Schiff (PAS) stain may also be helpful in highlighting and eliciting the pulmonary alveolar proteinosis (PAP) material and helps to distinguish it from hyaline membranes or edema fluid. Accompanying the alveolar proteinosis is the presence of diffuse marked alveolar epithelial hyperplasia, a feature that serves as an important histologic clue to diagnosis and distinguishes the infantile disorders from the adult type of immune-mediated PAP. Likely depending upon time of biopsy and length of survival, patients with genetic disorders of surfactant metabolism may later show evidence of chronic lobular remodeling within the lungs, evidenced by enlarged and disordered alveolar spaces, interstitial widening due to smooth muscle and fibrosis, and variable inflammation (Fig. 3B).
FIG. 3.
Genetic disorders of surfactant metabolism. (A) In early infancy, the genetic surfactant disorders typically demonstrate diffuse alveolar epithelial hyperplasia, abundant alveolar proteinosis material, and increased foamy macrophages. (B) In older infants, children, and adolescents, the histologic pattern is dominated by chronic lobular remodeling with variable interstitial fibrosis, smooth muscle, interstitial inflammation, and lesser amounts of alveolar proteinosis material. Cholesterol clefts may be prominent in some cases. (C) In patients with ABCA3 mutations, electron microscopy is useful for detection of characteristic dense round structures within small lamellar bodies (*).
The second type of genetic surfactant disorders to be discovered was the autosomal dominant mutation in surfactant protein C (SFTPC), described in 2001.17 SFTPC mutations have now been recognized as a cause of chronic diffuse lung disease in both the pediatric population and the adult population.18,19 As this disorder is inherited in an autosomal dominant pattern, families have been described with multiple generations affected by severe lung disease. Typically, when this disease presents in the pediatric age group, the age of biopsy and/or presentation is typically later than seen in SFTPB gene mutation, for example, lung biopsy may not be performed until after 6 months of age. At the time of biopsy, there may be a much greater amount of chronic lobular remodeling, interstitial fibrosis, and inflammation. With chronicity, another common pathologic feature is the presence of endogenous lipoid pneumonia, characterized by clusters of foamy alveolar macrophages, cholesterol clefts, and foreign body giant cell reaction. This pattern has also been described as “chronic pneumonitis of infancy” (CPI).20,21 Other histologic patterns described in older children and adults include a nonspecific interstitial pneumonia (NSIP) pattern and usual interstitial pneumonia (UIP).22 SFTPC mutations are associated typically with lesser amounts of alveolar proteinosis material, often quite focal and more globular in nature than that seen in SFTPB mutations. This feature can be easily missed in adolescents and adults in whom the histologic picture is dominated by end-stage pulmonary fibrosis. Careful search, in particular in the subpleural region, may yield focal proteinosis material and should prompt further evaluation for a SFTPC gene mutation.
In 2004, the third gene causing the disorders of surfactant metabolism was described involving the ATPase-binding cassette transporter subfamily A3 (ABCA3) gene.23 This discovery in many ways has revolutionized the approach to this class of disorders, as it is now recognized as the most common of the mutations resulting in abnormal surfactant metabolism. The histologic features associated with ABCA3 mutations include many of the features previously described for SFTPB and SFTPC mutations. The clinical and histologic manifestation in fact appears to overlap between these 2 entities.24 Histologic patterns associated with ABCA3 mutations include PAP, desquamative interstitial pneumonia (DIP), CPI, and NSIP with endogenous lipoid pneumonia. It is also now well recognized that ABCA3 mutations cause disease early in infants similar to SFTPB mutations, but also may produce chronic lung disease in adolescents and young adults.25 ABCA3 mutations are inherited in an autosomal recessive pattern, similar to SFTPB mutations, and therefore there may be no family history of interstitial lung disease in these children.
Electron microscopy is an important diagnostic tool in the evaluation of lung biopsies from children with surfactant disorders.26–29 SFTPB mutations are associated with multivesicular and multilamellated lamellar bodies. Patients with ABCA3 mutations often show virtually pathognomonic findings of unique dark round dense bodies within the lamellar structures, resembling a “fried egg” appearance (Fig. 3C). Many of the lamellar bodies in ABCA3 disease are also small in size and may be decreased in number or absent. It should be noted that these dense bodies in ABCA3 mutation patients are variable in distribution and there may be some normal lamellar bodies admixed with the abnormal structures.
While the mutations in SFTPB, SFTPC, and ABCA3 genes explain the majority of cases with this clinical and histologic pattern, it is clear that there is a smaller subset with histologic features of the genetic disorders of surfactant metabolism and yet these patients have normal mutation testing. This remains an area of active investigation. In recent years, it has been recognized that there are at least 2 other genes affecting surfactant metabolism in humans. Mutations in the granulocyte macrophage colony-stimulating factor receptor α chain (CSF2RA) gene were described as an X-linked form of PAP in 2008.30,31 The morphology of this disease appears to be similar to the immune-mediated PAP described in adults associated with antibodies to granulocyte macrophage colony-stimulating factor (GMCSF) (see below). It has also recently been recognized that mutations and deletions in the TTF1/NKX2.1 gene affect the development and function of the lung, thyroid gland, and brain, and also impact the metabolism of surfactant proteins.32,33 Neurologic dysfunction (chorea, developmental delay) and hypothyroidism may be a clue to the genetic etiology in these patients.
The differential diagnosis of the genetic disorders of surfactant metabolism includes other forms of PAP in children, either associated with antibodies against the GMCSF receptor or associated with alveolar macrophage dysfunction due to immunosuppression (human immunodeficiency virus (HIV), leukemia, bone marrow or solid organ transplant, rheumatologic disorders).34–36 Unlike the genetic SFTPB and ABCA3 abnormalities, these forms of “acquired” alveolar proteinosis typically show more homogeneous “smooth” proteinosis material, less pronounced alveolar epithelial hyperplasia, and no evidence of chronic lobular remodeling of the lung. The rare disorder lysinuric protein intolerance has also been associated with an alveolar proteinosis pattern in children.37 In older children and adolescents, the differential diagnosis of the genetic surfactant disorders also includes other processes resulting in interstitial lymphocytic infiltrates (NSIP pattern, Table 2)or interstitial fibrosis (Table 3).
Table 2.
Differential Diagnosis of Interstitial Lymphocytic Inflammation in Children
| Nonspecific interstitial pneumonia (NSIP) pattern (mild to moderate inflammation) |
| Autoimmune/rheumatologic disease |
| Genetic disorders of surfactant metabolism in older children/adolescents (ABCA3 or SFTPC mutations) |
| Hypersensitivity pneumonitis |
| Viral pneumonitis |
| Lymphocytic interstitial pneumonia (LIP) pattern (severe inflammation) |
| Autoimmune/rheumatologic disease (eg, Sjogren's syndrome) |
| Human immunodeficiency virus (HIV) infection |
| Lymphoproliferative disorders |
Table 3.
Differential Diagnosis of Interstitial Fibrosis in Children
| Bronchopulmonary dysplasia |
| Autoimmune/rheumatologic disease |
| Genetic disorders of surfactant metabolism |
| Chronic hypersensitivity pneumonitis |
| Other |
Systemic Metabolic and Storage Diseases
Lysosomal storage diseases and glycogen storage disorders typically manifest in the lung with foamy or vacuolated macrophages. While it is not uncommon to see foamy macrophages within the airspaces, for example in the setting of aspiration, airway obstruction, or the genetic surfactant disorders described earlier, the location of foamy macrophages in the interstitial, interlobular septal, and pleural compartments serves as an important clue to involvement by metabolic or storage disease. In some cases, there is little diagnostic difficulty for the pathologist, as the diagnosis has already been made in other ways by blood testing and other clinical manifestations. In other cases, however, lung involvement may be the earliest or primary manifestation of disease. The most common metabolic storage diseases seen within the lung include Niemann–Pick disease, glycogen storage disease, and mucopolysaccharidoses or mucolipidoses. Tissue retained in glutaraldehyde for electron microscopy may be useful for diagnosis of these disorders.
Alveolar Capillary Dysplasia
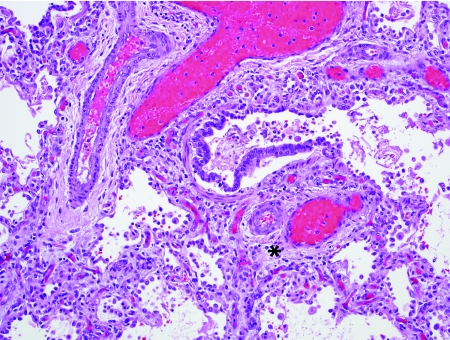
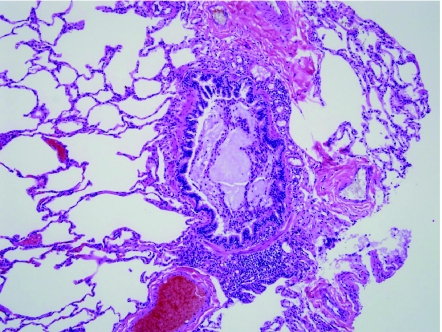
Alveolar capillary dysplasia (ACD) with misalignment of pulmonary veins is a genetic disorder characterized by abnormal vascular development in the lung.38–46 Clinically, these infants present very soon after birth sometimes with a short “honeymoon” period of wellness, followed by development of severe pulmonary hypertension. Greater than 80% of children with ACD also have extrapulmonary malformations, including congenital heart disease (hypoplastic left heart syndrome, aortic arch coarctation or hypoplasia, tetralogy of Fallot, other), intestinal malrotation, genitourinary tract abnormalities, and others. Diagnosis depends on demonstration of abnormal vasculature on lung biopsy and it is not uncommon for biopsies to be performed in the neonatal period to exclude the diagnosis of ACD and to determine the utility of further life-sustaining measures. Histologically, ACD is characterized by deficient alveolar capillaries within the alveolar walls, as well as misplacement of veins adjacent to arteries and arterioles (Fig. 4). Normally, the position of the pulmonary veins is within the interlobular septa. In ACD, the vein branches can be seen as dilated structures adjacent to the muscular pulmonary arteries within bronchovascular bundles and within the lobules, paired with muscularized arterioles. In addition to these venous and capillary abnormalities, the pulmonary arteries show severe medial hypertrophy with prominent muscularization of the intralobular arterioles. The airspace architecture is also typically disordered in ACD, demonstrating simplified lobules with deficient alveolarization, mimicking congenital alveolar dysplasia or acinar dysgenesis. In approximately one-third of cases, there is associated lymphangiectasia. ACD is fatal typically within the first weeks of life, although rare reports of late presentation or prolonged survival into the first year of life have been described.47–49 In 2009, Stankiewicz et al. discovered that many patients with ACD have either deletions of the FOX transcription factor gene cluster (FOXF1, FOXC2, FOXL1) on chromosome 16q24.1 or point mutations of the FOXF1 gene.50 As might be expected, patients with associated malformations often had deletions involving >1 gene in this cluster.
