Abstract
We identified 480 persons with positive thick smears for asexual Plasmodium falciparum parasites, of whom 454 had positive rapid diagnostic tests (RDTs) for the histidine-rich protein 2 (HRP2) product of the hrp2 gene and 26 had negative tests. Polymerase chain reaction (PCR) amplification for the histidine-rich repeat region of that gene was negative in one-half (10/22) of false-negative specimens available, consistent with spontaneous deletion. False-negative RDTs were found only in persons with asymptomatic infections, and multiplicities of infection (MOIs) were lower in persons with false-negative RDTs (both P < 0.001). These results show that parasites that fail to produce HRP2 can cause patent bloodstream infections and false-negative RDT results. The importance of these observations is likely to increase as malaria control improves, because lower MOIs are associated with false-negative RDTs and false-negative RDTs are more frequent in persons with asymptomatic infections. These findings suggest that the use of HRP2-based RDTs should be reconsidered.
Introduction
The diagnosis of malaria is typically based on microscopy, which is economical, widely accepted in malaria-endemic areas, and generally regarded as the gold standard.1 However, alternative rapid diagnostic tests (RDTs) have been developed for use in non-endemic countries, where skilled microscopists are less available, and endemic countries for malaria control programs. A number of the most widely used RDTs are based on detection of the histidine-rich protein 2 (HRP2) product of the Plasmodium falciparum hrp2 gene.2,3 Previous reports suggest that the sensitivities and specificities of these tests are 90–95% and 85–92%, respectively, relative to the thick smear.4–7
In these studies, we performed thick smears and HRP2-based RDTs on samples from 723 subjects in Mali. Specimens that produced false-negative RDT results (positive thick smear and negative RDT) were examined using polymerase chain reaction (PCR) for the histidine-rich repeat region of the hrp2 gene to determine whether the spontaneous deletion of that region was causing false-negative RDT results.
Materials and Methods
Overview
During the malaria transmission season (July to November) in 1996, finger stick blood samples were obtained from 723 subjects. One drop of blood was used to prepare a thick smear, 2–3 drops (50 μL) were used for the HRP2 RDT, and 2–3 drops were used for filter paper blots in the three villages noted below. After approval by Institutional Review Boards in Mali and New Orleans, the protocol used for these studies was presented to the chiefs and elders of the villages of Bancoumana, Donéguébougou, and Sirakoro, the directors of the Hôpital Gabriel Touré and Hôpital Point G in Bamako, and the director of the National Blood Bank in Bamako before it was discussed with potential subjects or their parents and guardians. Informed consent was obtained before enrollment and participation in the study.
Populations studied
Finger stick blood samples were obtained from asymptomatic children 6 months to 9 years of age with positive smears for P. falciparum infection in the suburban village of Sirakoro (8 km east of Bamako) and two rural villages (Bancoumana and Donéguébougou; 60 km south and 22 km north of Bamako, respectively). Samples were also obtained from asymptomatic blood donors in Bamako (adults ≥ 18 years) and symptomatic subjects who presented with fever and a presumptive diagnosis of malaria at either the Hôpital Gabriel Touré (children 6 months to 9 years) or the Hôpital Point G (adults ≥ 18 years), which are the major pediatric and adult teaching hospitals for the University of Bamako.
Microscopy
Thick smears were stained with 3% Giemsa (Sigma, St. Louis, MO) in phosphate buffer (pH 7.0) and examined using oil immersion magnification (1,000×). Each slide was examined by two microscopists, who estimated the parasite density by counting the number of asexual P. falciparum parasites in fields containing 300 white blood cells and multiplying by 25 to estimate the number of P. falciparum parasites per microliter (based on an average white blood cell [WBC] count of 7,500/μL).8 Slides were considered positive or negative after two readers examined fields containing 300 WBCs using oil immersion magnification (1,000×) and agreed that they were negative or within 10% (for positive slides). Slides on which there was disagreement were examined by a third senior microscopist (A.D.).
The HRP2-based RDT examined (ParaSight F; Becton-Dickinson, Abidjan, Cote D'Ivoire) was performed according to the manufacturer's instructions using a test strip coated with an immobilized immunoglobulin G1 (IgG1) monoclonal antibody against the central, histidine-rich repeat region of HRP2 (AHH[AHHAAD]2)12. The 50-μL blood sample used for this testing was obtained by applying a capillary tube to the site of the finger stick. After the blood sample had been lysed, applied to the test strip, and developed using the reagents provided, the test strips were examined and interpreted by two independent observers within 2 hours.
