Abstract
Home management of malaria is recommended for prompt, effective antimalarial treatment in children less than five years of age. Compliance, safety, and effectiveness of the new fixed-dose artesunate-amodiaquine regimen used to treat suspected malaria were assessed in febrile children enrolled in a 24-month cohort study in two settings in Madagascar. Children with fever were asked to visit community health workers. Presumptive antimalarial treatment was given and further visits were scheduled for follow-up. The primary endpoint was the risk of clinical/parasitologic treatment failure. Secondary outcomes included fever/parasite clearance, change in hemoglobin levels, and frequency of adverse events. The global clinical cure rate was 98.4% by day 28 and 97.9% by day 42. Reported compliance was 83.4%. No severe adverse effects were observed. This study provides comprehensive data concerning the clinical cure rate obtained with artesunate-amodiaquine and evidence supporting the scaling up of home management of malaria.
Introduction
Despite the large increase in the resources assigned to malaria by the national and international community (from US $6 billion in 2000 to US $16 billion in 2006), and the possibility of cure by the prompt administration of effective antimalarial treatment, malaria remains a major public health problem.1 Globally, this situation results mostly from an inability of many countries to provide reliable health services. There are several reasons for this, including a lack of access to health facilities, inadequacy of the health-care infrastructure and the poor performance of health staff.2–5 This problem is particularly marked in remote, rural areas of sub-Saharan Africa, such as Madagascar.
For several years, the World Health Organization (WHO) has advocated a strategy based on the home management of fever (HMM) to improve the situation.6 This strategy, based on the assumption that most cases of fever are caused by malaria in areas in which the disease is endemic, promotes the administration of appropriate treatment (after parasitologic confirmation if available) at home by care providers as soon as possible after the onset of symptoms.7,8 However, there is only limited evidence to demonstrate the effectiveness of this strategy, and policy decisions to introduce artemisinin combination therapies (ACTs) in community settings have been based on several untested assumptions.9 Only a few studies have assessed the impact of this strategy on health outcomes in Madagascar, and the only treatment administered in these studies was chloroquine, which has since become obsolete because of drug resistance.10
Madagascar has implemented a policy of HMM as part of its National Malaria Control Program since 1998. This strategy has involved the distribution of chloroquine within the community by health providers and community schools. This strategy successfully contained the deadly epidemic of malaria that spread through the central highlands of Madagascar during the 1980s.11 In 2004, prepackaged chloroquine was introduced and distributed through social marketing channels or health facilities.10 However, since 2005, the emergence and spread of chloroquine-resistant parasites12 has led to a change in the national policy, which now advocates the replacement of chloroquine with artesunate-amodiaquine (ASAQ) as the first-line treatment for uncomplicated malaria. However, the feasibility and impact of HMM with ACT in terms of compliance with treatment and the safety and effectiveness of the HMM strategy must be confirmed, in areas with different epidemiological characteristics.13
In this context and during a 24-month cohort study conducted in children less than five years of age in two settings (high and low levels of transmission), we have assessed the impact of the new fixed-dose ASAQ regimen on clinical outcomes to obtain evidence supporting (or ruling out) the use of ACT in the HMM strategy in Madagascar.
Methods
Sites and participants
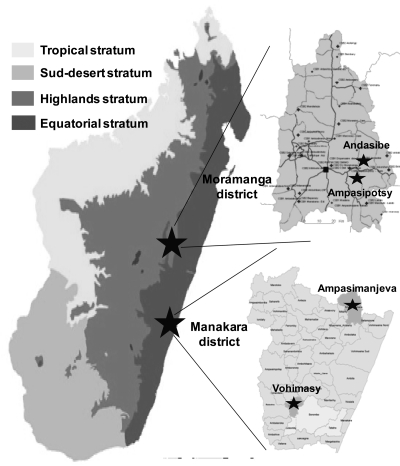
The study was conducted in two settings in Madagascar: two rural communities (Ampasimpotsy and Andasibe) in Moramanga District and two rural communities (Ampasimanjeva and Vohimasy) in Manakara District (Figure 1).
Figure 1.
Map of Madagascar showing malarious epidemiologic strata and four selected study sites, 2008–2009.
Moramanga District is located in the eastern foothills of the central highlands of Madagascar, 110 km east of Antananarivo (8°56'S, 48°12'E, elevation = 900 meters). At this site, malaria transmission levels are low and unstable. Moramanga District is characterized by an austral climate (mean temperature = 19.4°C, annual rainfall = 1,500 mm) and rainfall patterns extracted from data obtained over the past 30 years show that there is a five-month rainy season, extending from November through March. The annual entomologic inoculation rate in this area has been estimated to be 2–3 infectious bites per person per year, mostly from Anophelus funestus. The Bezanozano and Merina are the main ethnic groups in this area.
