Abstract
Chronic infection over a 16-month period and stunting of preschool children were compared between more spatially dense versus dispersed households in rural Panamá. Chronic protozoan infection was associated with higher household density, lower household wealth index, poor household water quality, yard defecation, and the practice of not washing hands with soap before eating. Models for chronic diarrhea confirmed the importance of household wealth, water quality, sanitation, and hygiene practices. Furthermore, chronic protozoan infection was an important predictor for low height-for-age, along with low household wealth index scores, but not household density. Thus, despite better access to health related infrastructure in the more densely populated households, chronic protozoan infection was more common, and was associated with higher rates of child stunting, compared with more dispersed households.
Introduction
Improved child health in combination with greater financial capital is considered integral in breaking the intergenerational cycle of poverty.1,2 As such, development of physical infrastructure is central to poverty alleviation. Not only does it increase access to economic opportunities through road construction and access to financial markets and employment opportunities,3–5 but physical infrastructure also improves health through the addition of sanitation and water systems as well as providing better access to schools and health facilities.6 Access to health facilities reduces child morbidity and mortality7,8 particularly when education initiatives enhance the use of the available services.9 Moreover, access to sanitation and water infrastructure, considered forgotten foundations in health,10 reduces child morbidity and mortality by encouraging the formation of well-being behaviors.11–13
However, infrastructure development is usually coupled with increased population density and spatial proximity of households, which can increase transmission of infectious diseases.14,15 For enteric pathogens, in particular, factors such as crowding within the home16,17 and higher household density have been associated with increased risk of infection.18 This finding has been shown to be of particular importance in urban areas where sanitation infrastructure is insufficient,19,20 but household spatial dispersion is also of likely relevance for studying child health in rural areas. Child growth, in particular, is indicative of the long-term impact of a child's environment on their health where growth failure is associated with factors such as prolonged dietary insufficiency or frequent infection.21,22 Thus, examining frequency of infection and child height for age can contribute to an understanding of the cumulative impact of physical assets (including infrastructure) and spatial proximity of households on child health.
Furthermore, as economic opportunities increase in extremely impoverished rural areas, accurately measuring household wealth is important to fully characterize the risk factors associated with child health outcomes. Measuring wealth in areas of extreme poverty is considered a challenge, primarily caused by the fact that household income is often inconsistent, making reported income or consumption estimations of wealth inaccurate.23 Statistically weighted asset-based indices of wealth24,25 are considered a proxy for long-term income to determine relative poverty23 and may have stronger explanatory power than expenditure data for rural health outcomes.25
Our investigation in western Panamá provided an opportunity to compare access to physical infrastructure and child health outcomes between more spatially concentrated and spatially dispersed rural areas and furthermore to examine the influence of household wealth on child stunting and infection outcomes in a region of extreme poverty. In the comarca Ngäbe–Buglé, contemporary livelihoods range from subsistence farming with minor cash inputs in spatially dispersed households to short-term wage labor from small stores or temporary employment in more spatially concentrated households. Importantly, recent studies in the comarca reported that more than half of Ngäbe children are stunted.26,27 Thus, our study aimed to 1) compare intestinal protozoan infection, diarrhea, and stunting in preschool children between spatially dispersed and more spatially concentrated households; and 2) determine whether household density and degree of poverty were associated with severity of stunting and chronicity of protozoan infections and diarrhea. Specifically, we hypothesized that households in the more densely populated regions would have higher average wealth, better access to sanitation, hygiene and health care, and therefore that children living in these households would have less severe stunting, and less chronic gastrointestinal protozoan infections and diarrhea.
Materials and Methods
Study population.
The comarca Ngäbe–Buglé is a semi-autonomous political region in Panamá inhabited primarily by the Ngäbe and Buglé indigenous groups (2004 population estimate of 128,978) of whom 91% live in extreme poverty (< US$1.75/day).28 In 2005, the Conditional Transfer (CT) program Red de Oportunidades began in the comarca, providing an additional US$ 50/month in either cash or food vouchers to the more than 90% of families in extreme poverty in exchange for participation in health and education programs.
The data presented are a subset of a larger study that was conducted in 2008 and 2009 in two corregimientos or political regions (Soloy: cash transfer; Emplanada de Chorcha: food voucher) within the district of Besiko within the CT project area (Figure 1). Using a random number generator, we randomly selected six villages within each corregimiento from those that were within two hours walking distance of the health center and had 25–100 households registered in a recent government survey. All households in selected villages were approached and those that met the inclusion criteria of having at least one child ≤ 4 years of age and living in extreme poverty, defined as having participated in a CT program since 2005, were given an orientation to the study in Spanish and Ngäbere (the local language). All but 3 eligible households agreed to participate, resulting in a total of 262 households with 373 preschool children (161 households with 1 eligible child, 91 with 2 eligible children, and 10 with 3 eligible children). Approximately 50–60% of the households from 11 villages and 37% of households from 1 village (Cerro Viejo) participated in the study (range = 7–41 households/village). The lower proportion of participating households in Cerro Viejo was caused by a lower proportion of households with children < 5 years of age.
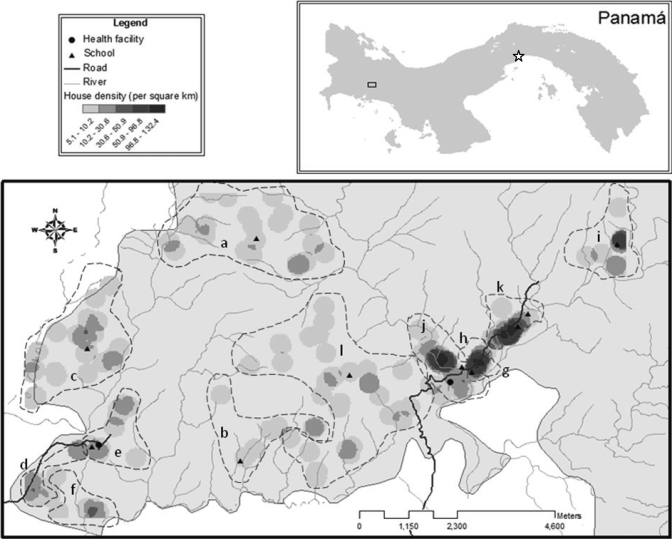
Figure 1.