PCR was used to amplify the histidine-rich repeat region of the hrp2 gene. Parasite DNA was extracted from filter paper blots with Chelex-100 as described previously.9 PCR amplification was performed using primers specific for the conserved 5′ and 3′ regions of the hrp2 gene that flank the central histidine-rich repeat region (Figure 1 and Table 1). Allotype-specific primers for the polymorphic block 2 region of merozoite surface protein 1 (MSP-1)10 and species-specific primers for ribosomal DNA11 were used as controls for smear-positive specimens, which yielded negative results with the HRP2-based RDT to determine whether P. falciparum DNA was present and thus, exclude nonspecific inhibition of the PCR.
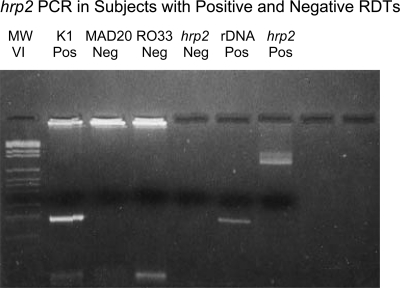
Figure 1.
hrp2 PCR in subjects with positive and negative HRP2 RDTs. Lane 1: DNA molecular weight markers VI (Roche, Indianapolis, IN). Lanes 2–6: results of PCR with DNA from a thick smear-positive subject with a negative HRP2 RDT using allotype-specific primers for the block 2 region of msp1 (lanes 2–4 show one K1 amplicon and no MAD20 or Ro33 amplicons), forward and reverse primers for hrp2 (lane 5 shows the absence of hrp2 amplicons), and species-specific primers for P. falciparum ribosomal DNA (lane 6 shows an amplicon of the expected size of 206 bp). In contrast, lane 7 provides a positive control for the hrp2 PCR based on DNA from a thick smear-positive subject with a positive HRP2 RDT (same forward and reverse primers as in lane 5 and Table 1).
Table 1.
Primers and conditions used to amplify the central repeat region of HRP2 and the polymorphic block 2 region of MSP1
| Primers | Primer sequences | Size (bp) | PCR conditions |
|---|---|---|---|
| HRP2 F | 5′-ATTCCGCATTTAATAATAACTTGTGTAGC-3′ | 74–968 | 95°C×2 minutes, 35 cycles of 94°C×30 seconds, 55°C×30 seconds, 72°C×60 seconds×35 cycles, and 72°C×10 minutes |
| HRP2 R | 5′-ATGGCGTAGGCAATGTGTGG-3′ | ||
| MSP1, conserved blocks 1 and 3 | |||
| MSP1 CONS F | 5′-ACTAGAAGCTTTAGAAGATGCAGTA-3′ | 522–639 | 95°C×2 minutes, 35 cycles of 94°C×30 seconds, 55°C×30 seconds, 72°C×60 seconds×35 cycles, and 72°C×10 minutes |
| MSP1 CONS R | 5′-AGTACGTCTAATTCATTTGCACGAA-3′ | ||
| MSP1, block 2 of K1 allotype parasites | |||
| MSP1K1 F | 5′-CTTAAATGAAGAAGAAATTACAAAAGGTGC-3′ | 140–266 | 95°C×2 minutes, 35 cycles of 94°C×30 seconds, 57°C×30 seconds, and 72°C×60 seconds |
| MSP1K1 R | 5′-GAGGGCTTGCACCAGATGAAGT-3′ | ||
| MSP1, block 2 of MAD20 allotype parasites | |||
| MSP1MAD F | 5′-GTATTAAATGAAGGAACAAGTGGAACAG-3′ | 157–193 | 95°C×2 minutes, 35 cycles of 94°C×30 seconds, 57°C×30 seconds, and 72°C×60 seconds |
| MSP1MAD R | 5′-TATCTGAAGGATTTGTACGTCTTGAATT-3′ | ||
| MSP1, block 2 of Ro33 allotype parasites | |||
| MSP1Ro33 F | 5′-AATAAAGGATGGAGCAAATACTCAAGTTGT-3′ | 153 | 95°C×2 minutes, 35 cycles of 94°C×30 seconds, 57°C×30 seconds, and 72°C×60 seconds |
| MSP1Ro33 R | 5′-TCTGAAGGATTTGCAGCACCTGGAGA-3′ | ||
Data analysis
The sensitivity and specificity of HRP2-based testing were estimated based on the thick smear. McNemar's test and the Fisher's exact test were used to test for significant differences (P < 0.05).