Manakara District is located on the southeastern coast of Madagascar (22°09S, 48°E). Malaria transmission rates in this area are stable and high. Transmission occurs throughout the year and the entomologic inoculation rate has been estimated to be 35–50 infectious bites per person per year, mostly from Anophelus gambiae. This area has an equatorial climate (mean temperature = 26.8°C, annual rainfall = 2,000 mm and 175 days of rain per year). The population is composed of the Antaisaka, Antaifasy, and Zafisoro ethnic groups.14
We first carried out a census of children 2–59 months of age at the selected sites. Standard questionnaires reporting information about household socioeconomic and demographic features, malaria-related knowledge, attitudes and practices and the use of preventive measures, such as bed nets, were recorded. Each child included in the cohort was assigned a unique identification code once his or her parents or guardians had completed and signed an informed consent form.
The parents and guardians of the participants were asked to take their children to the community health worker (CHW) if the children had a fever. All CHWs (two at each site) involved in the study received training concerning the signs and symptoms of malaria, the information to be given to parents and guardians if no regression of clinical signs was observed or if adverse events occurred during treatment. They were also trained to record the temperature of the child, weigh the child, manage ACT treatment, complete the forms used to compile information and data, prepare and store blood films and blood spots collected on filter paper correctly, and use a hemoglobinometer to determine hemoglobin concentration.
Both medical officers (MOs; one per site) were trained to follow-up enrolled children during home visits on days 3, 28, and 42, complete information and data forms, record the child's temperature, weigh the child, ask about compliance, prepare and store blood films and blood spots collected on filter paper, and report adverse events.
Study design
Children enrolled in the cohort who had a febrile episode were included in the study if they satisfied the following criteria: age ≥ 2 months, axillary temperature ≥ 37.5°C or had a fever within the past 24 hours, and capable of receiving oral treatment. Children with any clinical sign of severe malaria or serious concomitant disease were excluded from the study and immediately referred to the nearest health center. At each visit, CHWs prepared blood slides from the child for microscopy and collected blood on filter paper for polymerase chain reaction (PCR) analysis for further laboratory analysis. Parasitologic diagnosis was not used as a decision to treat, according to the Malagasy National Malaria Control Program guidelines. The first dose of the treatment (fixed dose of ASAQ) was administered to the child in the presence of the CHW. Subsequent doses were administered unsupervised at home, according to the instructions given by the CHW to parents and guardians. All parents and guardians were instructed to report to the CHW or the nearest health facility if the child had not improved after 48 hours or if the child show deterioration of his or her condition at any time. The CHWs were asked to complete forms regarding clinical (axillary temperature, weight) and biological (blood spot, slides, and hemoglobin concentration) information on day 0.
Children were followed-up by an MO through scheduled home visits on days 3, 28, and 42. The MO completed forms on days 3, 28, and 42 by recording clinical (axillary temperature, weight) and biological (blood spot, slides and hemoglobin concentration) data and information about compliance with treatment and adverse effects.
Treatment
According to the national malaria control policies in Madagascar, a fixed dose of ASAQ (Coarsucam™; Sanofi-Aventis, Paris, France) was used. The recommended dose for children 2–11 months of age was one tablet containing 25 mg of artesunate and 67.5 mg of amodiaquine per day for three days. The recommended dose for children 1–5 years of age was one tablet containing 50 mg of artesunate and 135 of mg amodiaquine per day for three days. Tablets were administered orally by the CHW on day 0 with drinking water. Patients and guardians were advised that the child should resume a normal diet as soon as possible. They were also issued the drug and were responsible for subsequent administration. All patients were monitored for 30 minutes after drug administration to ensure that the drug was not lost through vomiting. If the child vomited during this observation period, the same dose of the drug was administered again. If vomiting recurred, the subject was referred to the nearest health center.
Compliance with treatment
Consistent with previous studies15–17 based on home visits at day 3 and questionnaires addressed to the parents, compliance with treatment was classified into four categories: 1) certain compliance for patients for whom complete treatment was reported, with the treatment protocol followed exactly, supported by the evidence of an empty blister pack for the drug; 2) probable compliance for patients for whom complete treatment was reported with the treatment protocol followed exactly but without the supporting evidence of the empty blister pack; 3) probable non-compliance in cases in which all tablets were reported to have been taken, but not consistent with the prescribed time/dosage schedule; and 4) certain non-compliance for patients for whom tablets remained in the blister pack at the time of the home visit. Compliance was assessed by the MO on day 3.
Effectiveness and safety
A simplified WHO 2003 protocol,18 with scheduled home visits on days 3, 28, and 42 was used to assess the effectiveness and safety of the fixed dose of ASAQ. Treatment outcomes were assessed on days 28 and 42, as early treatment failure (ETF; danger signs, complicated malaria or inadequate response to treatment on days 0 to 3), late clinical failure (LCF; danger signs, complicated malaria or fever and parasitemia on days 4–28 or 4–42 in patients not previously meeting the criteria for ETF), late parasitologic failure (asymptomatic parasitemia on days 4–28 or 4–42 in patients not previously meeting the criteria for ETF or LCF), and adequate clinical and parasitologic response (absence of parasitemia on days 28 or 42 in patients not previously meeting the criteria for ETF, LCF, or late parasitologic failure).