Household density clusters, health facilities, and schools in the region participating in the study located in western Panama. The capital of Panama City is indicated by a star and dashed lines indicate villages. Emplanada de Chorcha: a = Alto Caña, b = Cerro Viejo, c = Chorchita, d = La Juventud, e = Plan de Chorcha, f = Quebrada de Lajas. Soloy: g = Barrio 19 Abril, h = Barrio 2000, I = Boca de Huso, j = Boca de Jebay, k = Boca Miel, l = Israel.
Ethical considerations.
Ethical approval was obtained from the Instituto Conmemorativo de Gorgas in Panamá and McGill University in Canada. Community interactions were established in accordance with the Guía para Realizar Estudios e Investigaciones en los Pueblos Indígenas de Panamá, which included participation in introductory and results workshops in each village. Written informed consent was obtained from primary caregivers during a household visit that included an explanation of study significance, participant requirements, and rights, and an opportunity to ask questions in Spanish and Ngäbere. According to Panamanian Ministry of Health (MINSA) protocol, primary caregivers received verbal explanation of results after each sample and a MINSA laboratory diagnostic form enabling them to seek treatment at a health center. In addition, at the end of each year, names of infected children were given to MINSA personnel at the health centers.
Study procedure.
The results presented are part of a larger collaborative investigation of child health conducted by MINSA, the University of Panamá, and McGill University. For the purpose of this report, long-term estimates of chronicity of protozoan infection and diarrhea were obtained by using data from 7 fecal samples collected over 16 months (Table 1). In addition, child anthropometry, household demographic data, spatial survey data, and child and household sanitation and hygiene practices were recorded during visits throughout the study period (Table 1). Data collection was conducted by MINSA personnel who had previous experience in the area together with local translators who had been oriented to the specific project goals and methods before initiation of the study, during which previously untested questionnaires were piloted with local communities. Quality of data collection was verified by rotating field supervisor visits and questionnaire revision after each day of field work. Laboratory procedures were conducted during each week of field work.
Table 1.
Timeline of sample collection during 10 household visits in western Panamá, June 2008–October 2009
| Characteristic | 2008 | 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 June | Visit 2 July | Visit 3 July | Visit 4 July | Visit 5 August | Visit 6 October | Visit 7 April | Visit 8 May | Visit 9 August | Visit 10 October | |
| Household, # | ||||||||||
| Recruit | 262 | |||||||||
| Household wealth | 229 | |||||||||
| Water quality | 206 | |||||||||
| Mother's education | 240 | |||||||||
| Household GPS* | 250 | |||||||||
| Child, # | ||||||||||
| Recruit | 373 | |||||||||
| Fecal sample† | 229 | 223 | 237 | 238 | 244 | 260 | 244 | |||
| Defecation practices | 279 | |||||||||
| Hand washing behaviors | 268 | |||||||||
| Anthropometry‡ | 280–288 | |||||||||
Global positioning system (GPS) coordinates were recorded during visits 7–10.
Children that provided fecal samples in ≥ 4 sample periods were included in the Chronic Protozoan and Diarrhea Index calculations (n = 292).
Variability in anthropometry sample sizes was caused by an error in data recording (height n = 7, weight n = 4).
Spatial survey.
Geographic longitude and latitude points were recorded during visits 7–10 at households (n = 250), health facilities (n = 2), and schools (n = 10); access roads were recorded as tracks with a handheld geographic positioning system (eTrex Vista HCX; Garmin, Olathe, KS). Geographic coordinates were converted to metric coordinates by using the Universal Transverse Mercator 17N projection and analyzed in ArcGIS Map 9.3.1 (Environmental Systems Research Institute, Redlands, CA). Household geographic coordinates were used to characterize the spatial dispersion of participant homes and distances from roads, health facilities, and schools. Distances were calculated by using proximity analysis, and household density was calculated by using a point density estimate for a circular neighborhood with a 250-meter radius. Resulting density estimates for each household describe the number of other study participant homes found within this circular area around their home, expressed in homes per square kilometer. Because household density is one of the primary comparisons in the current study, analysis was restricted to households for which spatial data were available (250 households and 356 children; 153 households with 1 eligible child, 88 with 2 eligible children, and 9 with 3 eligible children).
Household wealth characterization.
The primary caregivers of participating children were interviewed in Spanish or Ngäbere at visit 5 by using field-tested wealth characterization questionnaires based on government questionnaires previously used in the area. Demographic information on household construction (dirt floor, absence of walls, solid walls), possessions (radio, cell phone, bicycle, sewing machine, stove, hoe) and access to running water and latrine was used to calculate a Household Wealth Index (HWI) according to Demographic Health Survey methods,25 which were based on principal components analysis as developed by Filmer and Pritchett.24 The HWI was calculated for each house as the sum of the possessions and housing quality scores after being multiplied by their relative weights, as determined by the first component of principal components analysis (n = 229). Households were then subdivided into quintiles and categorized as lowest (lowest 40%), middle (middle 40%) and highest (top 20%) wealth groupings, as suggested by Filmer and Pritchett.24
Anthropometry.
Weight and height/length of participating children were measured at visit 4 by trained nutritionists using Seca anthropometry scales (Seca 750; Seca, Birmingham, United Kingdom), portable stadiometers (Seca 214; Seca), and measuring mats (Seca 210; Seca). Using sex and birth date information taken from health cards, we calculated child height-for-age (n = 285), weight-for-age (n = 288), and weight-for-height Z scores (n = 280)1 from World Health Organization (WHO) growth reference standards29 by using WHO Anthro 3.1. Children were classified as stunted if their height-for-age Z score (HAZ) was ≤ –2 SD, underweight if their weight-for-age Z score (WAZ) was ≤ –2 SD, and wasted or overweight/obese if their weight-for-height Z score (WHZ) was ≤ –2 SD or ≥ 2 SD, respectively. Years of school attended by the mother was also collected at visit 4 (n = 240).