FIG. 4.
Alveolar capillary dysplasia with misalignment of pulmonary veins. Alveolar capillary dysplasia (ACD) is characterized by deficient and centrally placed capillaries within the alveolar walls, medial hypertrophy of muscular pulmonary arteries and muscularization of arterioles, and misalignment of congested pulmonary veins and venules adjacent to the hypertrophied pulmonary arteries and arterioles (*). Abnormal airspace development is also a typical feature.
Disorders of Alveolar Growth and Development
The disorders of lung growth and development include a group of disorders characterized histologically by impaired alveolarization and/or simplification of the lobules. The disorders of major intrauterine impairment of lung development such as acinar dysgenesis and congenital alveolar dysplasia are beyond the scope of this review. The disorders that will be described in more detail are alveolar growth abnormalities associated with prematurity, pulmonary hypoplasia, chromosomal syndromes, and congenital heart disease. These disorders are grouped due to morphologic similarity on lung biopsy, although they may be clinically distinguished by perinatal history in many cases. It is also convenient to describe them together also because of the multifactorial pathogenesis in some cases,51 for example, a premature Down syndrome neonate with atrioventricular canal, or a premature infant with congenital diaphragmatic hernia. Furthermore, it is not uncommon for the histologic pattern of an “alveolar growth abnormality” to be detected pathologically, despite lack of clinical suspicion or known risk factors, in which case this diagnosis serves as a descriptive category requiring further clinical correlation.
In terms of pathogenesis, the alveolar growth abnormalities are unified by incomplete or insufficient alveolarization of either prenatal or postnatal origin. Morphologically, the airspaces are enlarged, round or elongated, and “simplified,” meaning that there is deficient alveolar septation. Alveolar simplification may be difficult for pathologists to detect without some experience in examining infant lung, as it can be easily mistaken for normal adult morphology. As a result, the size and shape of airspaces should be critically evaluated in all pediatric lung biopsies.
Bronchopulmonary dysplasia (BPD) refers to the form of chronic lung disease due to prematurity that was most common prior to the artificial surfactant era. Classically, BPD is characterized by alternating areas of hyperinflation (hyper-expansion) and collapse (atelectasis), accompanied by alveolar enlargement and variable interstitial fibrosis.52–55 The pathogenesis is in part due to varying degrees of airway injury with hyperinflated areas occurring distally to regions of small airway stenosis and areas of atelectasis in lung parenchyma distal to obliterated fibrotic airways. Following the advent of artificial surfactant supplementation, the lung histology associated with prematurity changed in parallel with the improvements in clinical outcome. The lung morphology most often seen in the post-surfactant era is therefore described as “new” BPD or simply chronic neonatal lung disease. This morphology differs in that there is less lobular injury and airway injury. The principle manifestations are alveolar simplification without significant interstitial or septal fibrosis.56,57 While “classic” BPD is still encountered occasionally in severely premature infants or in infants with prematurity and other superimposed risk factors for impaired lung growth (see discussion below), “new” BPD (alveolar simplification) is now the predominant pathologic finding associated with prematurity.
Similar to chronic neonatal lung disease due to prematurity, pulmonary hypoplasia results in a morphologic pattern of alveolar simplification. Pulmonary hypoplasia refers to underdevelopment of the lung typically due to mechanical space constraints within the thoracic cavity or impaired lung excursion in utero. Common etiologies include congenital diaphragmatic hernia, thoracic dystrophies with a small ribcage, chronic oligohydramnios, intrathoracic or intra-abdominal mass lesions, and neuromuscular disorders resulting in poor diaphragm function. The most dramatic examples of lung hypoplasia are seen in congenital diaphragmatic hernia with intrathoracic displacement of abdominal organs, especially if the liver is included in the herniated organs.58 The ipsilateral lung is markedly decreased in size (15%–25% expected lung volume), and the contralateral lung is also typically hypoplastic (50%–70% expected lung volume). While lung size can be determined at autopsy using measurements of lung weight and volume, the diagnosis of lung hypoplasia is more challenging on lung biopsy. Histologically, the hallmark of pulmonary hypoplasia is simplification of lobules and insufficient alveoli within the acinar unit. Over time, the hypoplastic lungs may become hyperinflated with markedly enlarged, round, and simplified airspaces histologically identical to the end effects of chronic neonatal lung disease due to prematurity (Fig. 5A). The radial alveolar count (RAC) is a method of documenting this lobular simplification and is performed by counting the number of alveoli that are intercepted by a line drawn from the center of a respiratory bronchiole to the nearest interlobular septum or pleural surface. The RAC is ∼5 in normal term infants and ∼9–10 by 1 year of age. This type of morphometric analysis is most useful in the research setting where assessment of large numbers of acinar units increases reproducibility of results. From a practical standpoint, the RAC is less useful for diagnostic wedge lung biopsies due to the small size of the tissue, and acinar hypoplasia can be detected by simply evaluating airspace architecture (size and shape) and recognizing the pattern of alveolar simplification caused by deficient secondary septation.
FIG. 5.
Alveolar growth and development. Abnormal alveolar growth and development is a major cause of chronic lung disease in infants and young children. (A) Hypoplastic lungs and lungs of premature infants show abnormal alveolarization, manifesting as enlarged, round, and poorly septated (“simplified”) alveolar spaces, as in this example from a 30-week gestation premature infant with chronic neonatal lung disease. (B) Down syndrome patients classically have exaggerated subpleural cystic airspaces on computed tomography (CT) imaging and on lung biopsy, sometimes associated with endogenous lipoid pneumonia (cholesterol clefts, foamy macrophages, and lymphocytic inflammation).
In addition to prematurity and hypoplasia, chromosomal syndromes and/or congenital heart disease are additional risk factors for abnormal alveolar growth and development. The factors that result in alveolar simplification in these disorders are not completely understood, but it is recognized that some children with these disorders have enlarged alveoli despite term gestation and lack of risk factors for hypoplasia. Down syndrome (trisomy 21) characteristically is associated with enlarged airspaces and widened alveolar ducts, which appear to be changes reflecting poor postnatal alveolarization rather than primary lung hypoplasia.59–61 In some babies and children with Down syndrome, there may also be both radiographic and pathologic evidence of subpleural cysts within the periphery of the lung (Fig. 5B).62,63 Lung biopsies from Down syndrome patients tend to be one of the most complicated for the pathologist to analyze as there are often multiple superimposed forms of injury. For example, a Down syndrome patient with atrioventricular canal and aspiration may have impaired lung function and morphology due in part to the underlying chromosomal syndrome as well as hemodynamic effects of the underlying congenital heart disease and chemical injury from repeated aspiration and/or infection. The mechanisms of impaired alveolar development associated with other forms of congenital heart disease are not well understood although it is likely that the intrauterine hemodynamics within the pulmonary vasculature influences the degree of alveolarization.