Patients found to have had treatment failure were treated with quinine (10 mg/kg, three times a day for seven days) at the nearest health center. Their response to repeat therapy was not assessed. Patients were excluded after enrollment if any of the following occurred: 1) use of antimalarial drugs outside of the study protocol; 2) detection during follow-up of mixed malarial infections; 3) parasitemia in the presence of a concomitant febrile illness likely to interfere with the classification of treatment outcome; 4) withdrawal of consent; 5) loss to follow-up, 6) protocol violation, or 7) death caused by an illness other than malaria. Clinical safety was assessed by the MO through regular interviews with the patients and their families, at which the occurrence of adverse events since the previous visit was recorded.
Laboratory analyses
Finger prick blood samples were collected by CHWs and MOs at enrollment and at successive follow-up visits. Thick blood films and blood spots on Whatman (Maidstone, United Kingdom) filter paper were prepared for parasitologic examination.
Hemoglobin measurement
Hemoglobin was measured on day 0 by CHWs and on day 28 by MOs by using a HemoCue hemoglobinometer (HemoCue AB, Ängelholm, Sweden).
Microscopic examination
At the Malaria Research Unit of the Pasteur Institute of Madagascar, blood films were stained with 10% Giemsa for 10 minutes and screened by examination under a light microscope with a 100× oil immersion lens. Parasite densities were determined from thick blood smears by counting the number of asexual parasites per 200 leukocytes or per 500 leukocytes if there were less than 10 parasites/200 leukocytes), assuming a leukocyte count of 8,000 cells/μL. A blood smear was considered negative if no parasites were seen after the review of 100 fields. Thin blood smears were used to detect infections other than those with Plasmodium falciparum.19 All slides were read by two expert microscopists, and discrepancies were resolved by a third microscopist. For quality control purposes, a random sample of 10% of the slides analyzed at each site and of all slides classified as positive were re-evaluated blind to the original result and the source study center at the National Malaria Control Program Center.
DNA extraction and PCR analysis
Parasite DNA was extracted from blood spots collected on day 0 and on the day of recurrence by using the Instagene Matrix (Bio-Rad, Marnes la Coquette, France) according to the manufacturer's instructions. Plasmodium species were detected by real-time PCR20 with a RotorGene 3000 thermocycler (Corbett Life Science, Sydney, New South Wales, Australia), and technicians were blinded to the results of microscopy as described.21 Molecular genotyping techniques were used to distinguish recrudescence from new infection in all patients with treatment failure after day 7. Filter paper blood samples collected on the day of enrollment and on the day of treatment failure were analyzed for polymorphisms in the genes for merozoite surface protein-1 (msp-1) and msp-2 by nested PCR, as described.22 We first compared msp-2 genotyping patterns on the day of treatment failure with those at treatment initiation. If all of the msp-2 alleles present on the day of treatment failure were present at the time of treatment initiation, genotyping was repeated with msp-1. An outcome was defined as recrudescence if all msp-1 and msp-2 alleles present at the time of treatment failure were present at the time of treatment initiation and as a new infection if this was not the case.
Statistical analysis
Data were input and verified with EpiInfo 6.04 software (Centers for Disease Control and Prevention, Atlanta, GA), and analyzed with MedCalc software version 9.1.0.1 (MedCalc Software, Mariakerke, Belgium).
Effectiveness data were assessed with a per-protocol analysis that included all patients who completed the study. The study was designed such that the primary analysis was stratified by site, malaria status on the day of enrollment and compliance with treatment. Parasite densities were normalized by using logarithmic transformation. Categorical variables were compared by chi-square or Fisher's exact tests, and continuous variables were compared by using independent-sample t-tests.
The primary effectiveness outcomes were the likelihood of 28-day and 42-day clinical and parasitologic failure that was unadjusted and adjusted for genotype. Risks of clinical and parasitologic treatment failure after adjustment for genotype were estimated by Kaplan-Meier survival analysis, in accordance with the new WHO protocol.18 Data for new infections were censored in survival analysis. Secondary outcomes included fever clearance, parasite clearance, change in hemoglobin concentration between the measurements on days 0, 28 and 42, and the incidence of adverse events. Hypotheses were tested by calculating differences in risk, exact 95% confidence intervals, and P values. A P value (two-tailed) < 0.05 was considered statistically significant.
Ethical issues
This study was conducted in accordance with the Helsinki Declaration and the national laws and regulations in force. The protocol was submitted to and approved by the Malagasy National Ethics Committee (No. 153/SANPF/2007). Written informed consent was obtained from each parent or guardian of the participants. If a parent or guardian was unable to sign, a fingerprint was made on the form to indicate consent. If parents or guardians were unable to read, the information was read and explained in the appropriate language. In such cases, the presence of a witness was required, and this witness signed the consent form to confirm that the patient's representatives had freely given consent. This protocol was also registered with the www.clinicaltrials.gov open clinical trials registry under identifier NCT00612547.