Fecal samples.
During household visits, labeled collection containers were given to each caregiver on the day before fecal sample collection with instructions on how to collect fecal samples. The following morning samples were collected from the home and transported on ice to the Parasitology Laboratory at the Hospital General del Oriente (Chiriqui, Panama) where MINSA technicians followed the MINSA standard protocol for clinical diagnosis of diarrhea (single, liquid or semi-liquid stool sample). One direct smear per sample was analyzed to record the presence or absence of the protozoan infections (Giardia spp. and/or Entamoeba histolytica/dispar). Up to seven fecal samples per child were collected during July 2008–October 2009 (Table 1). The number and timing of stool samples was defined by the design of the larger study.
Chronic protozoan and diarrhea indices
To assess risk factors for frequent protozoan infection or symptomatic diarrhea, we calculated a Chronic Protozoan Index (CPI) and a Chronic Diarrhea Index (CDI) as the number of positive stool samples divided by the total number of samples provided by each child for whom we had ≥ 4 fecal samples (n = 292). The provision of ≥ 4 fecal samples was deemed sufficient to reflect frequency of infection over the 16 months of the study. It is important to note that the CPI and CDI did not distinguish between new or persistent infections.
Water quality and behavioral risk factors.
To assess risk factors for stunting and infection, child defecation habits (n = 279) and hand washing practices (n = 268) were recorded as part of questionnaires administered to the mother during visits 2 and 3, respectively. Water samples were collected during visit 6 from the point of consumption in each household by using sealed and sterile IDEXX 100-mL collection containers. Samples were analyzed within six hours by using a Colilert/Quanti–Tray® (IDEXX, Westbrook, ME) according to manufacturer's instructions. The Colibert/Quanti–Tray uses a modified most probable number assay to estimate the concentration of Escherichia coli (colony-forming units [CFU]/100 mL) in samples from each home (n = 206). The method has a range of < 1 to 2,049 organisms/mL of water, and has been shown to produce similar results to traditional assays.29
Data analysis.
All statistical comparisons were conducted by using STATA version 11.1 (STATA Corporation, College Station, TX). In all cases, the level of significance was set at P < 0.05. Data were reported as mean ± SEM unless otherwise stated. Continuous data were compared by using Student t-tests and two-way analysis of variance for normally distributed variables (e.g., HAZ, WAZ) or non-parametric Mann-Whitney and Kruskal-Wallis tests for non-normally distributed variables (e.g., water quality, CPI, CDI, HWI, maternal education, distances, age). In the case of multiple comparisons, if initial test results were significant, Tukey or Mann-Whitney tests were conducted as appropriate. Binomial 95% confidence limits for prevalence data were determined by using the Agresti–Coull calculation, and comparisons were considered significant where confidence limits did not overlap. To examine the association of child health outcomes with individual and household traits, regression models were conducted on the total child sample and on a randomly selected index child from each household. To determine risk factors for chronic malnutrition, stepwise multiple regression analyses were conducted with HAZ as the dependent variable. The final model included independent variables with P < 0.10.
Of the 285 children for whom HAZ data were available (202 index children), a complete set of data for regression analysis was available for 213 persons (156 index children). Poisson models determined the risk factors for chronicity of diarrhetic stool and protozoan infection in which the dependent variable was the CPI or CDI score multiplied by100 to satisfy the count criteria. Because of the large number of observed zeros in the CPI, zero-inflated Poisson models were used to distinguish between factors that influenced presence or absence of protozoan infections (logistic portion) from those that determined the degree of chronicity of infection (Poisson portion). The final model was chosen based on the lowest Akaike's Information Criteria value. Of the 292 children for whom CPI/CDI indices could be calculated (216 index children), a complete set of data for regression analysis was available for 204 persons (149 index children). Our power calculations indicated that we required a sample size of 113 to detect an effect size of 0.15 in regression models with 9 independent variables (β = 0.80, α = 0.05).
Results
Household and child variables: overview and comparisons between political regions.
Overall, mothers had completed 3.8 ± 0.2 years of education. Most (91%) houses had dirt floors and 82% had walls. Latrines were found in 31% of households, but only 11% of children reportedly used them. Instead, most defecation occurred in nearby woods and streams (45%) and in the yard (28%). Piped water was available through aqueducts to only 35% of homes and 65% obtained their water from natural sources that included small streams or springs. Escherichia coli was found in 98% of the household water samples, with a mean ± SEM of 230 ± 23 CFU/100 mL (range = 0–1,012 CFU/100 mL). Hand washing with soap before eating was reported for 27% of children. The HWI ranged from –0.82 to 2.26 (mean ± SEM = 0.21 ± 0.04). Households from the lowest wealth category were characterized by the absence of an aqueduct, latrine, and stove, and those with the highest scores typically had a radio, a latrine, and an aqueduct, and many had a cell phone, a sewing machine, and a stove (Table 2). Households in Soloy were generally better off than those in Emplanada de Chorcha (a mean ± SEM HWI of 0.41 ± 0.06 compared with –0.12 ± 0.04 = (Table 3). These households were closer to the road, health facility, and the school, they had greater access to an aqueduct and a latrine, and mothers had nearly two years more education than those in Emplanada de Chorcha (Table 3).
Table 2.