Pulmonary Interstitial Glycogenosis
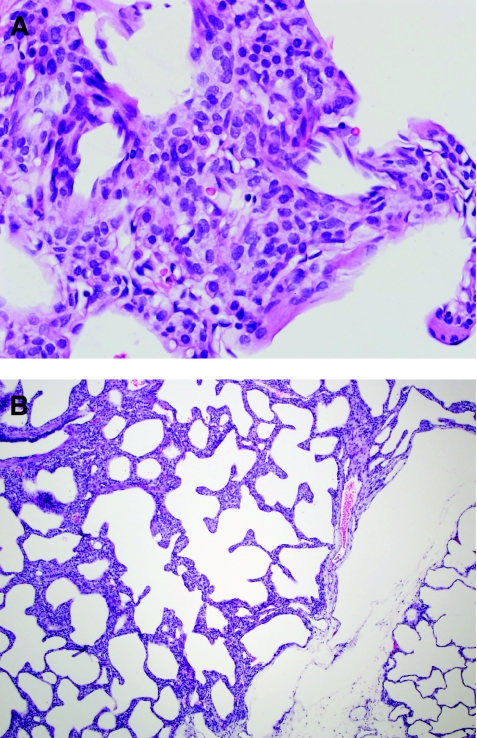
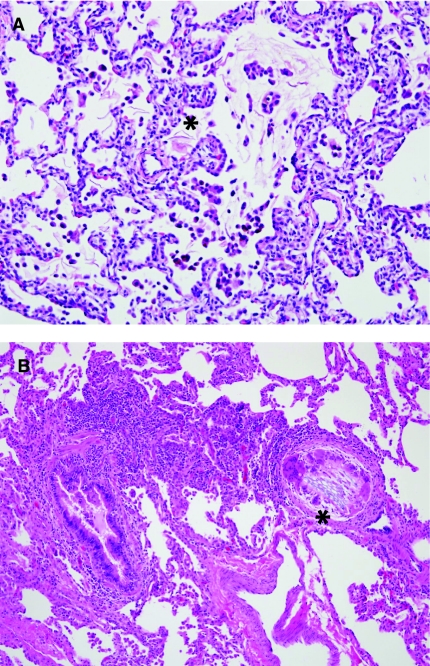
Also relevant to a discussion of alveolar growth abnormalities is the relatively recently recognized entity called “PIG” also previously described as infantile cellular interstitial pneumonia (ICIP).64–67 This entity is characterized by increased numbers of oval mesenchymal cells within the alveolar walls, expanding the interstitial space (Fig. 6). These cells have been shown not to be lymphocytic or histiocytic, but instead are thought to be reactive mesenchymal cells responding to various triggers (Table 4). While the etiology and pathogenesis of this cellular proliferation remains poorly understood, it is clear that this finding occurs not only as an isolated phenomenon but also more commonly in association with other forms of lung injury. PIG is most often seen as a patchy process in association with the disorders of alveolarization previously described (pulmonary hypoplasia, chronic neonatal lung disease due to prematurity, and pulmonary hypertension), but also has been seen in the lung adjacent to masses or cystic lesions. This phenomenon of interstitial cellular proliferation occurs only in infant lungs, with highest incidence in young infants (<6 months of age). This has led to the hypothesis that these cells may be a reactive proliferation within a growing lung, as the phase of most rapid postnatal alveolarization is within the infantile period. Electron microscopy in PIG demonstrates mesenchymal type cells with increased glycogen within cytoplasm as well as occasional lipid droplets, leading to the suggestion that these cells may be “lipofibroblast,” although exact histogenesis remains unknown. PIG explains the clinical exacerbation of chronic lung disease in some children presumably due to interstitial widening and impairment of diffusion capacity. Anecdotally, some children clinically respond to steroids, although in other cases the natural history seems to be one of spontaneous resolution over time, confirmed histologically in one case.68
FIG. 6.
Pulmonary interstitial glycogenosis. (A) Pulmonary interstitial glycogenosis (PIG), also called infantile cellular interstitial pneumonia (ICIP), is characterized by increased numbers of bland ovoid mesenchymal cells within the interstitium of the lung. It is thought to be a secondary reaction to a variety of forms of lung injury in neonates and young infants. (B) This process is often patchy in distribution and is commonly associated with disorders of alveolar growth.
Table 4.
Pulmonary Interstitial Glycogenosis: Diagnostic Features
| Interstitial widening with increased cellularity |
| Mesenchymal cells: vimentin-positive, leukocyte common antigen (LCA)-negative. |
| Cytoplasmic glycogen: Periodic acid–Schiff (PAS)-positive or demonstrated by electron microscopy |
| Often associated with other disease processes: alveolar growth abnormalities, congenital heart disease, pulmonary hypertension, congenital lung malformations |
Neuroendocrine Cell Hyperplasia of Infancy
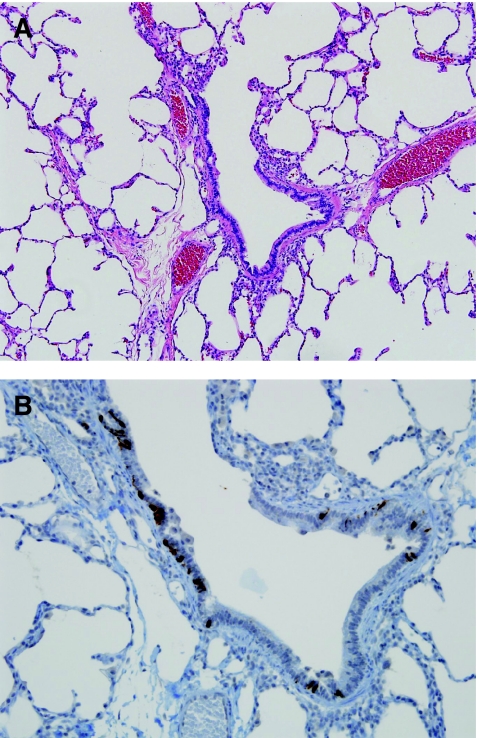
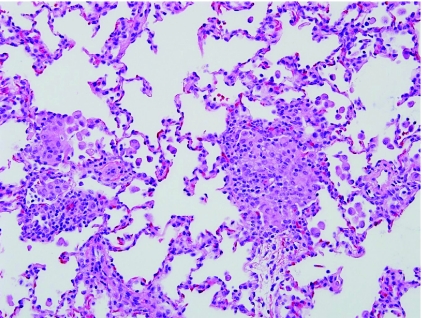
Neuroendocrine cell hyperplasia of infancy (NEHI) is a form of small airway disease (Table 5), described in 2005, characterized by increased numbers of neuroendocrine cells within the airways of infants and toddlers.69,70 Clinically, these children often have an oxygen requirement and the clinical picture is dominated by tachypnea and hypoxia. Characteristic imaging findings include hyperinflation on chest X-ray and patchy predominantly hilar ground-glass opacities on high-resolution chest CT.71 The severity of clinical features is disproportionate to the virtually normal histologic appearance of the lung biopsy tissue. Other forms of interstitial lung disease are excluded by examining the size of alveolar spaces, interstitial compartment, and vascular disease. When no other histologic features of chronic lung disease are identified in this clinical setting, immunohistochemical staining for bombesin is necessary to highlight the neuroendocrine cells within airways (Fig. 7). It is recognized from control tissues that the normal percentage of neuroendocrine cells within the respiratory epithelium is ∼2% to 8%. Diagnostic guidelines for histologic diagnosis of NEHI have been developed (Table 6). Exclusion of other forms of chronic lung disease is also an important consideration, as airway neuroendocrine cell hyperplasia has been described as a secondary finding in a wide variety of pediatric lung disorders.72–78 Some cases have mild airway-associated lymphoid hyperplasia, but significant fibrosis is absent. The etiology of NEHI remains unknown and it has been suggested that this may be a developmental or genetic disorder due to familial descriptions of NEHI, or may also be a secondary reaction to prior airway injury, for example a postinfectious process or post-aspiration process. It is also possible that this process represents an interaction of both genetic and environmental factors, for example, hereditary predisposition to neuroendocrine cell proliferation as a reaction to airway injury. The clinical importance of this diagnosis is that prognosis is excellent, and there is no associated mortality, unlike many other forms of diffuse lung disease in infants. There are no known effective therapies and recognition of this entity, either clinically or pathologically, prevents unnecessary use of steroid therapy or other more aggressive treatment. Most children experience symptomatic improvement over a period of years.
Table 5.
Differential Diagnosis of Airway Disease in Children
| Asthma |
| Neuroendocrine cell hyperplasia of infancy |
| Lymphocytic bronchiolitis (viral, aspiration, hypersensitivity pneumonitis, autoimmune) |
| Necrotizing bronchiolitis (viral, Stevens–Johnson syndrome) |
| Follicular bronchiolitis (Epstein-Barr virus (EBV), immunodeficiency, autoimmune disease) |
| Constrictive and obliterative bronchiolitis (post-viral, chronic aspiration, graft-versus-host disease (GVHD), allograft rejection) |
| Chronic bronchiolitis with bronchiectasis (cystic fibrosis, primary ciliary dyskinesia, immunodeficiency) |
FIG. 7.
Neuroendocrine cell hyperplasia of infancy. (A) Neuroendocrine cell hyperplasia of infancy (NEHI) is a clinicopathologic syndrome that should be suspected in any infant lung biopsy with near-normal histology. (B) Increased numbers of neuroendocrine cells within the small airways are demonstrated by bombesin immunohistochemistry.
Table 6.
Neuroendocrine Cell Hyperplasia of Infancy: Diagnostic features
| Increased percentage of airways with neuroendocrine cells, ≥75% |
| Increased percentage of neuroendocrine cells per airway, ≥10% |
| Large neuroepithelial bodies |
| Absence of other associated disease processes |
Infectious and Postinfectious Diseases
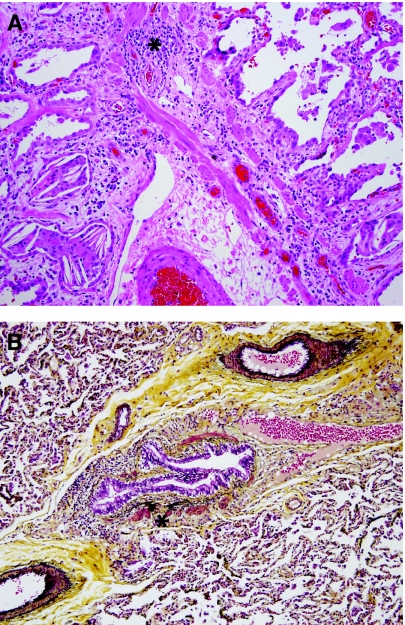
The spectrum of respiratory viral infections in children has been reviewed elsewhere,79 and is beyond the scope of this review. The typical findings are that of lymphocytic bronchiolitis, as well as mucosal necrosis and interstitial pneumonitis in some cases. Specific viral cytopathic effect may be seen with herpes simplex virus, adenovirus, and cytomegalovirus. Multinucleation of alveolar epithelial cells (giant cell pneumonia pattern) is an important clue to infection with one of the following viruses, particularly in immunocompromised patients: respiratory syncytial virus, human metapneumovirus, parainfluenza virus, herpes simplex virus, varicella zoster virus, and measles virus. Because acute respiratory viral illness is commonly diagnosed by clinical, serologic, and/or microbiological studies, there is little role for diagnostic lung biopsy, except perhaps for some immunocompromised children in whom multiple infectious agents may be suspected. In otherwise healthy children, lung biopsy is more likely to be performed for evaluation of postinfectious complications, for example, confirmation of suspected bronchiolitis obliterans syndrome (BOS), especially when preceding history of viral bronchiolitis is not elicited. Clinically, BOS is characterized by obstructive pulmonary function testing and expiratory chest CT imaging showing mosaic perfusion, corresponding to variable air-trapping and shunting of blood flow. The pathologic correlate of BOS is constrictive or obliterative bronchiolitis, typically a complication associated with viruses that produce extensive mucosal necrosis, for example adenovirus or influenza virus.80 The mucosal and submucosal injury induces a fibroblastic reparative response that eventually produces complete fibrosis of small airways (Fig. 8A). The mucosa may be completely absent, making the obliterated small airways difficult to visualize. Low-power histologic clues include the presence of an unpaired pulmonary artery profile, isolated foci of smooth muscle or cartilage without associated mucosa or bronchiolar lumen, and secondary evidence of airway obstruction (airspace hyperinflation, periairway foamy macrophages, and cholesterol clefts). Connective tissue stains such as trichrome stain or Movat pentachrome stain are very useful in further highlighting airway fibrosis (Fig. 8B). It should be noted that the distribution of obliterated airways is variable within the lung and leads to sampling error within lung biopsies. In some cases, complete obliteration of airways is readily demonstrated, while in other cases there may be only mild fibrosis (constrictive bronchiolitis) that nevertheless helps to support a clinical and radiographic suspicion of BOS. In addition to respiratory viral infections, other disorders that may produce obliterative bronchiolitis include graft-versus-host disease, chronic airway rejection in lung transplant patients, and Stevens–Johnson syndrome.
