Abstract
African histoplasmosis, caused by Histoplasma capsulatum var. duboisii, is endemic in Africa. The disease usually involves the skin, subcutaneous tissue, and bones. A case of African histoplasmosis presenting as a cutaneous tumor and non-healing wound in a 66-year-old immunocompetent male residing in Africa, the first ever reported following mudbaths and acupuncture, is hereby reported. Diagnosis was confirmed by means of polymerase chain reaction performed on tissue material. The patient was started on long-term itraconazole therapy and he responded well. African histoplasmosis should be included in the differential diagnosis of non-healing wounds or tumor-like lesions, especially in the context of mudbaths in an endemic area.
Introduction
African histoplasmosis, caused by Histoplasma capsulatum var. duboisii, is endemic in Africa, usually in areas located between 20° North and 20° South of the Equator, as well as in Madagascar.1 The disease is likely underreported, presumably because cases are not recognized or correctly diagnosed. It is sometimes associated with immunosupression and has rarely been reported outside Africa.1–3 We herein report a case in an immunocompetent male, with a rare clinical presentation of a cutaneous tumor; moreover, this is (to our best knowledge) the first case ever reported following mudbaths.
Case Report
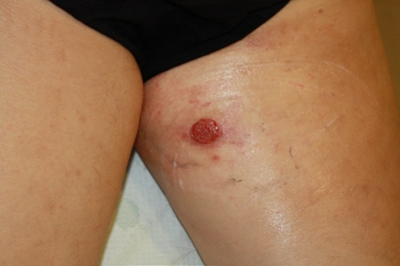
A 66-year-old male, resident of the Democratic Republic of Congo (DRC) for the last 42 years first noted, in September 2010, a pustule in his right upper thigh; at the time, he was taking mudbaths and local acupuncture, because of lumbago and sciatica-like pain. By the time he visited Rhodes (Greece), in January 2011, the pustule had evolved into a 2 cm large protruding, ulcerated tumor surrounded by a reddened area (Figure 1). A local general practitioner prescribed a short course of cefuroxime treatment, with no apparent improvement, after which the tumor was excised. Post-surgical treatment, first with cefaclor and then with ciprofloxacin and doxycycline (for 2 weeks each) was initiated, again with minimal improvement.
Figure 1.
Skin lesion at the time of the initial evaluation.
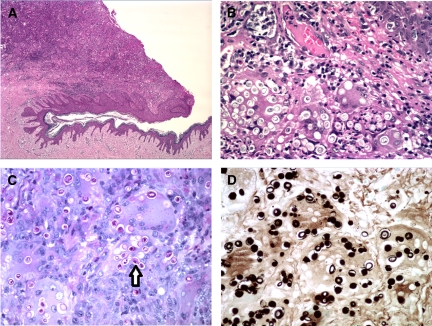
A 4 × 3 cm wide, 1.5 cm thick cutaneous ellipse containing a 1.9 cm large, ulcerated tumor was received at the Department of Pathology. Histology showed the tumor to consist of a diffuse, granulomatous inflammation of the dermis, with a prominent multinucleate giant cell component, both of Langhans and foreign body type. Many oval fungi, around 10 μm in diameter were identified, both within giant cells and outside; some formed budding pairs, with a narrow connection. Fungi were highlighted by means of both periodic acid Schiff (PAS) and Grocott stains (Figure 2). The overlying epidermis was focally ulcerated. The diagnosis of African histoplasmosis was suggested, after which the patient was admitted to the Department of Internal Medicine for a further workup. Material from the paraffin block was sent to an international reference center for mycology, where the diagnosis was confirmed by means of an in-house real-time polymerase chain reaction (PCR), according to a previously described protocol.4 This method detects both Histoplasma spp. varieties (var. capsulatum, var. duboisii) and targets the ITS2 region of the ribosomal DNA. The PCR results were confirmed by sequencing the amplicon and comparing the sequence with the nucleotide sequence database available in the Mycology Laboratory (Majadahonda, Madrid; more than 5,000 entries) and with the GenBank database (http://www.ncbi.nih.gov/GenBank/). The sequence matched that of H. capsulatum var. duboisii (GenBank accession no.: AB071834.1; patient’s isolate sequence European Nucleotide Archive accession no.: HE602531). The percentage of identity with both databases was 99%.
Figure 2.