Results
Microscopy
Microscopy was performed with 723 specimens, of which 480 (66%) were positive by thick smear. More than 80% of blood smears from asymptomatic children < 10 years of age in suburban and rural areas of Mali between July and November of 1996 were positive (477/584 = 82% and 58/68 = 85%, respectively) as were 53% (231/433) of smears from persons with fever and a presumptive diagnosis of malaria at both teaching hospitals (Tables 2 and 3). The lowest frequency of infection (21/382 = 5.5%) was among asymptomatic adult blood donors (persons ≥ 18 years of age).
HRP2-based RDTs were performed on the same 723 specimens, of which 506 (70%) were positive. As with microscopy, more than 80% of HRP2-based RDTs were positive with specimens from asymptomatic children in suburban and rural villages (317/375 = 85% and 70/73 = 96%, respectively) and > 50% of specimens from persons who presented to hospitals in Bamako with fever and a clinical syndrome consistent with malaria (e.g., headache, myalgias, or arthralgias; 264/465 = 57%). The lowest frequency of positive HRP2-based RDTs was in asymptomatic adults who were potential blood donors (39/267 = 15%).
Sensitivity, specificity, and positive and negative predictive values for the HRP2-based RDT
Based on the thick smear, the sensitivity and specificity of the HRP2-based RDT were 95% and 79%, respectively, with positive and negative predictive values of 90% and 88% (Table 2). Similar estimates were obtained in relation to the thin smear: sensitivity and specificity of 95% and 70%, respectively, and positive and negative predictive values of 81% and 91%, respectively (data not shown).
Table 2.
Thick smear and HRP2-based RDT results for 723 subjects and thin smear and HRP2-based RDT results for 641 subjects
| Positive | Negative | Totals | |
|---|---|---|---|
| Thick smear for asexual P. falciparum parasites | |||
| Positive HRP2-based RDT | 454 | 52 | 506 |
| Negative HRP2-based RDT | 26 | 191 | 217 |
| Totals | 480 | 243 | 723 |
| Thin smear for asexual P. falciparum parasites | |||
| Positive HRP2-based RDT | 355 | 81 | 436 |
| Negative HRP2-based RDT | 18 | 187 | 205 |
| Totals | 373 | 268 | 641 |
Comparison of thick smear and HRP2-based RDT results. The results obtained using McNemar's test for paired samples indicate that there were significant differences in the frequencies of positive and negative test results for the thick smear and the HRP2-based RDT [χ2 = 8.013, P = 0.005, odds ratio = 2.000 (95% confidence interval [CI] = 1.226–3.337)]. Comparison of thin smear and HRP2-based RDT results. The results obtained using McNemar's test for paired samples indicate that there were significant differences in the frequencies of positive and negative test results for the thick smear and the HRP2-based RDT (χ2 = 38.828, P < 0.001, odds ratio = 4.500 [95% CI = 2.676–7.971]).
Smear-positive specimens negative with the HRP2-based RDT
Of the 480 specimens with positive thick smears for P. falciparum, 26 (5%) had negative HRP2-based RDTs (Table 2). Review of those 26 smears revealed that all were positive for asexual P. falciparum parasites by microscopy, which eliminated false-positive smears as an explanation. To test for the deletion of hrp2, we used PCR to amplify the histidine-rich repeat region of hrp2 (Figure 1 and Table 1). Negative results were obtained with the hrp2 PCR (consistent with hrp2 deletion) for 10 of 22 RDT-negative specimens available. In contrast, positive results were obtained with the hrp2 PCR for the remaining 12 smear-positive and RDT-negative specimens and 15 of 15 smear-positive and RDT-positive controls (Table 4) (P < 0.01). To exclude nonspecific inhibition of the PCR, we used allotype-specific primers for the block 2 region of msp1 and species-specific primers for parasite ribosomal DNA.10,11 Those results were positive for each of the 10 smear-positive specimens that were negative in the hpr2 PCR.