Results
Population characteristics
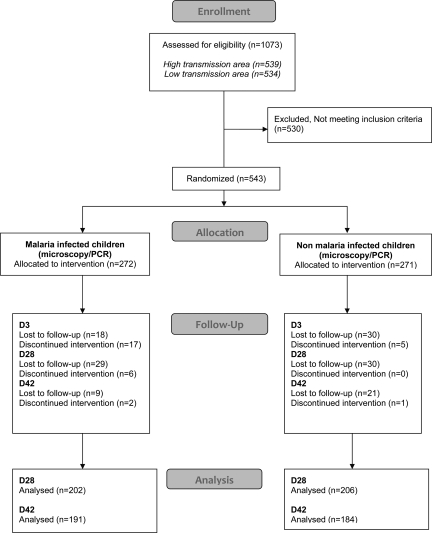
A total of 1,073 children enrolled in the cohort were followed-up for 24 months. During this period, 543 febrile children were seen at the various study sites and treated with fixed-dose ASAQ: 422 children had a one febrile episode, 99 had two febrile episodes, 19 had three febrile episodes, 2 had four febrile episodes, and 1 had five febrile episodes. No significant difference in the number of febrile episode was found between sites (P = 0.8).
The mean annual incidence of febrile episodes in children was estimated to be 0.55 for Ampasimanjeva (309 febrile episodes; 239 children with one febrile episode, 52 with two febrile episodes, 15 with three febrile episodes, 2 with four febrile episodes, and 1 with five febrile episodes), 0.23 for Vohimasy (118 febrile episodes; 92 children with one febrile episode, 24 with two febrile episodes, 2 with three febrile episodes), 0.11 for Ampasimpotsy (63 febrile episodes; 48 children with one febrile episode, 13 with two febrile episodes, 2 with three febrile episodes), and 0.11 for Andasibe (53 febrile episodes; 43 children with one febrile episode, 10 with two febrile episodes).
The characteristics of the cohort population are summarized in Table 1. Significant differences were found between sites. First, the mean age of the children was higher in areas of low-level malaria transmission. Second, the use of bed nets (including insecticide-treated bed nets) was much more frequent in areas with high levels of malaria transmission than in those with low levels of transmission. Third, the mean temperature of the child on day 0 was higher in areas of high-level malaria transmission than in areas with low levels of transmission. Fourth, the frequency of self-medication for malaria before consultation of the CHW ranged from 0% to 7.9%: chloroquine was more frequently used in areas of low-level malaria transmission, and cotrimoxazole was the first-line treatment used in areas with high levels of transmission.
Table 1.
Baseline characteristics of children involved in a 24-month cohort study of malaria, Madagascar, 2008–2009*
| Characteristic | Study sites | P | |||
|---|---|---|---|---|---|
| High transmission | Low transmission | ||||
| Ampasimanjeva | Vohimasy | Ampasimpotsy | Andasibe | ||
| Cohort population | 281 | 258 | 299 | 235 | |
| Mean age (months) | 28.6 | 26.5 | 30.1 | 31.5 | 0.009 |
| Sex ration (M:F) | 1 | 0.87 | 0.86 | 0.94 | NS |
| Bed nets availability (%) | 81.1 | 99.2 | 0 | 7.7 | < 0.0001 |
| Insecticide-treated bed net use (%) | 70.5 | 63.2 | 0 | 7.2 | < 0.0001 |
| No. febrile episode | 309 | 118 | 63 | 53 | |
| Mean temperature at day 0, °C (SD) | 38.4 (0.87) | 38.4 (0.86) | 38.3 (0.83) | 38.1 (0.57) | 0.02 |
| Previous antimalarial drug used (%) | 4.5 | 5.1 | 7.9 | 0 | < 0.0001 |
| CQ (%) | 47 | 43 | 71 | 33 | |
| CTX (%) | 53 | 57 | 29 | 67 | |
| Mean delay between fever and treatment in day (SD) | 1.3 (1.0) | 1.2 (0.9) | 1.5 (1.3) | 1.1 (1.1) | NS |
| % Positive slides for malaria | 36.9 | 43.2 | 31.7 | 24.5 | NS |
| Trophozoite density, mean (range), parasite/μL | 2,805 (48–76,760) | 2,835 (64–82,000) | 1,540 (64–37,250) | 2,190 (880–12,750) | NS |
| Hemoglobin, median (IQR), g/dL | 9.9 | 9.8 | 10.2 | 10.0 | NS |
| Anemia† at enrollment (%) | 51.1 | 50.9 | 44.6 | 53.8 | NS |
NS = not significant; CQ = chloroquine; CTX = cotrimoxazole; IQR = interquartile range.
Anemia was defined as a hemoglobin level < 10 g/dL.