FIG. 8.
Constrictive and obliterative bronchiolitis. (A) Obliterative bronchiolitis is the pathologic correlate of the clinical diagnosis of bronchiolitis obliterans syndrome (BOS). Histologically, it is characterized by fibrous obliteration of small airways, typically leaving a “footprint” of peripheral airway smooth muscle (*). Increased periairway macrophages and cholesterol clefts are common findings secondary to small airway obstruction. Potential etiologies include post-viral syndromes, chronic aspiration, chronic airway rejection in the setting of lung transplant, and graft-versus-host disease in the setting of bone marrow transplant. Asthma and cystic fibrosis occasionally produce focal obliterative bronchiolitis. (B) Constrictive bronchiolitis refers to partial obliteration (stenosis) of small airways and is a common finding in the spectrum of BOS. This lung biopsy from a child with chronic aspiration demonstrates increased subepithelial collagen and elastin (*) on Movat's pentachrome stain.
Asthma
While asthma is not a typical indication for lung biopsy, manifestations of asthma are occasionally observed in biopsies performed for other reasons and may aid in explaining obstructive pulmonary disease. The histologic features of small airways in asthma include smooth muscle hypertrophy, variable periairway lymphocytes and eosinophils, and intraluminal mucus plugging (Fig. 9). Large airways also typically show basement membrane thickening and goblet cell hyperplasia. In acute exacerbation, increased neutrophils, prominent eosinophils, and Charcot-Leyden crystals may be seen. It should also be noted that asthma patients are predisposed to allergic bronchopulmonary fungal disease, a diagnostic consideration in the setting of bronchiectasis or bronchial casts.
FIG. 9.
Asthma. Although not typically the indication for lung biopsy, features of asthma are sometimes seen in older children and adolescents. Periairway lymphocytes and eosinophils, mucus plugging, and alveolar distension (hyperinflation) are common findings. Airway smooth muscle hypertrophy and goblet cell hyperplasia are other typical features not demonstrated in this case.
Aspiration
Neonates with meconium aspiration are typically diagnosed clinically, and lung biopsy is not usually indicated.81,82 Occasionally, however, the pathologist may detect evidence of persistent meconium in lung biopsies, even several weeks after delivery. Perhaps due to transfer of care from a delivering hospital to the neonatal intensive care unit at another institution, a history of meconium-stained fluid or meconium aspiration at delivery may not be apparent from the medical record. Persistent infiltrates on imaging and/or persistent symptomatology raises a differential diagnosis with inherited disorders of surfactant metabolism or other interstitial lung disease in infants. Histologically, meconium aspiration can be recognized by the presence of loosely aggregated golden brown-pigmented macrophages and anucleate squames within a faintly basophilic mucoid fluid (Fig. 10A). Reactive alveolar epithelial hyperplasia, interstitial edema, or PIG may be associated findings.
FIG. 10.
Aspiration injury. (A) In neonates, meconium aspiration is occasionally detected in diagnostic lung biopsies. Meconium is identified by loose aggregates of myxoid material, pigmented macrophages, and keratin flakes (*). (B) Aspiration of food particles in older children is associated with a granulomatous tissue response (*). Other features of aspiration in this case include chronic airway injury and lymphocytic bronchiolitis.
More commonly, questions of chronic aspiration arise in older infants and children. Diagnosis of chronic aspiration remains a challenge for both pulmonologists and pathologists, as there are very few specific histologic features that confirm the diagnosis. In infants, milk or formula aspiration often remains occult on lung biopsy. Granular eosinophilic debris, increased foamy macrophages, cholesterol clefts, or intra-alveolar droplets may be seen, but are not specific findings. Oil red O stain can be performed on frozen sections of lung tissue to detect increased lipid-laden macrophages, but it must be interpreted in context of other clinical and histologic features, as increased lipid-laden macrophages may be seen in association with a wide variety of processes, including resolving hemorrhage, resolving pneumonia, and surfactant disorders. Aspiration can be diagnosed with confidence in older children when there are food particles (vegetable or skeletal muscle) within the alveolar parenchyma, often associated with a giant cell or granulomatous response (Fig. 10B).83 Lymphocytic bronchiolitis and/or remote airway injury with reactive lymphoid hyperplasia may be seen in some cases. A pattern of exogenous lipoid pneumonia is seen in cases of mineral oil aspiration, characterized by alveolar and interstitial lipid vacuoles associated with inflammation and variable interstitial fibroplasia. Attempts at identifying a specific immunohistochemical marker of milk or gastric acid aspiration have not been fruitful up to this point and in most cases, the diagnosis of aspiration cannot be excluded on lung biopsy.
Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis (HP) is often diagnosed clinically by history of environmental exposure and by imaging and serologic studies.84 Occasionally, a lung biopsy is performed if the history of exposure is not elicited or if other features are atypical. The characteristic histologic features of HP are lymphocytic bronchiolitis, periairway (bronchiolocentric), lymphocytic interstitial infiltrates, as well as small subtle poorly formed granulomas and giant cells in a periairway distribution (Fig. 11). Chronic HP may also show significant interstitial fibrosis and cystic remodeling of the lung. This diagnosis, although well described, may be challenging nevertheless as some cases may show only the interstitial lymphocytic infiltrates, and the giant cells and granulomas are difficult to recognize. Even if a specific diagnosis cannot be reached on biopsy, the presence of NSIP pattern (Table 2)should raise the possibility of HP in the differential diagnosis and prompt further questioning and laboratory evaluation for environmental exposures.
FIG. 11.
Hypersensitivity pneumonitis. Hypersensitivity pneumonitis in the subacute to chronic phase demonstrates mild to moderate interstitial lymphocyte infiltrates and inconspicuous poorly formed granulomas and giant cells, often in a periairway distribution. Chronic airway injury is a common associated finding.
Eosinophilic Pneumonia
Eosinophilic pneumonia is a histologic pattern of prominent eosinophils in the interstitium and alveolar spaces (Fig. 12). In the acute phase, the eosinophil infiltrates are accompanied by hyaline membranes (diffuse alveolar damage). Eosinophilic pneumonia may be the result of a drug reaction, although the etiology is not apparent in many cases. Peripheral blood eosinophilia is often associated.85
FIG. 12.
Eosinophilic pneumonia. Eosinophilic pneumonia is characterized by prominent eosinophil infiltrates in the interstitium and alveolar spaces, as well as hyaline membranes (acute phase) and interstitial organization (chronic phase). Eosinophilic pneumonia may be a manifestation of drug reactions, but is idiopathic in many cases.
Autoimmune and Rheumatologic Disease
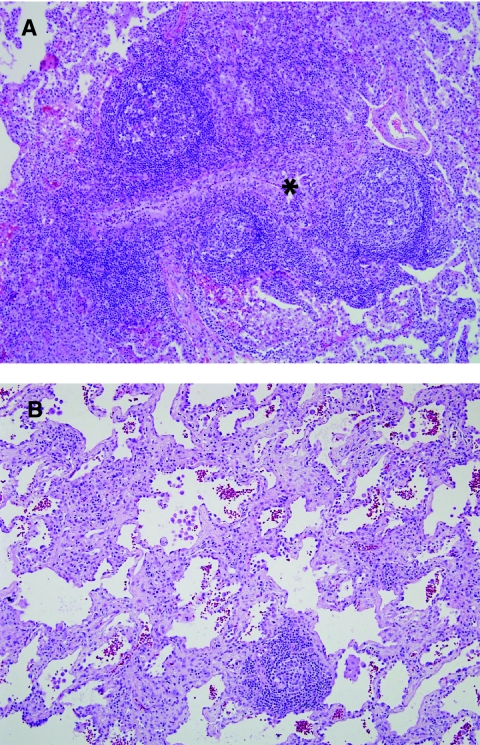
Autoimmune and rheumatologic (collagen vascular) diseases represent a challenging and dynamic field within diagnostic lung pathology. Just as there is a wide distribution of autoimmune disease based on clinical and serologic findings, similarly the pathologic findings show a wide spectrum of disease, often with involvement of multiple anatomic compartments of the lung (alveoli, interstitium, airways, vasculature, and/or pleura). Nevertheless, there are some common histologic features that suggest an immunologic or autoimmune process. Histologic patterns that are common to rheumatologic disorders include follicular bronchiolitis and other forms of lymphoid hyperplasia (Fig. 13A), NSIP pattern, lymphocytic interstitial pneumonia (LIP) pattern (Fig. 13B), interstitial fibrosis, chronic pulmonary vascular disease, pleuritis, and pleural fibrosis.86–89 Interstitial plasma cells lend support for an autoimmune process. In general, juvenile rheumatoid (idiopathic) arthritis in children is associated with lymphoid hyperplasia and chronic airway disease. Dermatomyositis may also show prominent lymphoid hyperplasia and interstitial fibrosis. Systemic lupus erythematosus shows a wide variety of manifestations, including both chronic lung disease and an acute pneumonitis pattern with hyaline membrane formation, reactive alveolar epithelium, and active inflammation. Pleuritis and pleural fibrosis also tend to be prominent features of lupus. Scleroderma is one of the more severe forms of rheumatologic lung disease, associated with interstitial fibrosis and characteristically severe arterial disease with arterial luminal stenosis by dense intimal fibrosis. Sjogren's syndrome may be associated with patchy or diffuse LIP pattern (Table 2), lymphocytic bronchiolitis, and follicular bronchiolitis. It should be noted that chronic lung disease is occasionally the first manifestation of systemic autoimmune disease and the associated histologic patterns are therefore important to recognize in order that further evaluation for autoimmune disease can be suggested in a diagnostic report.