Household variables included in the Household Wealth Index (HWI), their respective scoring factors, and the percentage of households in each wealth category owning items, western Panamá
| Variable | Scoring factor* | Lowest (lower 40%) | Middle (middle 40%) | Highest (upper 20%) |
|---|---|---|---|---|
| Aqueduct | 0.3911 | 2% | 41% | 90% |
| Latrine | 0.4114 | 2% | 29% | 92% |
| Radio | 0.1595 | 71% | 82% | 98% |
| Cell phone | 0.2948 | 2% | 7% | 42% |
| Bicycle | 0.1358 | 1% | 2% | 6% |
| Hoe | −0.1156 | 38% | 50% | 17% |
| Sewing machine | 0.1802 | 38% | 59% | 71% |
| Stove | 0.3888 | 0% | 7% | 43% |
| No walls | −0.2779 | 40% | 4% | 0% |
| Solid walls | 0.298 | 5% | 59% | 71% |
| Dirt floor | −0.429 | 100% | 98% | 54% |
| HWI | ||||
| Mean (SE) | −0.36 (0.02) | 0.25 (0.02) | 1.27 (0.18) | |
| Range | −0.82 to −0.089 | −0.087 to 0.67 | 0.69–2.26 |
Scoring factors were determined by using the variable coefficients from the first component of a Principle Component Analysis that explained 26% of the variability in the 11 variables.
Table 3.
Summary of household and child characteristics and health outcomes according to political region and household density category, western Panamá*
| Characteristic | Emplanada de Chorcha† | Soloy† | P | Dispersed households (< 50 houses/km2) | Dense households (> 50 houses/km2) | P |
|---|---|---|---|---|---|---|
| Household | ||||||
| Distance to road, km | 2.4 (0.2) 96 | 0.9 (0.09) 154 | < 0.001 | 1.9 (0.1) 181 | 0.2 (0.02) 69 | < 0.001 |
| Distance to health facility, km | 2.9 (0.2) 96 | 2.0 (0.1) 154 | < 0.001 | 2.8 (0.1) 181 | 1.1 (0.07) 69 | < 0.001 |
| Distance to school, km | 1.1 (0.06) 96 | 0.6 (0.04) 154 | < 0.001 | 0.9 (0.04) 181 | 0.4 (0.02) 69 | < 0.001 |
| Household density,‡ homes/km2 | 14.5 (1.3) 96 | 48.1 (3.0) 154 | < 0.001 | NA | NA | |
| Households in Soloy, % | NA | NA | 48 (40–55) 181 | 100 (94–100) 69 | < 0.001 | |
| Household Wealth Index§ | −0.12 (0.04) 90 | 0.41 (0.06) 139 | < 0.001 | 0.097 (0.05) 172 | 0.51 (0.09) 57 | < 0.001 |
| Maternal education, years | 2.7 (0.3) 94 | 4.6 (0.3) 146 | < 0.001 | 3.6 (0.3) 176 | 4.6 (0.5) 64 | 0.05 |
| Latrine, % | 15 (9–24) 93 | 41 (34–49) 142 | < 0.001 | 26 (20–33) 175 | 45 (33–58) 60 | < 0.001 |
| Aqueduct, % | 12 (7–20) 94 | 49 (42–57) 146 | < 0.001 | 27 (21–34) 176 | 52 (40–63) 64 | < 0.001 |
| Water quality, Escherichia coli CFU/100 mL | 270 (39) 83 | 184 (28) 123 | 0.002 | 243 (28) 152 | 151 (38) 54 | 0.004 |
| Child | ||||||
| Age, months | 26.3 (1.4) 139 | 25 (1.1) 217 | 0.5 | 25.5 (1.0) 268 | 25.7 (1.6) 88 | 0.93 |
| Female, % | 47 (39–55) 139 | 50 (44–57) 217 | 0.6 | 48 (42–54) 268 | 51 (41–61) 88 | 0.58 |
| Protozoan infection, CPI¶ | 0.24 (0.02) 122 | 0.31 (0.02) 170 | 0.005 | 0.25 (0.02) 228 | 0.37 (0.03) 64 | < 0.001 |
| Diarrhetic stool, CDI# | 0.3 (0.02) 122 | 0.3 (0.02) 170 | 0.92 | 0.30 (0.02) 228 | 0.30 (0.03) 64 | 0.69 |
| HAZ | ||||||
| Stunting (< −2 SD), % | 61 (53–70) 122 | 60 (52–67) 163 | 0.74 | 58 (51–64) 225 | 70 (57–80) 60 | 0.09 |
| Mean Z score | −2.33 (0.12) 122 | −2.18 (0.1) 163 | 0.33 | −2.16 (0.08) 225 | −2.56 (0.18) 60 | 0.03 |
| WAZ | ||||||
| Underweight (< −2 SD), % | 22 (16–31) 125 | 22 (16–29) 163 | 0.95 | 20 (16–26) 225 | 29 (19–41) 63 | 0.17 |
| Mean Z score | −0.94 (0.13) 125 | −0.99 (0.1) 163 | 0.74 | −0.87 (0.09) 225 | −1.3 (0.2) 63 | 0.03 |
| WHZ | ||||||
| Wasting (< –2 SD), % | 7 (3–13) 121 | 4 (2–9) 159 | 0.4 | 6 (3–10) 221 | 3 (0–12) 59 | 0.45 |
| Overweight (> 2 SD), % | 17 (12–25) 121 | 7 (4–12) 159 | 0.007 | 14 (10–19) 221 | 3 (0–12) 59 | 0.03 |
Values are mean or % (± SE binomial 95% confidence interval). Sample size is indicated after the summary statistics. NA = not available; CFU = colony-forming units; CPI, Chronic Protozoan Index; CDI = Chronic Diarrhea Index; HAZ = height-for-age Z score; WAZ = weight-for age Z score; WHZ = weight-for-height Z score.
Summary data presented for Emplanada de Chorcha and Soloy are restricted to households for which spatial data were available.
Density based on households participating in the study.
Asset-based index weights were derived from the first component of Principle Components Analysis.
Proportion of positive samples provided (for children who provided ≥ 4 of 7 possible sampling periods).
Proportion of positive samples provided (for children who provided ≥ 4 of 7 possible sampling periods).