Enrollment
For the 543 children treated with ASAQ on day 0, retrospective microscopy examination and parasite detection by PCR showed positive results in 198 children (M+/PCR+), 74 children were negative by microscopy and positive by PCR (M–/PCR+), and 271 were negative by both techniques (M–/PCR–).
A total of 375 (69.0%) of the 543 children completed treatment and follow-up to day 42. We identified 168 patients who were either lost to follow-up (n = 137; 48 on day 3, 59 on day 28, and 30 on day 42) or excluded/withdrawn from the trial (n = 31; 22 on day 3, 6 on day 28, and 3 on day 42). The flow of patients through the trial is shown in Figure 2.
Figure 2.
Flow of patients in the study in Madagascar, 2008–2009.
Compliance
Compliance with the correct treatment protocol by parents and guardians was assessed for the 543 children at the four study sites. Overall, 90.0% (95% confidence interval [CI] = 87.2–92.5%) of the children were treated correctly in terms of drug dose and treatment duration (certain and probable compliance groups). The levels of compliance did not differ significantly between sites (P = 0.5) or diagnosis groups (P = 0.8). We identified 46 cases of non-compliance: 42 children who had not taken the correct dose (some tablets remained in the blister), and four who had taken the correct amount of the drug but not according to the prescribed time schedule.
Primary outcome: treatment effectiveness
Treatment effectiveness results are shown by day of follow-up and study site in Table 2. Among children with malarial infections, the overall treatment failure rate was 10 of 183 (5.5%; 95% CI = 2.6–10.1%) on day 28 and 20 of 172 (11.6%; 95% CI = 7.1–17.9%) on day 42. After PCR analysis, only 3 of 183 (1.6%; 95% CI = 0.3–4.8%) children on day 28 and 3 of 143 (2.1%; 95% CI = 0.4–6.1%) children on day 42 were classified as displaying recrudescence. Treatment outcomes (on days 28 and 42, adjusted or not adjusted for PCR results) did not differ significantly between sites (P = 0.6).
Table 2.
Malaria treatment outcomes and day of follow-up (day 28 and day 42, unadjusted and adjusted by genotyping) according to the epidemiologic settings, Madagascar, 2008–2009*
| Characteristic | Outcome | High transmission | Low transmission | ||
|---|---|---|---|---|---|
| Ampasimanjeva | Vohimasy | Ampasimpotsy | Andasibe | ||
| Day 3 positive slide (%) | 1.0 | 2.3 | 0 | 0 | |
| Outcome measure by day | |||||
| Day 28 unadjusted by PCR | n = 113 | n = 50 | n = 12 | n = 8 | |
| Clinical failure | ETF | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF | 4 (3.5) | 0 (0) | 0 (0) | 0 (0) | |
| Parasitologic failure | LPF | 4 (3.5) | 2 (4.0) | 0 (0) | 0 (0) |
| Overall treatment failure | 8 (7.0) | 2 (4.0) | 0 (0) | 0 (0) | |
| Day 28 adjusted by PCR | n = 113 | n = 50 | n = 12 | n = 8 | |
| Clinical failure | ETF | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | |
| Parasitologic failure | LPF | 1 (0.9) | 1 (2.0) | 0 (0) | 0 (0) |
| Overall treatment failure | 2 (1.8) | 1 (2.0) | 0 (0) | 0 (0) | |
| Day 42 unadjusted by PCR | n = 104 | n = 48 | n = 12 | n = 8 | |
| Clinical failure | ETF | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF | 7 (6.7) | 0 (0) | 0 (0) | 0 (0) | |
| Parasitologic failure | LPF | 7 (6.7) | 4 (8.3) | 0 (0) | 0 (0) |
| Overall treatment failure | 14 (13.4) | 4 (8.3) | 1 (8.3) | 1 (12.5) | |
| Day 42 adjusted by PCR | n = 75 | n = 48 | n = 12 | n = 8 | |
| Clinical failure | ETF | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | |
| Parasitologic failure | LPF | 1 (1.3) | 1 (2.1) | 0 (0) | 0 (0) |
| Overall treatment failure | 2 (2.6) | 1 (2.1) | 0 (0) | 0 (0) | |
PCR = polymerase chain reaction; ETF = early treatment failure; LCF = late clinical failure; LPF = late parasitologic failure.
Secondary outcomes
Parasite clearance occurred rapidly in response to ASAQ treatment and was detected on day 3 in children with malaria. Only two children had parasitemia on microscopic examination on day 3 (690–3,600 parasites/μL in the first case and 150–2,750 parasites/μL in the second case). Fever clearance was more rapid in areas with low levels of malaria transmission (0% of children with a temperature ≥ 37.5°C on day 3) than in areas with high transmission rates (8.6% in Ampasimanjeva and 4.2% in Vohimasy), but this difference was not significant. On day 42, the extent of hematologic recovery (median individual increase in hemoglobin levels) was 0.8 g/dL (95% CI = 0.5–1.0 g/dL). Significant differences were observed between sites (Ampasimanjeva = 0.7 g/dL, Vohimasy = 1.3 g/dL, Ampasimpotsy = 2.0 g/dL, and Andasibe = 4.3 g/dL; P < 0.001).