FIG. 13.
Autoimmune and rheumatologic disease. Histologic patterns associated with autoimmune disease include prominent lymphoid hyperplasia, nonspecific interstitial pneumonia (NSIP) pattern, lymphocytic interstitial pneumonia pattern, interstitial fibrosis, pulmonary arterial disease, and pleural disease. Increased numbers of interstitial plasma cells may be a clue to autoimmune etiology. (A) Follicular bronchiolitis is one form of lymphoid hyperplasia in the lung (compressed bronchiole, *). (B) This patient with juvenile idiopathic arthritis showed a pattern of cellular and fibrosing NSIP on lung biopsy.
Immunodeficiency Disorders
In addition to the autoimmune diseases described earlier, pulmonary lymphoid hyperplasia is a common manifestation of primary immunodeficiency disorders. HIV infection may result in follicular lymphoid hyperplasia and/or LIP pattern.90,91 Common variable immunodeficiency classically results in an airway-centered lymphoid hyperplasia with follicular bronchiolitis.92 The lymphoid hyperplasia may be so pronounced that it mimics a lymphoproliferative process and malignancy should be carefully excluded when there are confluent sheets of lymphocytes in the lung. Some T-cell immunodeficiencies may also produce lymphocytic bronchiolitis and perivascular lymphocyte infiltrates. Other disorders associated with immune dysregulation and lymphoid hyperplasia in the pediatric lung are autoimmune lymphoproliferative syndrome, autoimmune polyendocrinopathy syndrome, and IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome. Finally, Epstein-Barr virus (EBV) should be considered in any lung biopsy with significant follicular lymphoid hyperplasia, whether in a healthy child or immunodeficient child.
Pulmonary Vascular Disease
Pulmonary vascular disease may mimic interstitial lung disease clinically and by imaging.93 Pulmonary vascular disease in children most often takes the form of secondary pulmonary arterial hypertension in the setting of chronic lung disease and hypoxemia due to prematurity, hypoplasia, or other interstitial lung disease (Table 7).94 The primary (idiopathic) and familial forms of pulmonary arterial hypertension are relatively rare in children and typically manifest in older children and adolescents. This diagnosis is made often on clinical grounds and lung biopsy is typically not necessary for diagnosis. Nevertheless, the pathologist should be familiar with chronic pulmonary hypertensive changes within the arteries including medial hypertrophy of the small muscular pulmonary arteries (Fig. 14A), variable intimal proliferation, and muscularization within the intralobular (intra-acinar) arterioles. Normally, the arterioles have a very thin-walled and any smooth muscle coat of these vessels should be considered abnormal. Notoriously, there is a poor clinical pathologic correlation between histologic and clinical findings of pulmonary arterial hypertension. For example, there are some cases in which medial hypertrophy of pulmonary arteries is observed and yet there are no echocardiographic features to suggest pulmonary hypertension. Conversely, there may be some cases in which there is clinical evidence of pulmonary hypertension, and yet the pulmonary arteries histologically do not manifest with histologic changes. If severe histologically then this typically does correlate with clinical evidence of pulmonary hypertension and regardless of the clinical findings is an important observation to report within any pediatric lung biopsy, particularly those with chronic interstitial changes.
Table 7.
Clinicopathologic Differential Diagnosis of Pulmonary Arteriopathy in Children
| Pulmonary arterial hypertension |
| Idiopathic pulmonary arterial hypertension |
| Familial pulmonary arterial hypertension |
| BMPR2 gene mutation-associated, other |
| Associated with systemic disease |
| Congenital systemic to pulmonary shunts |
| Congenital heart disease with Eisenmenger's syndrome. Examples: atrial septal defect, ventricular septal defect, atrioventricular septal defect, patent ductus arteriosus, other |
| Systemic arteriovenous shunt (vein of Galen malformation) |
| Portal (portopulmonary) hypertension |
| Collagen vascular disease |
| Human immunodeficiency virus infection |
| Drugs and toxins |
| Other systemic disease |
| Sickle cell disease/hemoglobinopathies |
| Metabolic storage diseases (glycogen storage disease, Gaucher's disease, other) |
| Hereditary hemorrhagic telangiectasia |
| Langerhans cell histiocytosis |
| Myeloproliferative disorders |
| Splenectomy |
| Associated with significant intrapulmonary venous or capillary involvement: |
| Alveolar capillary dysplasia with misalignment of pulmonary veins |
| Pulmonary veno-occlusive disease |
| Pulmonary capillary hemangiomatosis |
| Pulmonary venous hypertension |
| Pulmonary vein obstruction |
| Pulmonary vein stenosis |
| Anomalous pulmonary venous connection with obstructive physiology |
| Left atrial or ventricular disease heart disease |
| Cardiomyopathy |
| Other left ventricular failure |
| Left-sided valvular heart disease |
| Mitral stenosis |
| Aortic stenosis |
| Pulmonary arterial hypertension associated with hypoxemia |
| Persistent pulmonary hypertension of the newborn |
| Alveolar growth abnormalities (prematurity, hypoplasia, other) |
| Interstitial lung disease |
| Chronic obstructive pulmonary disease (cystic fibrosis, other) |
| Other |
| Sleep-disordered breathing |
| Alveolar hypoventilation disorders |
| Chronic exposure to high altitude |
| Pulmonary hypertension due to chronic thrombotic and/or embolic disease |
| Pulmonary arterial thromboembolism, proximal or distal |
| Other embolism |
| Examples: tumor, parasites, foreign material/intravenous drug abuse |
| Miscellaneous |
| Mediastinal/hilar pulmonary vascular compression, lymphangiomatosis, sarcoidosis |
Modified from 2003 revised World Health Organization classification of pulmonary hypertension, Proceedings of the 3rd World Symposium on Pulmonary Arterial Hypertension, Venice, Italy, June 23–25, 2003. J Am Coll Cardiol 2004; 43(Suppl. 12):1S–90S.
FIG. 14.
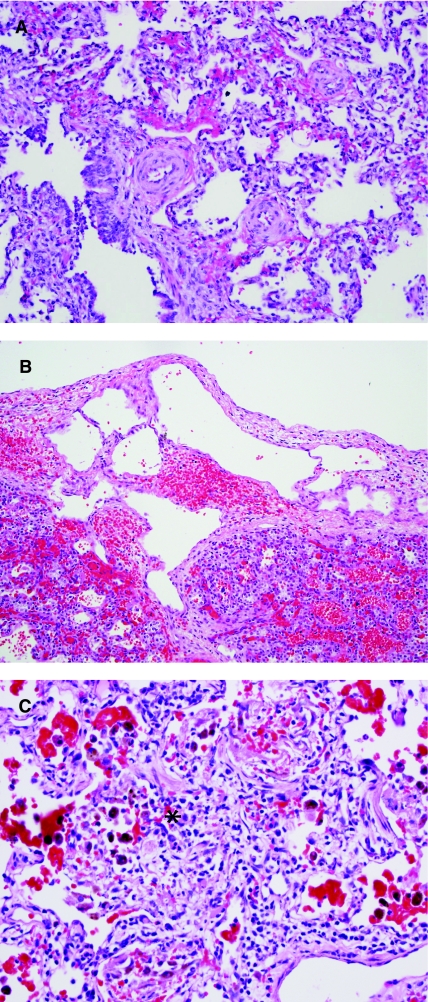
Pulmonary vascular disease. (A) Pulmonary arterial medial hypertrophy is commonly associated with forms of congenital heart disease causing chronic pulmonary over circulation, as in left-to-right shunt lesions. Chronic congestive vasculopathy due to pulmonary venous hypertension should also be excluded in any biopsy demonstrating pulmonary arteriopathy. (B) Lymphangiectasia is best demonstrated in the pleura and interlobular septa and may be a primary developmental abnormality or a secondary hemodynamic process. (C) Pulmonary capillaritis should be sought in patients with pulmonary hemorrhage syndromes, with or without other systemic manifestations of autoimmune disease. Histologically, it is characterized by increased neutrophils in the interstitium and alveolar spaces (*), associated with focal alveolar fibrin, extravasated red blood cells, and increased hemosiderin-laden macrophages.
Other common settings for pulmonary arterial disease is congenital heart disease.94–96 A histologic classification of pulmonary vascular disease in this setting has been described as the Heath Edwards classification, Grades I through VI. This classification is still useful in characterizing the morphology of pulmonary arterial hypertension associated with congenital heart disease, but has not reliably correlated with other forms of pulmonary arterial hypertension and does not appear to have prognostic significance in that setting. While lung biopsy has been historically performed to assess the degree of pulmonary hypertension in patients with congenital heart disease, this practice has fallen out of favor and is performed uncommonly in most children's hospitals.