Overview of the ulcerated cutaneous tumor (A, Hematoxylin-Eosin,×4); fungi within giant cells, epidermis at upper right (B, Hematoxylin-Eosin, ×40); fungi highlighted by periodic acid Schiff (PAS) (C, ×40, arrow at budding form) and Grocott (D, ×40).
Upon admission, the patient had a past medical history of type II diabetes mellitus for the last 10 years, in addition to persistent lumbago and right sciatica pain. He was on metformin, carvedilol, clopidogrel, and enteric-coated aspirin. No risk factors for human immunodeficiency virus (HIV) infection or exposure to pets or soil (other than the mudbaths previously reported) were disclosed. Physical examination disclosed a systolic heart murmur consistent with aortic stenosis with normal S1 and S2 and hepatosplenomegaly without concomitant lymphadenopathy. No spinal tenderness was found; neurologic assessment was unremarkable. Complete blood count, serum protein electrophoresis, and biochemical examinations were within normal limits. Serologic evaluation for hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV, as well as specific PCR for Plasmodium spp. performed in a blood specimen were negative. Fungal blood cultures were negative. Chest computer tomography was unremarkable, whereas abdominal computer tomography disclosed hepatosplenomegaly with no enlarged lymph nodes. Bone scan showed increased gallium citrate concentration at the L4 and S1 vertebrae, right sternoclavicular joint and both knees, interpreted as of a degenerative origin.
The patient was initiated on itraconazole capsules at a dose of 200 mg tid for 3 days, followed by 200 mg bid for the following 6 months. The surgical wound gradually healed with no recurrence of the original skin lesion. He has since returned to DRC, where repeat ultrasound examinations (the last one 4 months after treatment initiation) showed a significant reduction of both liver and spleen size with no evidence of punctuate calcifications. Complete blood count during follow-up was normal. He is still under treatment and fares well.
Discussion
First described in 1952 by Dubois and others,5 African histoplasmosis (also known as histoplasmosis duboisii) is a rare deep mycosis. It usually occurs in the tropical belt of Africa, between the Tropics of Cancer and Capricorn, and in Madagascar. It has rarely been reported outside Africa, mainly in migrants or former inhabitants of the continent.1–3 Fewer than 300 cases had been reported up to 2007, only a few of them arising in the context of HIV infection.1,3
The causal agent is H. capsulatum var. duboisii, which differs in some aspects, such as fatty acid profile, cell wall glycan structure, or lack of urease6,7 from the more commonly encountered Histoplasma capsulatum var. capsulatum. The fungus has recently been isolated from soil containing bat excrements in Nigeria6 thus accounting for the association of cases with a history of previous exposure to caves infested with bat droppings or to contaminated soil, like classical histoplasmosis.1
Unlike classical histoplasmosis, which mainly occurs in the lungs, tissues most frequently involved in African histoplasmosis include skin, subcutaneous tissue, and bones, although lymph node, spleen, hepatic, pulmonary or gastrointestinal lesions have also been described in disseminated disease.5,6,8,9 Unusual clinical presentations so far reported include Addison’s disease,10 a gastric ulcer,11 peritonitis caused by perforation of an intestinal lesion,12 a colonic tumor,13 and an orbital cyst.14 Unlike the case hereby reported, cutaneous lesions are usually multiple, appear simultaneously, and incubation can be very long going, sometimes several months or years after exposure.1
Histology shows a mainly granulomatous inflammation, with a prominent component of huge (up to 150 μm) multinucleate giant cells, both of foreign body and Langhans type, containing many (up to 10 or more) oval or lemon-shaped, thick-walled, 8–15 μm large yeast cells, in contrast to those of H. capsulatum var. capsulatum, which do not exceed 5 μm. Fungal cells divide by narrow budding. They are easily identified in tissue sections by virtue of time-honored histochemical stains, such as PAS or Grocott methenamine-silver. Differential diagnosis mainly includes Cryptococcus and Penicillium species: the lack of a mucicarminophilic halo excludes Cryptococcus, whereas Penicillium yeasts divide by intracellular septation, not by narrow budding.15–18
Although specific primary antibodies for immunostaining of H. capsulatum var. capsulatum yeasts in tissue sections are available,19 no such report exists up to now for H. capsulatum var. duboisii.
There is a more prominent giant cell component in histologic sections of African, compared with classical histoplasmosis. Although the reason for this difference has not yet been elucidated, recently identified differences in fatty acid profile or cell wall glycan structure between the two varieties of H. capsulatum7 might play a role, probably along with other, as yet undetermined factors.