Adverse events
Adverse events were systematically reported throughout this study. The proportion of children for whom an adverse event was reported to the CHW was low in the M+/PCR+ group (3.5%; 95% CI = 1.5–7.2%). Diarrhea and sleepiness were the most frequently recorded adverse events. In the other groups, adverse events were more frequent: 5.3% (95% CI = 1.5–13.1%) in the M–/PCR+ group and 30.4% (95% CI = 21.8–41.2%) in the M–/PCR– group. Only two of these adverse events were considered serious (one child in the M+/PCR+ group and one child in M–/PCR– group). Both of these children subsequently died of presumed severe pneumonia after referral to the health center.
Discussion
Home management of malaria, as advocated by the National Malaria Control Program in Madagascar, remains an essential strategy for malaria control. In this strategy, the aim is to administer effective antimalarial treatment (after parasitologic confirmation if available), promptly in all children five years of age with fever lasting 24 hour. Home management of malaria has been used for several decades, but its impact on malaria mortality or morbidity has never been assessed in Madagascar. All available data for Madagascar concerning the feasibility of this strategy were obtained in studies in which chloroquine was used as the antimalarial drug.10 According to previous publications studies by Hopkins and others23 and Pagnoni,13 the safety and effectiveness of HMM with ACT must be confirmed in various epidemiologic settings to provide policy makers with better information about opportunities for adopting and extending this strategy for malaria control. The objective of this study was to provide detailed data concerning the annual incidence of malaria infections in febrile children less than five years of age in various rural settings in Madagascar, and data on the safety and effectiveness of fixed-dose ASAQ recommended for the presumptive antimalarial treatment of febrile children and the degree of compliance with treatment.
The overall mean annual incidence of febrile episodes in children less than five years of age has been estimated to be 0.25, ranging from 0.11 in areas with low rates of transmission to 0.23–0.55 in areas with high transmission rates. No significant differences were found between the two settings, which was likely caused by greater use of insecticide-treated bed nets (approximately 67%) in areas of high transmission rates than in areas with low transmission rates (approximately 3.1%)). This finding is of fundamental importance for the National Malaria Control Program in Madagascar because it can improve planning for the number of treatments annually purchased for HMM, in particular with support from the Global Fund to Fight AIDS, Tuberculosis and Malaria.
This study also made it possible to quantify the frequency of malaria infection (by using microscopy and PCR for parasite detection) among febrile children. Malaria prevalence among febrile children ranged from 38.6% in areas with high levels of transmission to 28.4% in areas of low transmission and was considerably lower than that reported in studies in Tanzania (42%), Uganda (61%), Nigeria (71%), and Ghana (71%).24 Malaria prevalence estimates among febrile children obtained by PCR were significantly higher than those obtained by microscopy in areas with high transmission rates (difference between positive microscopy results and positive PCR results was 17.1% in high transmission areas versus 3.6% in low transmission areas; P < 0.0001). Moreover, the pyrogenic threshold was approximately two-fold higher in high transmission areas than in low transmission areas (mean parasitemia in symptomatic children = 3,064 parasites/μL in areas with high transmission rates versus 1,302 parasites/μL in areas with low transmission rates), which indicates that anti-disease immunity to malaria may have occurred earlier among children living in areas of highest transmission of malaria in Madagascar.
In this study, we provide the first reliable data concerning the effectiveness of the new fixed dose of ASAQ prescribed by CHWs and administered by parents and guardians at home without supervision in Madagascar. The PCR-adjusted cure rate on day 42 varied between sites from 97.6% to 100%, which was higher than the threshold of 90% recommended by WHO.18,24 The failure rates recorded were consistent with previous reports of therapeutic efficacy from studies carried out at the health-center level with non-fixed doses of ASAQ11 and at the community level with various ACTs in Africa.25 In the non-malaria group (M–/PCR–), more than 50% of the children were asymptomatic by day 42, which is consistent with febrile viral infections in these cases. We recorded only a few minor adverse events, such as diarrhea and sleepiness, and it was not possible to determine whether these adverse events were truly related to the antimalarial treatment. The levels of compliance with treatment reported during interviews with parents or guardians was consistently high (90.0%; 95% CI = 87.2–92.5%) and did not differ between sites. Our results were similar to those recently reported for Africa (81% in Uganda, 93% in Nigeria, and 97% in Ghana).26
As suggested by Pagnoni,13 these data provide some reassurance concerning the likelihood of selecting drug-resistant parasites through exposure to sub-therapeutic levels of drugs.27 However, the high degree of compliance observed in this study, which was not obtained under real life conditions, strongly suggests that CHWs must be trained in the correct use of antimalarial drugs. The packaging of antimalarial drugs in fixed-dose combination presentations is particularly important in ensuring compliance.