In children with congenital heart disease, it should be emphasized that each component of the pulmonary vasculature should be evaluated histologically. The pulmonary arterial changes may be the most prominent abnormality in the biopsy, but should lead to further evaluation of pulmonary veins also in order to identify forms of pulmonary venous hypertension. Pulmonary venous hypertension may result from pulmonary veno-occlusive disease (PVOD) or more commonly pulmonary vein stenosis. Increased pulmonary venous pressure also occurs in any form of chronic left ventricular heart failure (eg, dilated cardiomyopathy) or left-sided cardiac obstruction. Pulmonary venous hypertension is manifested in lung tissues as increased muscularization and/or “arterialization” of the pulmonary veins within the interlobular septa. Severe pulmonary arterial disease is typically associated. Other markers of chronic passive congestion within the lung include alveolar capillary congestion, and increased numbers of hemosiderinladen macrophages. Iron stain is sometimes useful in this regard.
The other component of the vasculature that needs to be evaluated is the lymphatic system.97 Lymphatic dilatation and muscularization are also features of pulmonary venous hypertension. Lymphangiectasia may be either a primary developmental process or a secondary hemodynamic abnormality and the distinction may be difficult on histologic grounds alone (Fig. 14B). Again, assessment of other interstitial lung disease and other aspects of pulmonary vascular architecture are essential in resolving whether lymphangiectasia is primary or secondary. Another observation about pulmonary lymphangiectasia is that it may be associated with congenital chylothorax, in which case there may be associated lung hypoplasia.
The last category of pulmonary vascular disease that should be mentioned is the group of pulmonary vasculitides. Pulmonary hemorrhage syndromes are sometimes associated with capillaritis in the alveolar walls (Fig. 14C),98,99 a finding that may correlate with anti-neutrophil cytoplasmic antibodies (ANCA)-positive isolated pulmonary hemorrhage, microscopic polyangiitis, Wegener granulomatosis, and other autoimmune disease. Histologically, acute capillaritis demonstrates increased numbers of interstitial and alveolar neutrophils (acute alveolitis) and focal alveolar fibrin in areas of alveolar wall injury. Focal organizing pneumonia pattern is a commonly associated, but nonspecific, finding. The diagnosis of capillaritis can be difficult given the focality and variable activity of disease at any given point in time and is particularly challenging following steroid therapy. Hyperplastic lymphoid aggregates in a lung biopsy with chronic pulmonary hemorrhage may be a clue to immune-mediated disease. Other forms of pulmonary vasculitis involving medium-sized vessels include Wegener granulomatosis (granulomatous vasculitis) and Churg–Strauss syndrome (eosinophilic vasculitis). If vasculitis is not identified, other causes of chronic pulmonary hemorrhage or hemosiderosis should be considered, such as recurrent hemorrhage from chronic vasculopathy (eg, pulmonary venous hypertension), hemosiderosis secondary to aspiration (Heiner's syndrome), and idiopathic hemosiderosis.
Summary
Interpretation of pediatric lung biopsy remains a dynamic and challenging field that requires careful correlation with clinical and imaging features. The collection of tissues for special studies can be successfully performed with guidance of standardized protocols. Application of these special studies can be helpful in the diagnosis of the genetic disorders of surfactant metabolism, ACD, infectious processes, PIG, pulmonary hemorrhage syndromes, and autoimmune diseases. Remarkable advances have been made in the last 10 years, which have allowed further understanding of the biology and pathologic diagnosis of pediatric lung disease. The close collaboration between pediatric pulmonologists, neonatologists, pulmonary biologists, radiologists, and pathologists in this field holds tremendous promise for continued advancements in our ability not only to diagnose these disorders, but also to provide more effective individualized therapy.
References
- 1.Langston C. Patterson K. Dishop MK. Askin F. Baker P. Chou P. Cool C. Coventry S. Cutz E. Davis M. Deutsch G. Galambos C. Pugh J. Wert S. White F chILD Pathology Co-operative Group. A protocol for the handling of tissue obtained by operative lung biopsy: recommendations of the chILD pathology co-operative group. Pediatr Dev Pathol. 2006;9:173–180. doi: 10.2350/06-03-0065.1. [DOI] [PubMed] [Google Scholar]
- 2.Gianoulis M. Chan N. Wright JL. Inflation of lung biopsies for frozen section. Mod Pathol. 1988;1:357–358. [PubMed] [Google Scholar]
- 3.Deutsch GH. Young LR. Deterding RR. Fan LL. Dell SD. Bean JA. Brody AS. Nogee LM. Trapnell BC. Langston C. Albright EA. Askin FB. Baker P. Chou PM. Cool CM. Coventry SC. Cutz E. Davis MM. Dishop MK. Galambos C. Patterson K. Travis WD. Wert SE. White FV Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langston C. Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr Dev Pathol. 2009;12:421–437. doi: 10.2350/08-11-0559.1. [DOI] [PubMed] [Google Scholar]
- 5.Fan LL. Kozinetz CA. Deterding RR. Brugman SM. Evaluation of a diagnostic approach to pediatric interstitial lung disease. Pediatrics. 1998;101:82–85. doi: 10.1542/peds.101.1.82. [DOI] [PubMed] [Google Scholar]
- 6.Howenstine MS. Eigen H. Current concepts on interstitial lung disease in children. Curr Opin Pediatr. 1999;11:200–204. doi: 10.1097/00008480-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Coren ME. Nicholson AG. Goldstraw P. Rosenthal M. Bush A. Open lung biopsy for diffuse interstitial lung disease in children. Eur Respir J. 1999;14:817–821. doi: 10.1034/j.1399-3003.1999.14d16.x. [DOI] [PubMed] [Google Scholar]
- 8.Langston C. Fan LL. The spectrum of interstitial lung disease in childhood. Pediatr Pulmonol. 2001;(Suppl 23):70–71. [PubMed] [Google Scholar]
- 9.Fan LL. Langston C. Pediatric interstitial lung disease: children are not small adults. Am J Respir Crit Care Med. 2002;165:1466–1467. doi: 10.1164/rccm.2204012. [DOI] [PubMed] [Google Scholar]
- 10.Clement A. Henrion-Caude A. Fauroux B. The pathogenesis of interstitial lung diseases in children. Paediatr Respir Rev. 2004;5:94–97. doi: 10.1016/j.prrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Hilman BC. Amaro-Galvez R. Diagnosis of interstitial lung disease in children. Paediatr Respir Rev. 2004;5:101–107. doi: 10.1016/j.prrv.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Fan LL. Deterding RR. Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol. 2004;38:369–378. doi: 10.1002/ppul.20114. [DOI] [PubMed] [Google Scholar]
- 13.Clement A ERS Task Force. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J. 2004;24:686–697. doi: 10.1183/09031936.04.00089803. [DOI] [PubMed] [Google Scholar]
- 14.Nogee LM. de Mello DE. Dehner LP. Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 15.Nogee LM. Garnier G. Dietz HC. Singer L. Murphy AM. deMello DE. Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deMello DE. Nogee LM. Heyman S. Krous HF. Hussain M. Merritt TA. Hsueh W. Haas JE. Heidelberger K. Schumacher R. Molecular and phenotypic variability in the congenital alveolar proteinosis syndrome associated with inherited surfactant protein B deficiency. J Pediatr. 1994;125:43–50. doi: 10.1016/s0022-3476(94)70119-9. [DOI] [PubMed] [Google Scholar]
- 17.Nogee LM. Dunbar AE., 3rd Wert SE. Askin F. Hamvas A. Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 18.Tredano M. Griese M. Brasch F. Schumacher S. de Blic J. Marque S. Houdayer C. Elion J. Couderc R. Bahuau M. Mutation of SFTPC in infantile pulmonary alveolar proteinosis with or without fibrosing lung disease. Am J Med Genet A. 2004;126A:18–26. doi: 10.1002/ajmg.a.20670. [DOI] [PubMed] [Google Scholar]
- 19.Lawson WE. Grant SW. Ambrosini V. Womble KE. Dawson EP. Lane KB. Markin C. Renzoni E. Lympany P. Thomas AQ. Roldan J. Scott TA. Blackwell TS. Phillips JA., 3rd Loyd JE. du Bois RM. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzenstein AL. Gordon LP. Oliphant M. Swender PT. Chronic pneumonitis of infancy. A unique form of interstitial lung disease occurring in early childhood. Am J Surg Pathol. 1995;19:439–447. [PubMed] [Google Scholar]
- 21.Fisher M. Roggli V. Merten D. Mulvihill D. Spock A. Coexisting endogenous lipoid pneumonia, cholesterol granulomas, and pulmonary alveolar proteinosis in a pediatric population: a clinical, radiographic, and pathologic correlation. Pediatr Pathol. 1992;12:365–383. doi: 10.3109/15513819209023316. [DOI] [PubMed] [Google Scholar]