The case hereby presented is, to our best knowledge, the first ever reported following mudbaths, although the fungus could have also been introduced by needles used for acupuncture. However, no needle application occurred in close proximity to the area of the lesion. Although the portal of entry of H. capsulatum var. duboisii has not yet been firmly established, it is presumed that, like other Histoplasma spp., it enters the body by the respiratory tract. However, in our case no pulmonary focus was found. Moreover, the presence of a single cutaneous lesion, in contrast to multiple ones usually encountered in African histoplasmosis, makes plausible a transcutaneous portal of entry in our patient. Furthermore, this case is also unusual in that it manifested as a cutaneous tumor, its real identity having been established by histologic examination. Tumorous lesions of African histoplasmosis have seldom been previously reported, both in the skin20 and in other locations, e.g., the colon.13
Our patient had hepatosplenomegaly when first evaluated. Because no alternative cause was revealed during his workup and because repeat ultrasound examinations during treatment disclosed a significant reduction of the size of both liver and spleen, involvement of both organs seems plausible. Nevertheless, no evidence of punctuate calcifications, a sign further supporting visceral involvement was noted upon reimaging. Furthermore, one might wonder about the cause of (initially interpreted as degenerative) “hot” foci identified in the bone scan, osseous involvement being frequent in African histoplasmosis.
Regarding the laboratory diagnosis and in view of the positive PCR results, no further specific fungal investigation was performed. A urinary Histoplasma antigen was not performed because it is known to cross-react with both varieties of H. capsulatum spp3.
Regarding therapy, we opted for a regimen offered for classical disseminated histoplasmosis with an initial plan to continue for at least 6 months. The patient remains under close follow-up; if treatment continues to be well tolerated, it may be prolonged for up to 12 months, as recommended for disseminated histoplasmosis.21 A second admission for post-treatment reevaluation is scheduled.
In conclusion, although rare, African histoplasmosis should be included in the differential diagnosis of non-healing wounds or tumor-like lesions, especially in the context of mudbaths in an endemic area.
Footnotes
Authors’ addresses: Sotirios Tsiodras, Miranda Drogari-Apiranthitou, Konstantinos Leventakos, Theodoros Kelesidis, and Georgios Petrikkos, 4th Department of Internal Medicine, “Attikon” University Hospital, National and Kapodistrian University of Athens School of Medicine, Athens, Greece, E-mails: tsiodras@med.uoa.gr, mdrogari@hotmail.com, kostasleventakos@gmail.com, tkelesid@gmail.com, and petrikos@med.uoa.gr. Konstantinos Pilichos, Plastic Surgery Practice, Rhodes, Dodecanese, Greece, E-mail: kpilplast@yahoo.gr. Maria Jose Buitrago Serna, Mycology Reference Laboratory, National Centre of Microbiology, Istituto de Salud Carlos III, Madrid, Spain, E-mail: buitrago@isciii.es. Ioannis Panayiotides, 2nd Department of Pathology, ?Attikon? University Hospital, National and Kapodistrian University of Athens School of Medicine, Athens, Greece, E-mail: ioagpan@gmail.com.