However, like all studies carried out under operational conditions, this study was subject to several limitations. First, compliance was assessed by the MO during interviews as part of the visit on day 3. Plasma drug concentration was not determined on day 7, but this method would provide a more reliable means of measuring compliance. Second, the diagnosis of malaria infections was assessed retrospectively and patient follow-up was based exclusively on the day 28 and day 42 visits.
In conclusion, our study provides important data on the safety and the effectiveness of the fixed-dose ASAQ in the context of HMM in Madagascar and compliance with this treatment, given current calls for the widespread use of ACT in the community.6 This study provides evidence that the HMM program in Madagascar using fixed-dose ASAQ can result in the delivery of effective drugs for treating malaria infections. However, use of rapid diagnostic tests as part of the HMM program in Madagascar should be considered to improve the targeting of antimalarial treatment to true cases of malaria, particularly in areas with low rates of transmission. Further studies are also urgently need to identify the causes of acute fever when diagnosis is negative for malaria to implement official recommendations to guide the management of patients who show negative results for rapid diagnostic tests.
ACKNOWLEDGMENTS
We thank the villagers in Ampasimpotsy and Andasibe in Moramanga District and Andramora and Ambodivoahangy in Manakara District for their participation in the study; the medical team Dr. Gladys Somondrara, Dr. J. Randriamboavonjy, Gervais Razafimahatrata, Gladys Saroma, and Jasmin Velo for conducting field work; the Moramanga District Health Service, the Manakara District Health Service, the Ampasimanjeva Medical Foundation, SAF FJKM, Inter-Aide, the Reggio Terzzo Mondo, and the Madagascar National Malaria Control Program for their active involvement in the study; and Dr, Luciano Tuseo, Dr. Franco Pagnoni, and Professor Francois Dabis for their constructive advice.
Footnotes
Financial support: This study was supported by Population Service International, Inter-aide, the Ampasimanjeva Medical Foundation, the Ministry of Health of Madagascar (DULM, PNLP, DRS Moramanga, Manakara), ADRA, Sanofi Aventis Paris (for providing Coarsucam™), the local authorities (Ampasimpotsy, Mahatsara, Ambodivoahangy, Andramora), and SanteNet. Arsène Ratsimbasoa was supported by the Fondation Mérieux. Didier Ménard was supported by the French Ministry of Foreign Affairs.
Authors' addresses: Arsène Ratsimbasoa, Harintsoa Ravony, Jeanne-Aimée Vonimpaisomihanta, and Rogelin Raherinjafy, Ministère de la Santé, du Planning Familial et de la Protection Sociale, Antananarivo, Madagascar, E-mails: arsene.ratsimbasoa@laposte.net, niriharintsoa@yahoo.fr, vonimpaiso@yahoo.fr, and raherinjafy@yahoo.fr. Martial Jahevitra, Rabenja Rapelanoro, and Jean De Dieu Marie Rakotomanga, Université d'Antananarivo, Antananarivo, Madagascar, E-mails: mjahevitra@yahoo.fr, frapelanoro@yahoo.fr, and rktjdm@yahoo.fr. Denis Malvy, Centre René Labusquière, Université Victor Segalen Bordeaux 2, Bordeaux, France, E-mail: denis.malvy@chu-bordeaux.fr. Pascal Millet, EA Pharmaceuticals and Analytical Methods for Neglected Diseases and Counterfeit Drugs, Bordeaux 2 University, Bordeaux, France, E-mail: pascal.millet@u-bordeaux2.fr. Didier Ménard, Malaria Molecular Epidemilogy Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia, E-mail: dmenard@pasteur-kh.org.