- 22.Chibbar R. Shih F. Baga M. Torlakovic E. Ramlall K. Skomro R. Cockcroft DW. Lemire EG. Nonspecific interstitial pneumonia and usual interstitial pneumonia with mutation in surfactant protein C in familial pulmonary fibrosis. Mod Pathol. 2004;17:973–980. doi: 10.1038/modpathol.3800149. [DOI] [PubMed] [Google Scholar]
- 23.Shulenin S. Nogee LM. Annilo T. Wert SE. Whitsett JA. Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 24.Doan ML. Guillerman RP. Dishop MK. Nogee LM. Langston C. Mallory GB. Sockrider MM. Fan LL. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 25.Bullard JE. Wert SE. Whitsett JA. Dean M. Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172:1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards V. Cutz E. Viero S. Moore AM. Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol. 2005;29:503–509. doi: 10.1080/01913120500323480. [DOI] [PubMed] [Google Scholar]
- 27.deMello DE. Heyman S. Phelps DS. Hamvas A. Nogee L. Cole S. Colten HR. Ultrastructure of lung in surfactant protein B deficiency. Am J Respir Cell Mol Biol. 1994;11:230–239. doi: 10.1165/ajrcmb.11.2.8049084. [DOI] [PubMed] [Google Scholar]
- 28.Tryka AF. Wert SE. Mazursky JE. Arrington RW. Nogee LM. Absence of lamellar bodies with accumulation of dense bodies characterizes a novel form of congenital surfactant defect. Pediatr Dev Pathol. 2000;3:335–345. doi: 10.1007/s100249910048. [DOI] [PubMed] [Google Scholar]
- 29.Cutz E. Wert SE. Nogee LM. Moore AM. Deficiency of lamellar bodies in alveolar type II cells associated with fatal respiratory disease in a full-term infant. Am J Respir Crit Care Med. 2000;161:608–614. doi: 10.1164/ajrccm.161.2.9905062. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Moczygemba M. Doan ML. Elidemir O. Fan LL. Cheung SW. Lei JT. Moore JP. Tavana G. Lewis LR. Zhu Y. Muzny DM. Gibbs RA. Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T. Sakagami T. Rubin BK. Nogee LM. Wood RE. Zimmerman SL. Smolarek T. Dishop MK. Wert SE. Whitsett JA. Grabowski G. Carey BC. Stevens C. van der Loo JC. Trapnell BC. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maquet E. Costagliola S. Parma J. Christophe-Hobertus C. Oligny LL. Fournet JC. Robitaille Y. Vuissoz JM. Payot A. Laberge S. Vassart G. Van Vliet G. Deladoëy J. Lethal respiratory failure and mild primary hypothyroidism in a term girl with a de novo heterozygous mutation in the TITF1/NKX2.1 gene. J Clin Endocrinol Metab. 2009;94:197–203. doi: 10.1210/jc.2008-1402. [DOI] [PubMed] [Google Scholar]
- 33.Carré A. Szinnai G. Castanet M. Sura-Trueba S. Tron E. Broutin-L'Hermite I. Barat P. Goizet C. Lacombe D. Moutard ML. Raybaud C. Raynaud-Ravni C. Romana S. Ythier H. Léger J. Polak M. Five new TTF1/NKX2.1 mutations in brain–lung–thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 34.Bedrossian CW. Luna MA. Conklin RH. Miller WC. Alveolar proteinosis as a consequence of immunosuppression. A hypothesis based on clinical and pathologic observations. Hum Pathol. 1980;11:527–535. [PubMed] [Google Scholar]
- 35.Nachajon RV. Rutstein RM. Rudy BJ. Collins MH. Pulmonary alveolar proteinosis in an HIV-infected child. Pediatr Pulmonol. 1997;24:292–295. doi: 10.1002/(sici)1099-0496(199710)24:4<292::aid-ppul9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Samuels MP. Warner JO. Pulmonary alveolar lipoproteinosis complicating juvenile dermatomyositis. Thorax. 1988;43:939–940. doi: 10.1136/thx.43.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parto K. Svedström E. Majurin ML. Härkönen R. Simell O. Pulmonary manifestations in lysinuric protein intolerance. Chest. 1993;104:1176–1182. doi: 10.1378/chest.104.4.1176. [DOI] [PubMed] [Google Scholar]
- 38.Janney CG. Askin FB. Kuhn C., 3rd Congenital alveolar capillary dysplasia—an unusual cause of respiratory distress in the newborn. Am J Clin Pathol. 1981;76:722–727. doi: 10.1093/ajcp/76.5.722. [DOI] [PubMed] [Google Scholar]
- 39.Wagenvoort CA. Misalignment of lung vessels: a syndrome causing persistent neonatal pulmonary hypertension. Hum Pathol. 1986;17:727–730. doi: 10.1016/s0046-8177(86)80182-4. [DOI] [PubMed] [Google Scholar]
- 40.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Pediatr Pathol. 1991;11:163–170. doi: 10.3109/15513819109064753. [DOI] [PubMed] [Google Scholar]
- 41.Haraida S. Lochbühler H. Heger A. Nerlich A. Diebold J. Wiest I. Müller-Höcker J. Löhrs U. Congenital alveolar capillary dysplasia: rare cause of persistent pulmonary hypertension. Pediatr Pathol Lab Med. 1997;17:959–975. [PubMed] [Google Scholar]
- 42.Boggs S. Harris MC. Hoffman DJ. Goel R. McDonald-McGinn D. Langston C. Zackai E. Ruchelli E. Misalignment of pulmonary veins with alveolar capillary dysplasia: affected siblings and variable phenotypic expression. J Pediatr. 1994;124:125–128. doi: 10.1016/s0022-3476(94)70267-5. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez C. Rodriguez A. Palenzuela S. Forteza C. Rossello JL. Congenital misalignment of pulmonary veins with alveolar capillary dysplasia causing persistent neonatal pulmonary hypertension: report of two affected siblings. Pediatr Dev Pathol. 2000;3:271–276. doi: 10.1007/s100249910035. [DOI] [PubMed] [Google Scholar]
- 44.Garola RE. Thibeault DW. Alveolar capillary dysplasia, with and without misalignment of pulmonary veins: an association of congenital anomalies. Am J Perinatol. 1998;15:103–107. doi: 10.1055/s-2007-993907. [DOI] [PubMed] [Google Scholar]
- 45.Sen P. Thakur N. Stockton DW. Langston C. Bejjani BA. Expanding the phenotype of alveolar capillary dysplasia (ACD) J Pediatr. 2004;145:646–651. doi: 10.1016/j.jpeds.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 46.Eulmesekian P. Cutz E. Parvez B. Bohn D. Adatia I. Alveolar capillary dysplasia: a six-year single center experience. J Perinat Med. 2005;33:347–352. doi: 10.1515/JPM.2005.067. [DOI] [PubMed] [Google Scholar]
- 47.Licht C. Schickendantz S. Sreeram N. Arnold G. Rossi R. Vierzig A. Mennicken U. Roth B. Prolonged survival in alveolar capillary dysplasia syndrome. Eur J Pediatr. 2004;163:181–182. doi: 10.1007/s00431-003-1385-6. [DOI] [PubMed] [Google Scholar]
- 48.Al-Hathlol K. Phillips S. Seshia MK. Casiro O. Alvaro RE. Rigatto H. Alveolar capillary dysplasia. Report of a case of prolonged life without extracorporeal membrane oxygenation (ECMO) and review of the literature. Early Hum Dev. 2000;57:85–94. doi: 10.1016/s0378-3782(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 49.Abdallah HI. Karmazin N. Marks LA. Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit Care Med. 1993;21:628–630. doi: 10.1097/00003246-199304000-00026. [DOI] [PubMed] [Google Scholar]
- 50.Stankiewicz P. Sen P. Bhatt SS. Storer M. Xia Z. Bejjani BA. Ou Z. Wiszniewska J. Driscoll DJ. Maisenbacher MK. Bolivar J. Bauer M. Zackai EH. McDonald-McGinn D. Nowaczyk MM. Murray M. Hustead V. Mascotti K. Schultz R. Hallam L. McRae D. Nicholson AG. Newbury R. Durham-O'Donnell J. Knight G. Kini U. Shaikh TH. Martin V. Tyreman M. Simonic I. Willatt L. Paterson J. Mehta S. Rajan D. Fitzgerald T. Gribble S. Prigmore E. Patel A. Shaffer LG. Carter NP. Cheung SW. Langston C. Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenough A. Factors adversely affecting lung growth. Paediatr Respir Rev. 2000;1:314–320. doi: 10.1053/prrv.2000.0070. [DOI] [PubMed] [Google Scholar]
- 52.Margraf LR. Tomashefski JF., Jr Bruce MC. Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991;143:391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 53.O'Brodovich HM. Mellins RB. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. Am Rev Respir Dis. 1985;132:694–709. doi: 10.1164/arrd.1985.132.3.694. [DOI] [PubMed] [Google Scholar]
- 54.Bonikos DS. Bensch KG. Northway WH., Jr Edwards DK. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol. 1976;7:643–666. doi: 10.1016/s0046-8177(76)80077-9. [DOI] [PubMed] [Google Scholar]
- 55.Stocker JT. Pathologic features of long-standing “healed” bronchopulmonary dysplasia: a study of 28 3- to 40-month-old infants. Hum Pathol. 1986;17:943–961. doi: 10.1016/s0046-8177(86)80646-3. [DOI] [PubMed] [Google Scholar]
- 56.Husain AN. Siddiqui NH. Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 57.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 58.Thurlbeck WM. Kida K. Langston C. Cowan MJ. Kitterman JA. Tooley W. Bryan H. Postnatal lung growth after repair of diaphragmatic hernia. Thorax. 1979;34:338–343. doi: 10.1136/thx.34.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooney TP. Thurlbeck WM. Pulmonary hypoplasia in Down's syndrome. N Engl J Med. 1982;307:1170–1173. doi: 10.1056/NEJM198211043071902. [DOI] [PubMed] [Google Scholar]