Table 4.
hrp2 PCR results for thick smear-positive specimens with false-negative and true-positive HRP2-based RDTs
| Positive thick smear for asexual P. falciparum | |||
|---|---|---|---|
| Negative HRP2-based RDT | Positive HRP2-based RDT | Totals | |
| Positive hrp2 PCR | 12 | 15 | 27 |
| Negative hpr2 PCR | 10 | 0 | 10 |
| Totals | 22 | 15 | 37 |
hrp2 PCR results for smear-positive specimens with negative vs. positive HRP2-based RDT results. Among subjects with positive thick smears, positive hrp2 PCR results were found in all those subjects (15/15) with positive HRP2 RDTs but only one-half (12/22) of those subjects with negative HRP2 RDTs (McNemar's test, P = 0.002, χ2 = 10.083, degrees of freedom = 1).
Parasite densities (asexual parasite counts) and HRP2-based RDT results
There were no significant differences in asexual parasite counts between smear-positive persons with false-negative RDTs (8,459 ± 15,369 [N = 22]) and smear-positive persons with true-positive RDTs (8,048 ± 9,535 [N = 24]; P = 0.913).
Parasite density and multiplicity of infection
Based on the 31 smear-positive samples for which the multiplicity of infection (MOI) was available, there was no relationship between the MOI and parasite density (P > 0.40 based on analysis of variance).
Clinical illness and false-negative HRP2-based RDT results
None of the asymptomatic smear-positive children with a false-negative HRP2-based RDT were ill (symptomatic). In contrast, none of the smear-positive symptomatic subjects studied at the Hôpital Gabriel Touré or the Hôpital Point G had a false-negative RDT (0/201 versus 26/279). These results indicate that false-negative HRP2-based RDTs are more common in persons with asymptomatic P. falciparum infection (P < 0.001).
False-negative RDT results and MOI
Three sets of allotype-specific primers for the variable block 2 region of MSP-1 were used to estimate the MOI and minimal number of parasite genotypes in a single specimen based on the number of amplicons obtained from samples available for testing. Each of the smear-positive specimens with a negative HRP2-based RDT and negative hrp2 PCR consistent with gene deletion was from an infection with a single parasite genotype (i.e., only one amplicon was obtained using PCR with three sets of matched primers for K1, MAD20, and Ro33 allotype parasites; mean MOI = 1.00 ± 0.00 [N = 12]). The K1 and RO33 block 2 allotypes of msp1 (although not MAD20) were both identified in these specimens (RO33 = 7, K1 = 5). In contrast, smear-positive specimens that were also RDT-positive had a higher mean MOI (2.42 ± 0.84 [N = 19], P < 0.001), and MAD20 allotype parasites were present (RO33 = 10, K1 = 20, MAD20 = 13).
Discussion
Microscopy and RDT results
The high frequency of positive smears in these studies is consistent with previous studies in Mali and other regions of West Africa during the transmission season.12 Likewise, the sensitivity and specificity estimates for the HRP2-based RDT (using thick or thin smears) are consistent with previous reports.4–7 As noted in those reports, the sensitivity of the HRP2-based RDT decreases with asexual parasite densities less than 100/μL.
Deletion of the hrp2 histidine-rich repeat region as the basis of false-negative HRP2-based RDTs
Previous reports have examined potential causes of false-positive HRP2-based RDTs (e.g., rheumatoid factor).13 In contrast, this report examines potential causes of false-negative HRP2-based RDTs. Re-examination of the blood smears for the 26 smear-positive, RDT-negative specimens excluded false-positive readings by microscopy. Likewise, PCR amplification with primers for the polymorphic block 2 region of msp1 and species-specific primers for P. falciparum ribosomal DNA showed that P. falciparum DNA was present (consistent with true-positive blood smears) and excluded PCR inhibitors10,11 as an explanation for the negative hrp2 PCR results (for the 10 smear-positive specimens with false-negative RDTs).
Previous evidence for the deletion of hrp2
Deletion of hrp2 is known to occur, especially during prolonged in vitro culture of asexual P. falciparum parasites, and it is likely related to the subtelomeric location of the hrp2 gene.14,15 However, before these studies were performed, we did not know whether parasites with hrp2 deletions circulated in the blood of infected humans in sub-Saharan Africa. The results presented here suggest that spontaneous hrp2 deletions occur under field conditions and that P. falciparum parasites without the histidine-rich repeat region of hrp2 or its gene product produce bloodstream infections among human subjects in sub-Saharan Africa. Although the negative PCR results could have resulted from sequence variability in the upstream and downstream regions used for the primers, that finding alone would not produce a negative RDT for HRP2. For that reason, because we are not aware of sequence variation in those regions that interfere with this PCR and because previous reports detail hrp2 deletions from Papua New Guinea16 and Peru,17 these results suggest that spontaneous deletions of hrp2 occur in many (perhaps most) areas with P. falciparum transmission.