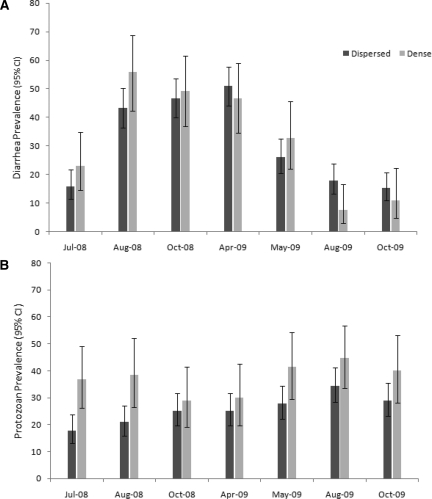
The population of preschool children in this study was 25.5 ± 0.8 months old and 49% were female. A high percentage (60%) of the preschool children was stunted, 5% were wasted, 22% were underweight, and interestingly, 11% were overweight. The prevalence or percentage of children with diarrhetic stool samples was high (45%) in samples collected during August 2008–April 2009, but low in July 2008 and August and October 2009 (Figure 2A). In contrast, the prevalence of protozoan infections at each of the seven sample periods was relatively consistent (22–37%); Giardia spp. in 18–34% of the children and E. histolytica/dispar in 2–5% of samples (Figure 2B). Comparisons between the political regions of Soloy and Emplanada de Chorcha showed no difference in age, sex ratios, stunting, underweight, and wasting or chronicity of diarrhetic stools. However, children from Soloy had higher CPIs and lower frequency of being overweight compared with those in Emplanada de Chorcha (Table 3).
Figure 2.
Prevalence of A, symptomatic diarrhea and B, protozoan infection over the duration of the study, western Panamá. Prevalence with 95% confidence intervals are presented for each sample period for children from dispersed (dark gray) and dense (light gray) households. Household density was classified as dispersed if < 50 houses/km2 (n = 178–200 children) or dense if > 50 houses/km2 (n = 45–60 children).
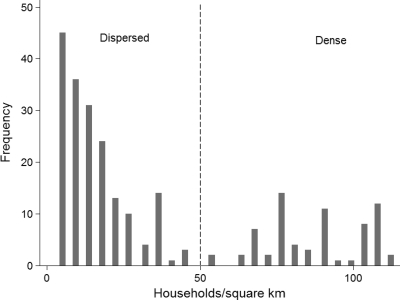
The study area was accessible through two dirt roads (Figure 1); the western road had limited access during the rainy season (July–November). The mean ± SEM direct line distance between homes and the nearest access road was 1.5 ± 0.1 km, the distance to the nearest health center was 2.3 ± 0.1 km, and the distance to the nearest elementary school was 0.8 ± 0.04 km. Household density varied considerably through the study area, and households were characterized as being dispersed or dense on the basis of a natural division in the frequency distribution (Figure 3); at 50 households/km2, 72% of the homes were considered to be dispersed and 28% were considered to be dense. Homes in Emplanada de Chorcha were more spatially dispersed than those in Soloy. Of the dispersed households, 48% were in Soloy, but Soloy also had 100% of the dense households (Figure 1 and Table 3).
Figure 3.
Frequency distribution of density of participating households, western Panamá. Household density was determined using ArcGIS point density estimates with a radius of 250 m around each home (n = 250, mean ± SD = 35.2 ± 2.2 houses/km2, minimum = 4.5, and maximum = 128 houses/km2). Households were classified as dispersed (n = 181) or dense (n = 69) according to the natural break in the frequency distribution at 50 houses/km2.
Spatial comparison of household characteristics.
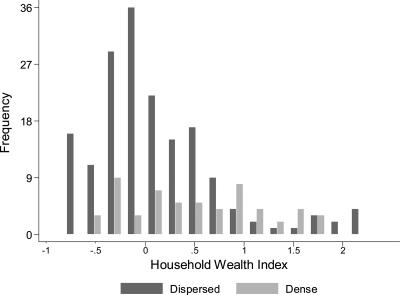
To address the first of our major objectives, we compared the biophysical/demographic conditions between the dispersed and dense households. A binary comparison confirmed that dispersed homes were approximately three times farther from health facilities and schools than dense homes, and 10 times farther from access roads (Table 3). Mothers in the dispersed households also had fewer years of education (P = 0.05). Household access to sanitation and hygiene infrastructure was greater in the dense households in which twice as many households had a latrine and aqueduct, and where water quality was also significantly better compared with dispersed households (Table 3). The HWI had a similar range in the dense and dispersed households (Figure 4). However, the average value was five times higher in dense households (Table 3).
Figure 4.
Frequency distribution of Household Wealth Index (HWI) by household density group, western Panamá. The HWI is an asset-based index derived from measures of household construction and ownership of durable goods. Household density was classified as dispersed if < 50 houses/km2 (n = 172 households) or dense if > 50 houses/km2 (n = 57 households).
Child health comparisons.
Anthropometry
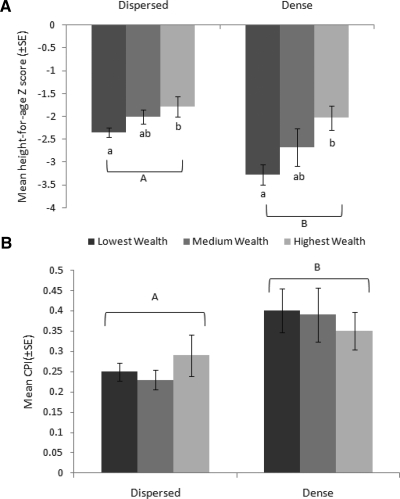
The prevalence of stunting and underweight was the same in children from dense and dispersed households. However, the frequency of being overweight was higher in the dispersed households, in which 14% of children had a WHZ score > 2 SD, compared with 3% in the dense households (Table 3). Despite better household access to physical infrastructure and greater household wealth, child mean WAZ was lower in the dense households. Furthermore, two-way analysis of variance confirmed that HAZ was significantly lower in children from dense households compared with those from dispersed households (F1,162 = 7.71, P = 0.006) and in children from the lowest wealth groupings compared with those from the highest wealth groupings (F2,162 = 18.49, P < 0.001); there was no interaction between these two factors (F2,162 = 2.69, P = 0.10) (Figure 5A). Risk factors associated with low HAZ were being older, living in a household with a lower HWI, and having chronic protozoan infections (Table 4). Household density entered the model, but only with P = 0.08. A virtually identical regression model was obtained on the basis of a single index child per household (Table 4).