- 60.Cooney TP. Wentworth PJ. Thurlbeck WM. Diminished radial count is found only postnatally in Down's syndrome. Pediatr Pulmonol. 1988;5:204–209. doi: 10.1002/ppul.1950050405. [DOI] [PubMed] [Google Scholar]
- 61.Schloo BL. Vawter GF. Reid LM. Down syndrome: patterns of disturbed lung growth. Hum Pathol. 1991;22:919–923. doi: 10.1016/0046-8177(91)90183-p. [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez OR. Gomez IG. Recalde AL. Landing BH. Postnatal development of the cystic lung lesion of Down syndrome: suggestion that the cause is reduced formation of peripheral air spaces. Pediatr Pathol. 1991;11:623–633. doi: 10.3109/15513819109064794. [DOI] [PubMed] [Google Scholar]
- 63.Tyrrell VJ. Asher MI. Chan Y. Subpleural lung cysts in Down's syndrome. Pediatr Pulmonol. 1999;28:145–148. doi: 10.1002/(sici)1099-0496(199908)28:2<145::aid-ppul11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 64.Schroeder SA. Shannon DC. Mark EJ. Cellular interstitial pneumonitis in infants. A clinicopathologic study. Chest. 1992;101:1065–1069. doi: 10.1378/chest.101.4.1065. [DOI] [PubMed] [Google Scholar]
- 65.Canakis AM. Cutz E. Manson D. O'Brodovich H. Pulmonary interstitial glycogenosis: a new variant of neonatal interstitial lung disease. Am J Respir Crit Care Med. 2002;165:1557–1565. doi: 10.1164/rccm.2105139. [DOI] [PubMed] [Google Scholar]
- 66.Smets K. Dhaene K. Schelstraete P. Meersschaut V. Vanhaesebrouck P. Neonatal pulmonary interstitial glycogen accumulation disorder. Eur J Pediatr. 2004;163:408–409. doi: 10.1007/s00431-004-1450-9. [DOI] [PubMed] [Google Scholar]
- 67.Onland W. Molenaar JJ. Leguit RJ. van Nierop JC. Noorduyn LA. van Rijn RR. Geukers VG. Pulmonary interstitial glycogenosis in identical twins. Pediatr Pulmonol. 2005;40:362–366. doi: 10.1002/ppul.20255. [DOI] [PubMed] [Google Scholar]
- 68.Deutsch GH. Young LR. Histologic resolution of pulmonary interstitial glycogenosis. Pediatr Dev Pathol. 2009;12:475–480. doi: 10.2350/08-12-0575.1. [DOI] [PubMed] [Google Scholar]
- 69.Deterding RR. Fan LL. Morton R. Hay TC. Langston C. Persistent tachypnea of infancy (PTI)—a new entity. Pediatr Pulmonol. 2001;(Suppl 23):72–73. [PubMed] [Google Scholar]
- 70.Deterding RR. Pye C. Fan LL. Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 71.Brody AS. Guillerman RP. Hay TC. Wagner BD. Young LR. Deutsch GH. Fan LL. Deterding RR. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cutz E. Yeger H. Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol. 2007;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- 73.Johnson DE. Anderson WR. Burke BA. Pulmonary neuroendocrine cells in pediatric lung disease: alterations in airway structure in infants with bronchopulmonary dysplasia. Anat Rec. 1993;236:115–119. doi: 10.1002/ar.1092360115. 172. [DOI] [PubMed] [Google Scholar]
- 74.Johnson DE. Wobken JD. Landrum BG. Changes in bombesin, calcitonin, and serotonin immunoreactive pulmonary neuroendocrine cells in cystic fibrosis and after prolonged mechanical ventilation. Am Rev Respir Dis. 1988;137:123–131. doi: 10.1164/ajrccm/137.1.123. [DOI] [PubMed] [Google Scholar]
- 75.Asabe K. Tsuji K. Handa N. Kajiwara M. Suita S. Immunohistochemical distribution of bombesin-positive pulmonary neuroendocrine cells in a congenital diaphragmatic hernia. Surg Today. 1999;29:407–412. doi: 10.1007/BF02483031. [DOI] [PubMed] [Google Scholar]
- 76.Alshehri M. Cutz E. Banzhoff A. Canny G. Hyperplasia of pulmonary neuroendocrine cells in a case of childhood pulmonary emphysema. Chest. 1997;112:553–556. doi: 10.1378/chest.112.2.553. [DOI] [PubMed] [Google Scholar]
- 77.Perrin DG. McDonald TJ. Cutz E. Hyperplasia of bombesin-immunoreactive pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome. Pediatr Pathol. 1991;11:431–447. doi: 10.3109/15513819109064779. [DOI] [PubMed] [Google Scholar]
- 78.Gillan JE. Cutz E. Abnormal pulmonary bombesin immunoreactive cells in Wilson-Mikity syndrome (pulmonary dysmaturity) and bronchopulmonary dysplasia. Pediatr Pathol. 1993;13:165–180. doi: 10.3109/15513819309048204. [DOI] [PubMed] [Google Scholar]
- 79.Hammond S. Chenever E. Durbin JE. Respiratory virus infection in infants and children. Pediatr Dev Pathol. 2007;10:172–180. doi: 10.2350/07-02-0238.1. [DOI] [PubMed] [Google Scholar]
- 80.Moonnumakal SP. Fan LL. Bronchiolitis obliterans in children. Curr Opin Pediatr. 2008;20:272–278. doi: 10.1097/MOP.0b013e3282ff62e9. [DOI] [PubMed] [Google Scholar]
- 81.Swaminathan S. Quinn J. Stabile MW. Bader D. Platzker AC. Keens TG. Long-term pulmonary sequelae of meconium aspiration syndrome. J Pediatr. 1989;114:356–361. doi: 10.1016/s0022-3476(89)80551-7. [DOI] [PubMed] [Google Scholar]
- 82.Nathan L. Leveno KJ. Carmody TJ., 3rd Kelly MA. Sherman ML. Meconium: a 1990s perspective on an old obstetric hazard. Obstet Gynecol. 1994;83:329–332. [PubMed] [Google Scholar]
- 83.Gadol CL. Joshi VV. Lee EY. Bronchiolar obstruction associated with repeated aspiration of vegetable material in two children with cerebral palsy. Pediatr Pulmonol. 1987;3:437–439. doi: 10.1002/ppul.1950030611. [DOI] [PubMed] [Google Scholar]
- 84.Fan LL. Hypersensitivity pneumonitis in children. Curr Opin Pediatr. 2002;14:323–326. doi: 10.1097/00008480-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Oermann CM. Panesar KS. Langston C. Larsen GL. Menendez AA. Schofield DE. Cosio C. Fan LL. Pulmonary infiltrates with eosinophilia syndromes in children. J Pediatr. 2000;136:351–358. doi: 10.1067/mpd.2000.103350. [DOI] [PubMed] [Google Scholar]
- 86.Katzenstein AL. Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18:136–147. [PubMed] [Google Scholar]
- 87.Yousem SA. Colby TV. Carrington CB. Follicular bronchitis/bronchiolitis. Hum Pathol. 1985;16:700–706. doi: 10.1016/s0046-8177(85)80155-6. [DOI] [PubMed] [Google Scholar]
- 88.Athreya BH. Doughty RA. Bookspan M. Schumacher HR. Sewell EM. Chatten J. Pulmonary manifestations of juvenile rheumatoid arthritis. A report of eight cases and review. Clin Chest Med. 1980;1:361–374. [PubMed] [Google Scholar]
- 89.Lovell D. Lindsley C. Langston C. Lymphoid interstitial pneumonia in juvenile rheumatoid arthritis. J Pediatr. 1984;105:947–950. doi: 10.1016/s0022-3476(84)80085-2. [DOI] [PubMed] [Google Scholar]
- 90.Grieco MH. Chinoy-Acharya P. Lymphocytic interstitial pneumonia associated with the acquired immune deficiency syndrome. Am Rev Respir Dis. 1985;131:952–955. doi: 10.1164/arrd.1985.131.6.952. [DOI] [PubMed] [Google Scholar]
- 91.Joshi VV. Oleske JM. Minnefor AB. Saad S. Klein KM. Singh R. Zabala M. Dadzie C. Simpser M. Rapkin RH. Pathologic pulmonary findings in children with the acquired immunodeficiency syndrome: a study of ten cases. Hum Pathol. 1985;16:241–246. doi: 10.1016/s0046-8177(85)80009-5. [DOI] [PubMed] [Google Scholar]
- 92.Kinane BT. Mansell AL. Zwerdling RG. Lapey A. Shannon DC. Follicular bronchitis in the pediatric population. Chest. 1993;104:1183–1186. doi: 10.1378/chest.104.4.1183. [DOI] [PubMed] [Google Scholar]
- 93.Sondheimer HM. Lung MC. Brugman SM. Ikle DN. Fan LL. White CW. Pulmonary vascular disorders masquerading as interstitial lung disease. Pediatr Pulmonol. 1995;20:284–288. doi: 10.1002/ppul.1950200505. [DOI] [PubMed] [Google Scholar]
- 94.Cool CD. Deutsch G. Pulmonary arterial hypertension from a pediatric perspective. Pediatr Dev Pathol. 2008;11:169–177. doi: 10.2350/07-12-0398.1. [DOI] [PubMed] [Google Scholar]
- 95.Rabinovitch M. Haworth SG. Castaneda AR. Nadas AS. Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978;58:1107–1122. doi: 10.1161/01.cir.58.6.1107. [DOI] [PubMed] [Google Scholar]
- 96.Haworth SG. Pulmonary vascular disease in different types of congenital heart disease. Implications for interpretation of lung biopsy findings in early childhood. Br Heart J. 1984;52:557–571. doi: 10.1136/hrt.52.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Faul JL. Berry GJ. Colby TV. Ruoss SJ. Walter MB. Rosen GD. Raffin TA. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med. 2000;161:1037–1046. doi: 10.1164/ajrccm.161.3.9904056. [DOI] [PubMed] [Google Scholar]
- 98.Susarla SC. Fan LL. Diffuse alveolar hemorrhage syndromes in children. Curr Opin Pediatr. 2007;19:314–320. doi: 10.1097/MOP.0b013e3280dd8c4a. [DOI] [PubMed] [Google Scholar]
- 99.Fullmer JJ. Langston C. Dishop MK. Fan LL. Pulmonary capillaritis in children: a review of eight cases with comparison to other alveolar hemorrhage syndromes. J Pediatr. 2005;146:376–381. doi: 10.1016/j.jpeds.2004.10.025. [DOI] [PubMed] [Google Scholar]