Frequency of hrp2-negative P. falciparum parasites
Simultaneous infection with more than one P. falciparum parasite (genotype) is common in Mali. At the peak of the transmission season, asymptomatic children may have six to eight different parasites in their blood at the same time. Thus, the 10 smear-positive, RDT-negative, and hrp2 PCR-negative specimens provide a minimal estimate of the frequency of hrp2-negative P. falciparum parasites (10/480 = 2%). This finding is because one hrp2-positive parasite in a specimen with numerous hrp2-negative parasites can produce a positive HRP2 test result. Thus, the actual frequency of HRP2-negative parasites in Mali at the time of this study may have been as high as 10–15%. In contrast, the remaining 12 specimens positive by thick smear and negative with the HRP2-based RDT were positive when tested with the hrp2 PCR. In those specimens, sequence variability may account for the negative HRP2-based RDT, with positive results on the hrp2 PCR.18 Alternatively, HRP2 may have been internalized into the digestive vacuole in some strains rather than released into the bloodstream.19
Conclusions and recommendations
These results confirm that hrp2 deletions occur under field conditions as well as in the laboratory14–16 and show that P. falciparum parasites without the histidine-rich repeat region of the hrp2 gene or its HRP2 gene product produce bloodstream infection in sub-Saharan Africa. In addition, recent reports indicate that hrp2 deletions are present in both Papua New Guinea and the Amazon region of Peru16,17 and thus, suggest that false-negative results for RDTs based on HRP2 may also occur in those regions. However, it is not clear whether parasites without hrp2 are at a selective disadvantage or whether they are more frequent at times of intense transmission, such as the rainy season, when these studies were performed in Mali. Finally, there is the question of how this information should affect the use of RDTs based on detection of the HRP2 gene product. These results suggest that the majority of persons with P. falciparum infection are positive using HRP2-based RDTs (454/480 = 95%) and thus, that HRP2-based RDTs are likely to be valuable, especially in regions where skilled microscopists are rare. However, the false-negative HRP2-based RDTs obtained for a minority (5%) of smear-positive specimens indicate that a negative test result with an RDT based on HRP2 (e.g., ParaSight F [Beckton-Dickinson, Franklin Lakes, NJ], Binax Now [Inverness Medical Innovations, Alere, Inc., Waltham, MA], CareStart [DiaSYS, Berkshire, England], ParaCheck Pf [Orchid Biomedical Systems, Goa, India], ParaHIT f [Span Diagnostic, Ltd., Udhna Surat, India], ICT-Pf/Pv [Binax, Inc., Portland, ME], Malaria Pf Rapid Test [Aluxbio, Ltd., Shanghai, China], or AZOG Malaria PF [AZOG Laboratories, Phillipsburg, NJ])3,20–26 does not exclude active bloodstream infection with asexual P. falciparum parasites. In addition, the association between false-negative RDTs and a low MOI suggests that this discrepancy may become increasingly important as malaria control becomes more effective. Based on these results, we suggest that the RDTs used to diagnose P. falciparum infection in humans should target parasite proteins thought to be essential (e.g., parasite lactic dehydrogenase)1,27–29 and should also consider proteins with identifiable (distinguishable) variants in other plasmodial species.
Table 3.
Sources, ages, and results of thick smears for asexual P. falciparum parasites
| Sources | Asymptomatic children and adults | ||
|---|---|---|---|
| Ages | Results | Percent | |
| Bancoumana | 6 months to 9 years | 124 of 145 | 86 |
| Donéguébougou | 6 months to 9 years | 60 of 76 | 79 |
| Sirakoro | 5–16 years | 49 of 60 | 82 |
| Adult blood donors (Bamako) | ≥ 18 years | 22 of 382 | 6 |
| Asymptomatic children and adults | 255 of 663 | 38 | |
| Hôpital Gabriel Touré (Bamako) | 6 months to 9 years | 115 of 255 | 45 |
| Hôpital Point G (Bamako) | ≥ 18 years | 110 of 174 | 63 |
| Symptomatic children and adults | 225 of 429 | 52 | |
ACKNOWLEDGMENTS
These studies were supported by a grant from Becton-Dickinson, Abidjan, Cote D'Ivoire; Grants TDR 920571 and TDR 920644 from the United Nations Development Program/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases; and a Cooperative Agreement with the National Institutes of Health (National Institute of Allergy and Infectious Disease P50 AI 39469) for the Mali–Tulane Tropical Medicine Research Center. The authors thank Joseph Perrone for his personal interest and support during the performance and interpretation of these studies. None of the authors have financial interests in any of the companies that produce these or other rapid tests for the diagnosis of malaria.