Figure 5.
Mean child health outcomes by household density group and wealth percentile, western Panamá. Child mean ± SE values for A, height-for-age Z-score (n from left = 99, 93, 25, 16, 18, 23) and B, chronicity of protozoan infection (CPI) (n from left = 107, 87, 28, 14, 19, 26) in the dispersed (< 50 houses/km2) and dense households (> 50 houses/km2). The Household Wealth Index (HWI), an asset-based index derived from measures of household construction and ownership of durable goods, was used to define wealth groups (bottom = 40%, middle = 40%, and top = 20%) and is represented by dark to light gray bars. Unique letters indicate significantly different groups (P < 0.05) determined by two-way analysis of variance and A, post hoc Tukey test or B, Mann-Whitney non-parametric test.
Table 4.
Predictors of height-for-age Z score in preschool children in western Panamá from a stepwise multiple regression model*
| Characteristic | Total sample | Index child | ||||
|---|---|---|---|---|---|---|
| Raw coefficient | Standardized coefficient | P | Raw coefficient | Standardized coefficient | P | |
| Age, months | −0.03 | −0.03 | < 0.001 | −0.04 | −0.03 | < 0.001 |
| Water quality, Escherichia CFU/100 mL | NE† | – | – | NE | – | – |
| Maternal education, years | NE | – | – | NE | – | – |
| Household Wealth Index‡ | 0.40 | 0.21 | 0.01 | 0.29 | 0.15 | 0.02 |
| Wash with soap§ | NE | – | – | NE | – | – |
| Yard defecation¶ | NE | – | – | NE | – | – |
| Protozoan infection, CPI# | −1.06 | −0.20 | 0.002 | −1.41 | −0.25 | < 0.001 |
| Diarrhetic stool, CDI** | NE | – | – | NE | – | – |
| Household density, homes/km2 | −0.005 | −0.11 | 0.08 | NE | – | – |
| Intercept | −1.08 | – | < 0.001 | −0.90 | – | < 0.001 |
| Adjusted R2 | 0.26 | 0.33 | ||||
| F4,208 | 19.50 | F3,152 | 26.44 | |||
| p > F | < 0.001 | <0.001 | ||||
| No. | 213 | 156 | ||||
Height-for-age Z score HAZ available: n = 285 children (202 index children), complete data set available: n = 213 (156 index children).CFU = colony-forming units; CPI = Chronic Protozoan Index; CDI = Chronic Diarrhea Index.
Excluded during stepwise process if P > 0.10.
Asset-based index of household wealth weights were derived from the first component of Principle Components Analysis.
Child washes hands with soap before eating (0 = no, 1 = yes), as reported by the mother.
Child defecation occurs primarily in the yard around the home (0 = no, 1 = yes), as reported by the mother.
Proportion of positive samples provided (for children who provided ≥ 4 of 7 possible sampling periods).
Proportion of positive samples provided (for children who provided ≥ 4 of 7 possible sampling periods).
Protozoan infection
The sample-specific prevalence of protozoan infections was higher in children from dense households than those from dispersed households in July 2008 but not in subsequent periods (Figure 2). However, chronicity of protozoan infections was higher in children living in dense households, in which the mean ± SEM CPI was 0.37 ± 0.03 compared with 0.25 ± 0.02 in the dispersed households (Table 3). There was no effect of wealth category on chronicity of protozoan infections (χ2 adjusted for ties = 2.51, degrees of freedom = 2, P = 0.28) (Figure 5B). The logistic portion of the zero-inflated Poisson model identified that risk factors for being infected with protozoans were being younger, having worse household water quality, and defecating in the yard. The associations of age and household density with being infected with protozoans were further supported by the index child model (Table 5). Predictors of chronicity of protozoan infections (Poisson portion of model) were the same for the total sample model and the index child model (Table 5). Chronicity of protozoan infection was associated with being older, not washing with soap before eating, being from a household with a lower HWI, poorer water quality (higher E. coli counts) or from a household with a greater surrounding population density. Surprisingly, having a mother with more years of education also emerged as a predictor of chronicity of protozoan infections. Results of analysis based on an index child per household were virtually identical to those of the total sample model.
Table 5.
Predictors of chronicity of protozoan infection in preschool children in Panamá based on a zero-inflated Poisson regression model*
| Characteristic | Total sample† | Index child‡ | ||
|---|---|---|---|---|
| Logistic portion§ | Coefficient | P | Coefficient | P |
| Age, months | −0.04 | 0.002 | −0.04 | 0.01 |
| Mother's education, years | NE¶ | – | NE | – |
| Wash with soap# | NE | – | NE | – |
| Water quality, CFU Escherichia coli/100 mL | −0.002 | 0.009 | NE | – |
| Household Wealth Index** | NE | – | NE | – |
| Yard defecation†† | −0.85 | 0.046 | −0.86 | 0.07 |
| Household density, homes/km2 | −0.01 | 0.06 | −0.02 | 0.05 |
| Intercept | 0.94 | 0.04 | 0.73 | 0.15 |
| Poisson portion‡‡ | ||||
| Age, months | 0.005 | < 0.001 | 0.007 | < 0.001 |
| Wash with soap | −0.088 | 0.003 | −0.21 | < 0.001 |
| Household Wealth Index | −0.06 | 0.01 | −0.10 | < 0.001 |
| Water quality, CFU E. coli/100 mL | 0.0001 | 0.008 | 0.0002 | < 0.001 |
| Household density, homes/km2 | 0.002 | < 0.001 | 0.003 | < 0.001 |
| Mother's education, years | 0.03 | < 0.001 | 0.03 | < 0.001 |
| Yard defecation | NE | – | NE | – |
| Intercept | 3.31 | < 0.001 | 3.23 | < 0.001 |
Chronicty of protozoan infection available: n = 292 children (216 index children), complete data set available: n = 204 (149 index children). CFU = colony-forming unit.