References
- 1.Gugnani HC. Histoplasmosis in Africa: a review. Indian J Chest Dis Allied Sci. 2000;42:271–277. [PubMed] [Google Scholar]
- 2.Nethercott JR, Schachter RK, Givan KE, Ryder DE. Histoplasmosis due to Histoplasma capsulatum var. duboisii in a Canadian immigrant. Arch Dermatol. 1978;114:595–598. [PubMed] [Google Scholar]
- 3.Loulergue P, Bastides F, Baudouin V, Chandenier J, Mariani-Kurkdjian P, Dupont B, Viard J-P, Dromer F, Lortholary O. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg Infect Dis. 2007;13:1647–1652. doi: 10.3201/eid1311.070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitrago MJ, Berenguer J, Mellado E, Rodríguez-Tudela JL, Cuenca-Estrella M. Detection of imported histoplasmosis in serum of HIV-infected patients using a real-time PCR-based assay. Eur J Clin Microbiol Infect Dis. 2006;10:665–668. doi: 10.1007/s10096-006-0207-y. [DOI] [PubMed] [Google Scholar]
- 5.Dubois A, Janssens PG, Brutsaert P, Vanbreuseghem R. A case of African histoplasmosis; with a mycological note on Histoplasma duboisii n. sp. [in French, Un cas d? histoplasmose africaine. Avec une note mycologique sur Histoplasma duboisii n. sp.] Ann Soc Belge Med Trop. 1952;32:569–584. [PubMed] [Google Scholar]
- 6.Gugnani HC, Muotoe-Okafor F. African histoplasmosis: a review. Rev Iberoam Micol. 1997;14:155–159. [PubMed] [Google Scholar]
- 7.Zarnowski R, Miyazaki M, Dobrzyn A, Ntambi JM, Woods JP. Typing of Histoplasma capsulatum strains by fatty acid profile analysis. J Med Microbiol. 2007;56:788–797. doi: 10.1099/jmm.0.47067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minta DK, Dembélé M, Lorre G, Diallo DA, Traoré HA, Chabasse D. African histoplasmosis (Histoplasma capsulatum var. duboisii): a case report from Mali [in French, Histoplasmose Africaine à Histoplasma capsulatum var. duboisii. A propos d? un cas au Mali] Cahiers Santé. 2005;15:195–199. [PubMed] [Google Scholar]
- 9.Binford CH, Dooley JR. Pathology of Tropical and Extraordinary Diseases. Vol. 2. Washington DC: Armed Forces Institute of Pathology; 1976. African histoplasmosis. Binford CH, Connor DH, eds; pp. 581–583. [Google Scholar]
- 10.Mudawi HM, Elamin EM, Baraka OZ, El-Hassan AM. Addison’s disease due to Histoplasma duboisii infection of the adrenal glands. Saudi Med J. 2008;29:904–906. [PubMed] [Google Scholar]
- 11.Sanguino JC, Rodrigues B, Baptista A, Quina M. Focal lesion of African histoplasmosis presenting as a malignant gastric ulcer. Hepatogastroenterology. 1996;43:771–775. [PubMed] [Google Scholar]
- 12.Pequignot H, Abelanet R, Christoforov B, Drouhet E, Duflo B, Gorin JP, Lapierre J, de Saint-Maur P, Tran-Vinh-Hien Peritonitis due to perforation of intestinal histoplasmosis due to Histoplasma duboisii [in French, Péritonite par perforation d?une histoplasmose intestinale à Histoplasma duboisii] Ann Med Interne (Paris) 1972;123:981–986. [PubMed] [Google Scholar]
- 13.Khalil M, Iwatt AR, Gugnani HC. African histoplasmosis masquerading as carcinoma of the colon: report of a case and review of the literature. Dis Colon Rectum. 1989;32:518–520. doi: 10.1007/BF02554509. [DOI] [PubMed] [Google Scholar]
- 14.Bansal RK, Suseelan AV, Gugnani HC. Orbital cyst due to Histoplasma duboisii. Br J Ophthalmol. 1977;61:70–71. doi: 10.1136/bjo.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weedon D, editor. Weedon’s Skin Pathology. Third edition. Churchill Livingstone Elsevier; 2010. pp. 581–606. (Mycoses and algal infections). [Google Scholar]
- 16.McKee PH, Calonje E, Grayson W. In: Pathology of the Skin with Clinical Correlations. Third edition. McKee PH, Calonje E, Granter SR, editors. Vol. 1. Philadephia, PA: Elsevier Mosby; 2005. pp. 837–992. (Infectious diseases of the skin). [Google Scholar]
- 17.Chandler FW, Kaplan W, Ajello L. Histoplasmosis duboisii. A Colour Atlas and Textbook of the Histopathology of Mycotic Diseases. London: Wolfe Medical Publications Ltd.; 1980. pp. 67–69. [Google Scholar]
- 18.Prieto-Granada CN, Lobo AZC, Mihm MC., Jr . In: Diagnostic Pathology of Infectious Disease. Kradin RL, editor. Saunders Elsevier; 2010. pp. 519–616. (Skin infections). [Google Scholar]
- 19.White CL. In: Theory and Practice of Histological Techniques. Sixth edition. Bancroft JD, Gamble M, editors. Churchill Livingstone Elsevier; 2008. pp. 493–516. (Immunohistochemistry applications in pathology). [Google Scholar]
- 20.Oguachuba HN, Gugnani HC. African histoplasmosis manifesting as a cutaneous tumour treated with econazole. J Trop Med Hyg. 1982;85:259–263. [PubMed] [Google Scholar]
- 21.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CA. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]