Footnotes
Authors' addresses: Ousmane A. Koita, Laboratory of Applied Molecular Biology, Faculty of Science and Techniques, University of Bamako, Bamako, Mali, E-mail: okoita@icermali.org. Ogobara K. Doumbo, Amed Ouattara, Lalla K. Tall, Aoua Konaré, Mahamadou Diakité, Mouctar Diallo, Issaka Sagara, Godfred L. Masinde, Safiatou N. Doumbo, Amagana Dolo, Anatole Tounkara, and Issa Traoré, Mali–Tulane Tropical Medicine Research Center, Faculty of Medicine, Pharmacy, and Dentistry, University of Bamako and the National Transfusion Centre, Bamako, Mali, E-mails: okd@icermali.org, aouattara@medicine.umaryland.edu, lallakass@yahoo.com, gafouk@yahoo.fr, mdiakite@icermali.org, mouctard@icermali.org, isagara@icermali.org, sdoumbo@icermali.org, adolo@icermali.org, anatol@icermali.org, and issacely@yahoo.fr. Godfred L. Masinde and Donald J. Krogstad, Department of Tropical Medicine and the Center for Infectious Diseases, Tulane University, New Orleans, LA, E-mail: gmasinde@hotmail.com and krogstad@tulane.edu.
References
- 1.Cooke AH, Chiodini PL, Doherty T, Moody AH, Ries J, Pinder M. Comparison of a parasite lactate dehydrogenase-based immunochromatographic antigen detection assay (OptiMAL) with microscopy for the detection of malaria parasites in human blood samples. Am J Trop Med Hyg. 1999;60:173–176. doi: 10.4269/ajtmh.1999.60.173. [DOI] [PubMed] [Google Scholar]
- 2.Beadle C, Long GW, Weiss WR, McElroy PD, Maret SM, Oloo AJ, Hoffman SL. Diagnosis of malaria by detection of P. falciparum HRP2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 3.Bechem NN, Leke RFG, Tietche F, Taylor DWT. Evaluation of a rapid test for histidine rich protein 2 for diagnosis of Plasmodium falciparum infection in Cameroonian children. Trans R Soc Trop Med Hyg. 1999;93:46. doi: 10.1016/s0035-9203(99)90175-x. [DOI] [PubMed] [Google Scholar]
- 4.Dietze R, Perkins M, Boulos M, Luz F, Reller B, Corey GR. The diagnosis of Plasmodium falciparum infection using a new antigen detection system. Am J Trop Med Hyg. 1995;52:45–49. doi: 10.4269/ajtmh.1995.52.45. [DOI] [PubMed] [Google Scholar]
- 5.Premji Z, Minjas JN, Shiff CJ. Laboratory diagnosis of malaria by village health workers using the rapid manual ParaSight™F test. Trans R Soc Trop Med Hyg. 1994;88:418. doi: 10.1016/0035-9203(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 6.Shiff CJ, Premji Z, Minjas JN. A new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1993;87:646–648. doi: 10.1016/0035-9203(93)90273-s. [DOI] [PubMed] [Google Scholar]
- 7.Watson PA, Laidoueu AB, Kacou G, Traoré M. Comparison of a rapid dipstick test and thick blood films for detecting parasites of Plasmodium falciparum used under typical conditions at a semi-rural hospital in Cote d'Ivoire. Trop Doct. 1998;28:85–88. doi: 10.1177/004947559802800210. [DOI] [PubMed] [Google Scholar]
- 8.Payne D. Use and limitations of light microscopy for diagnosis of malaria at the primary health care level. Bull World Health Organ. 1988;66:621–626. [PMC free article] [PubMed] [Google Scholar]
- 9.Wooden J, Gould EE, Pauli AT, Sibley CH. Plasmodium falciparum: a simple polymerase chain reaction method for differentiating strains. Exp Parasitol. 1992;72:207–212. doi: 10.1016/0014-4894(92)90180-i. [DOI] [PubMed] [Google Scholar]
- 10.Morata P, Queipo-Ortuno MI, de Dios Colmenero J. Strategy for optimizing DNA amplification in a peripheral blood assay used for diagnosis of human brucellosis. J Clin Microbiol. 1998;36:2443–2446. doi: 10.1128/jcm.36.9.2443-2446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snounou G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol. 1996;50:263–291. doi: 10.1385/0-89603-323-6:263. [DOI] [PubMed] [Google Scholar]