Total Sample Model Statistics: likelihood ratio χ2 = 105.46, P < 0.001, n = 204, zero observations = 55, Vuong statistic of zero-inflated Poisson vs. Poisson z = 10.94, p > z < 0.001.
Index Child Model Statistics: likelihood ratio χ2 = 141.51, P < 0.001, N = 149, zero observations = 43, Vuong statistic of zero-inflated Poisson vs. Poisson z = 9.42, P < 0.001.
Logistic portion of the model identifies variables associated with likelihood of being uninfected where positive coefficients indicate a greater likelihood of being uninfected.
Excluded during stepwise process if P > 0.10.
Child washes hands with soap before eating (0 = no, 1 = yes) as reported by the mother.
Asset-based index of household wealth derived using Principle Components Analysis.
Child defecates in the yard around the home (0 = no, 1 = yes) as reported by the mother.
Poisson Portion of the model determines variables associated with chronicity of protozoan infection.
Diarrhea
Prevalence of diarrhea at individual sampling points (Figure 2) or chronicity of diarrhea (Table 3) did not differ between dense and dispersed households; mean CDI = 0.30 in children from dense and dispersed households. Mean CDI was also similar for children from households in the lowest, medium, and highest wealth categories (χ2 = 0.57, P = 0.75). Several risk factors for chronicity of diarrhea were similar to those for protozoan infections. Specifically, greater chronicity of child diarrhea was associated with defecating in the yard and not washing with soap before eating (P = 0.05) (Table 6). Younger children had higher CDIs. In addition, the household factors related to chronic diarrhea were having poor water quality, having a lower HWI, and having a mother with more years of schooling. Household density did not enter the model (Table 6). Index child models supported the association of sanitation and hygiene behaviors with chronic diarrhea, but age, HWI, or maternal education did not enter as significant (Table 6).
Table 6.
Predictors of chronicity of diarrhetic stool in preschool children in western Panamá from a stepwise Poisson regression model*
| Characteristic | Total sample | Index child | ||||
|---|---|---|---|---|---|---|
| Raw coefficient | Standardized coefficient | P | Raw coefficient | Standardized coefficient | P | |
| Age, months | –0.008 | 0.99 | < 0.001 | −0.002 | 0.97 | 0.08 |
| Water quality, Escherichia coli CFU/100 mL | 0.0003 | 1.10 | < 0.001 | 0.00026 | 1.09 | < 0.001 |
| Maternal education, years | 0.01 | 1.01 | 0.01 | −0.008 | 0.97 | 0.07 |
| Household Wealth Index† | −0.10 | 0.90 | < 0.001 | NE | – | – |
| Wash with soap‡ | −0.06 | 0.97 | 0.05 | −0.11 | 0.95 | 0.002 |
| Yard defecation§ | 0.08 | 1.04 | 0.005 | 0.21 | 1.10 | < 0.001 |
| Household density, homes/km2 | NE¶ | – | – | NE | – | – |
| Intercept | 3.51 | – | < 0.001 | 3.30 | – | < 0.001 |
| Likelihood ratio χ2 | 197.13 | 92.82 | ||||
| P | < 0.001 | < 0.001 | ||||
| No. | 204 | 149 | ||||
Chronic Diarrhea Index available: n = 292 children (216 index children), complete data set available: n = 204 (149 index children). CFU = colony-forming units.
Asset based index weights were derived from the first component of Principle Components Analysis.
Child washes hands with soap before eating (0 = no, 1 = yes), as reported by the mother.
Child defecation occurs primarily in the yard around the home (0 = no, 1 = yes), as reported by the mother.
Excluded during stepwise process if P > 0.10.
Discussion
Our study provided an in-depth analysis of stunting, intestinal protozoan infection, and diarrhea in a rural indigenous area of Panamá. We have confirmed that in this area of extreme poverty, households from more densely populated areas had better access to health facilities, sanitation, and water infrastructure, less fecal contamination of drinking water, and greater, albeit still low, household wealth as measured by possession of durable goods such as a stove, a cell phone, a latrine, and aqueduct access. In contrast to our hypothesis, the better socioeconomic status of homes from more densely populated areas did not always correspond with better health outcomes. Rather, children from these households had a greater severity of stunting and more frequent protozoan infections over a 16-month period than children from more dispersed households. Second, we showed that within these spatially defined groups, child stunting was greater in the lowest wealth households than in the highest wealth households, but that chronicity of protozoan infections was equal across wealth groups. Regression models supported the link between infections and anthropometry outcomes by showing that chronic protozoan infections were a risk factor for low HAZ. The model for protozoan infections highlighted the role of greater household density, few assets, poor water quality, and poor hygiene practices in increasing the risk of protozoan infections. Chronicity of child diarrhea was also associated with poor sanitation and hygiene practices and poor water quality, but not with household density.