References
- 1.Snow RW, Guerra CA, Mutheu JJ, Hay SI. International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med. 2008;5:e142. doi: 10.1371/journal.pmed.0050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster S. Treatment of malaria outside the formal health services. J Trop Med Hyg. 1995;98:29–34. [PubMed] [Google Scholar]
- 3.Kager PA. Malaria control: constraints and opportunities. Trop Med Int Health. 2002;7:1042–1046. doi: 10.1046/j.1365-3156.2002.00981.x. [DOI] [PubMed] [Google Scholar]
- 4.Moerman F, Lengeler C, Chimumbwa J, Talisuna A, Erhart A, Coosemans M, D'Alessandro U. The contribution of health-care services to a sound and sustainable malaria-control policy. Lancet Infect Dis. 2003;3:99–102. doi: 10.1016/s1473-3099(03)00518-8. [DOI] [PubMed] [Google Scholar]
- 5.Wiseman V, Scott A, Conteh L, McElroy B, Stevens W. Determinants of provider choice for malaria treatment: experiences from The Gambia. Soc Sci Med. 2008;67:487–496. doi: 10.1016/j.socscimed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Scaling Up Home-Based Management of Malaria: from Research to Implementation. Geneva: World Health Organization; 2004. [Google Scholar]
- 7.World Health Organization . The Roll Back Malaria Strategy for Improving Access to Treatment through Home Management of Malaria. Geneva: World Health Organization WHO/HTM/MAL/2005.1101; 2005. [Google Scholar]
- 8.Greenwood B, Marsh K, Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991;7:277–281. doi: 10.1016/0169-4758(91)90096-7. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessandro U, Talisuna A, Boelaert M. Editorial: Should artemisinin-based combination treatment be used in the home-based management of malaria? Trop Med Int Health. 2005;10:1–2. doi: 10.1111/j.1365-3156.2004.01375.x. [DOI] [PubMed] [Google Scholar]
- 10.Ratsimbasoa A, Randrianarivelojosia M, Millet P, Soares JL, Rabarijaona L, Rakotoson B, Malvy D, Menard D. Use of pre-packaged chloroquine for the home management of presumed malaria in Malagasy children. Malar J. 2006;5:79. doi: 10.1186/1475-2875-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andriantsoanirina V, Menard D, Tuseo L, Durand R. History and current status of Plasmodium falciparum antimalarial drug resistance in Madagascar. Scand J Infect Dis. 2010;42:22–32. doi: 10.3109/00365540903289670. [DOI] [PubMed] [Google Scholar]
- 12.Menard D, Ratsimbasoa A, Randrianarivelojosia M, Rabarijaona LP, Raharimalala L, Domarle O, Randrianasolo L, Randriamanantena A, Jahevitra M, Andriantsoanirina V, Rason MA, Raherinjafy R, Rakotomalala E, Tuseo L, Raveloson A. Assessment of the efficacy of antimalarial drugs recommended by the National Malaria Control Programme in Madagascar: up-dated baseline data from randomized and multi-site clinical trials. Malar J. 2008;7:55. doi: 10.1186/1475-2875-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagnoni F. Malaria treatment: no place like home. Trends Parasitol. 2009;25:115–119. doi: 10.1016/j.pt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, Bertrand O, Picot J, King CL, Grimberg BT, Mercereau-Puijalon O, Zimmerman PA. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci USA. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Allen E, Dlamini SS, Tsoka J, Bredenkamp B, Mthembu DJ, White NJ, Sharp BL. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med. 2005;2:e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Depoortere E, Guthmann JP, Sipilanyambe N, Nkandu E, Fermon F, Balkan S, Legros D. Adherence to the combination of sulphadoxine-pyrimethamine and artesunate in the Maheba refugee settlement, Zambia. Trop Med Int Health. 2004;9:62–67. doi: 10.1046/j.1365-3156.2003.01157.x. [DOI] [PubMed] [Google Scholar]
- 17.Depoortere E, Salvador ET, Stivanello E, Bisoffi Z, Guthmann JP. Adherence to a combination of artemether and lumefantrine (Coartem) in Kajo Keji, southern Sudan. Ann Trop Med Parasitol. 2004;98:635–637. doi: 10.1179/000349804225021271. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated falciparum Malaria. Geneva: World Health Organization; 2003. [Google Scholar]
- 19.World Health Organization . Management of Uncomplicated Malaria and the Use of Antimalarial Drugs for the Protection of Travellers: Report of an Informal Consultation (WHO/MAL/96) Geneva: World Health Organization; 1996. [Google Scholar]
- 20.Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB, Jr, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, Jahevitra M, Andriantsoanirina V, Menard D. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. Am J Trop Med Hyg. 2008;78:217–221. [PubMed] [Google Scholar]
- 22.Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am J Trop Med Hyg. 2003;68:133–139. [PubMed] [Google Scholar]
- 23.Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home-based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malar J. 2007;6:134. doi: 10.1186/1475-2875-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajayi IO, Browne EN, Bateganya F, Yar D, Happi C, Falade CO, Gbotosho GO, Yusuf B, Boateng S, Mugittu K, Cousens S, Nanyunja M, Pagnoni F. Effectiveness of artemisinin-based combination therapy used in the context of home management of malaria: a report from three study sites in sub-Saharan Africa. Malar J. 2008;7:190. doi: 10.1186/1475-2875-7-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faucher JF, Aubouy A, Adeothy A, Cottrell G, Doritchamou J, Gourmel B, Houze P, Kossou H, Amedome H, Massougbodji A, Cot M, Deloron P. Comparison of sulfadoxine-pyrimethamine, unsupervised artemether-lumefantrine, and unsupervised artesunate-amodiaquine fixed-dose formulation for uncomplicated Plasmodium falciparum malaria in Benin: a randomized effectiveness noninferiority trial. J Infect Dis. 2009;200:57–65. doi: 10.1086/599378. [DOI] [PubMed] [Google Scholar]
- 26.Ajayi IO, Browne EN, Garshong B, Bateganya F, Yusuf B, Agyei-Baffour P, Doamekpor L, Balyeku A, Munguti K, Cousens S, Pagnoni F. Feasibility and acceptability of artemisinin-based combination therapy for the home management of malaria in four African sites. Malar J. 2008;7:6. doi: 10.1186/1475-2875-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]