- 12.Bouvier P, Rougemont A, Breslow N, Doumbo O, Delley V, Dicko A, Diakite M, Mauris A, Robert CF. Seasonality and malaria in a West African village: does high parasite density predict fever incidence? Am J Epidemiol. 1997;145:850–857. doi: 10.1093/oxfordjournals.aje.a009179. [DOI] [PubMed] [Google Scholar]
- 13.Bartoloni A, Strohmeyer M, Sabatinelli G, Benucci M, Serni U, Parasidi F. False positive ParaSight™F test for malaria patients with rheumatoid factor. Trans R Soc Trop Med Hyg. 1998;92:33–34. doi: 10.1016/s0035-9203(98)90945-2. [DOI] [PubMed] [Google Scholar]
- 14.Pologe LG, Ravetch JV. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature. 1986;322:473–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- 15.Kemp DJ, Thompson JK, Walliker D, Corcoran LM. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc Natl Acad Sci USA. 1987;84:7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biggs BA, Kemp DJ, Brown GV. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci USA. 1989;86:2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamboa D, Ho M-F, Bendezu J, Torres K, Chiodini PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J, Cheng Q. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, Bell D, Cheng Q. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 19.Howard RJ, Uni S, Aikawa M, Aley SB, Leech JH, Lew AM, Wellems TE, Rener J, Taylor DW. Secretion of a malarial Histidine-rich Protein (PfHRPII) from Plasmodium falciparum infected erythrocytes. J Cell Biol. 1986;103:1269–1277. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins H, Kambale W, Kamya MR, Staedke SG, Dorsey G, Rosenthal PJ. Comparison of HRP2 and pLDH-based rapid diagnostic tests for malaria with longitudinal follow-up in Kampala, Uganda. Am J Trop Med Hyg. 2007;76:1092–1097. [PubMed] [Google Scholar]
- 21.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–518. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 22.Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008;21:97–110. doi: 10.1128/CMR.00035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayang C, Soula G, Tahar R, Basco LK, Gazin P, Moyou-Somo R, Delmont J. Use of a histidine-rich protein 2-based rapid diagnostic test for malaria by health personnel during routine consultation of febrile outpatients in a peripheral health facility in Yaoundé, Cameroon. Am J Trop Med Hyg. 2009;81:343–347. [PubMed] [Google Scholar]
- 24.Swarthout TD, Counihan H, Senga RKK, van den Broek I. Paracheck-Pf® accuracy and recently treated Plasmodium falciparum infections: is there a risk of over-diagnosis? Malar J. 2007;6:58. doi: 10.1186/1475-2875-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Malaria Rapid Diagnostic Test Performance: Results of WHO Product Performance Testing: Round 1, 2008. 2008. http://www.finddiagnostics.orgexport/sites/default/media/press/pdf/Full-report-malaria-RDTs.pdf Available at. Accessed August 8, 2010.
- 26.World Health Organization List of Products Submitted to the WHO Malaria RDT Testing Programme—Round 1. 2009. www.wpro.who.int/sites/rdt/documents Available at. Accessed August 8, 2010.
- 27.Makler MT, Hinrichs DH. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 28.Makler MT, Piper RC, Milhous WK. Lactate dehydrogenase and the diagnosis of malaria. Parasitol Today. 1998;14:376–377. doi: 10.1016/s0169-4758(98)01284-8. [DOI] [PubMed] [Google Scholar]
- 29.Maltha J, Gillet P, Bottieau E, Cnops L, van Esbroeck L, Jacobs J. Evaluation of a rapid diagnostic test (CareStart™ Malaria HRP-2/pLDH (Pf Pan) Combo Test for the diagnosis of malaria in a reference setting. Malar J. 2010;9:171. doi: 10.1186/1475-2875-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]