The indigenous population of Panamá has disproportionately high levels of poverty characterized by lower access to physical infrastructure and worse health outcomes than their non-indigenous rural counterparts. According to the 2008 Niveles de Vida report,28 96.3% of the rural indigenous population lived in poverty compared with 50.7% of rural non-indigenous population and 18% of the urban population. These impoverished living conditions are accompanied by lower access to physical infrastructure; less than half of the indigenous population are reported to have access to an improved water supply and sanitation infrastructure.31 Child anthropometry outcomes in our study region were well below national averages and similar to rural indigenous statistics for Panamá.31 At the country level, 18% of preschool children were reported as chronically malnourished (HAZ < –2 SD)32 a figure that masks the fact that 56.6% of rural indigenous children are short for their age.26 In our study, 60% of children were stunted, which is similar to the prevalence of stunting (61%) reported in a nearby region within the Comarca.27 The previously reported prevalence of low WAZ nationally was 6% but as with HAZ, it was significantly higher in indigenous populations in which nearly 25% of preschool children were considered underweight,26 similar to the prevalence recorded in our study. The low level of wasting compared with the high prevalence of stunting observed in this population is a known pattern in Latin America.33 Stunting is often seen as an epidemiologic indicator of the accumulated, long-term effects of not only poor diet but also repeated infections.21,22 Thus, in this population, the primary nutritional problem is not acute weight loss but rather chronic restriction of the potential growth of a child.
Recently, it has been suggested that use of political regions as the basis for understanding health risks may not be as appropriate as contextual characteristics that more directly impact child health outcomes.34 By considering the spatial dispersion of households, we were able to identify two groups with distinct socioeconomic and physical infrastructure characteristics. Specifically, homes in the more densely populated areas were closer to a road, a health center, and a school. They also had a more complex set of durable goods including radios, cell phones, sewing machines, and stoves, and were more likely to have a latrine and aqueduct. However, despite the presence of aqueducts in more than 50% of homes in the dense areas, most household water samples were not potable. Escherichia coli counts in dense households, although lower than those in dispersed households, were 100 times higher than the WHO recommendation that E. coli should be absent from drinking water.35 This paradox is likely caused by the inadequate coverage and quality of the infrastructure in the more densely populated areas where less than half of the homes had access to latrines and most children did not use them. These numbers are well below the recommendation of 75% of community coverage considered to maximize community health.36 Low coverage and poor quality of sanitation infrastructure has been linked to higher prevalence of parasitic infections and diarrhea.19 Thus, it is believed that although homes in the more densely populated areas have better access to physical infrastructure, it was insufficient to meet the health needs of the population.
Despite the extreme poverty across our study area, characterizing household wealth was important for understanding the risk factors related to child health in the comarca Ngäbe–Buglé because of changes in economic flows associated with physical infrastructure in rural areas.3,4 Using our HWI, which was driven primarily by presence or absence of a latrine, an aqueduct, a stove, and a dirt floor, we were able to show that dense households had a greater number of assets than dispersed households. We also showed that many of the dense households were similar in HWI to those in dispersed households. Recently asset-based indices as a measure of wealth have been shown to not only be a more reliable index of wealth under conditions of extreme poverty, but also to be related to health outcomes, including improved nutritional status37,38 and reduced parasitic infections,39,40 an observation consistent with our findings. In rural areas where income is inconsistent, asset-based indices are considered a valuable proxy for relative standard of living, which was of particular interest in our study area where wage work is extremely rare and variables such as number of wage earners or income would not capture household financial status. Importantly, because of regional and cultural variability in the range of possessions, the resultant index is location specific.23,41
To ensure the construction of a regionally appropriate index, we used questionnaires previously designed to record possessions for the comarca Ngäbe–Buglé, and we also field tested the questionnaire before beginning the study. Our resultant HWI using data on 11 possession and household construction variables explained 26% of the variability among households in ownership of these items, similar to a study in rural Africa where 12 variables accounted for 24% of the population variance.39 The regional specificity in indices is highlighted by a recent study in Peru that used 36 variables to explain a similar degree of variance in the possession ownership (23%).40 This finding is likely caused by the urban location of the study where items such as televisions, blenders, and furniture were required to distinguish between households.
We found that the severity of stunting was higher in children from dense households and in those with lower HWIs. The finding that older children were shorter for their age is consistent with those of other studies21,41 and supports the hypothesis that low HAZ is believed to be caused by the cumulative effect of undernutrition42,43 for children living in deprived conditions. Furthermore, identification of chronic protozoan infections (primarily Giardia spp.) as a risk factor for low HAZ highlighted the connection between stunting and infection. Giardia spp. have been reported to have adverse affects on linear growth44,45 and possible consequences for psychomotor development.46 We therefore suggest that the greater chronicity of protozoan infection in children from dense homes is an important cause of the more severe stunting observed in these children.
Our concept of chronic protozoan infection and chronic diarrhea was based on the idea that repeated or sustained bouts of infection or diarrhea over an extended period would affect child development and could result from repeated pathogen exposure or differential ability to access treatment. Thus, the CPI and CDI were created as indicators of the frequency of positive samples for protozoan infection or diarrhea over the 16-month study period and were considered to represent potential chronic pathogenicity. Our indices differ from the operational research definitions for chronic and persistent diarrhea47 in that we did not measure the duration of diarrheal episodes or protozoan infection. Thus, although our indices cannot differentiate between new and sustained illness or classify illness as acute (abrupt onset and resolved ≤ 14 days), chronic (> 14 days caused by congenital defects in absorption), or persistent (> 14 days caused by infection or malnutrition),47 they provide a long-term estimate of the frequency of illness for each child. The fact that the CPI was a predictor of stunting is of particular interest, given that the diagnostic methods used by MINSA have a lower sensitivity than other research protocols and may have led to an underestimation of child infection burden. If the lower sensitivity of our analysis resulted in detection of only more severe infections, then the association of CPI with stunting may also be influenced by the severity of protozoan infection, which would be of interest to examine in future studies. Furthermore, the similarity between model results for chronic diarrhea and protozoan infection is encouraging for the non-diagnostic definition of diarrhea used in this study.
Using these indices, we found that protozoan infection and diarrhea were more chronic among children of more educated mothers, a somewhat unexpected finding, given that child health typically improves with increasing years of maternal education.48,49 In our study area, the more educated women were younger, less experienced mothers (personal observation). In addition to maternal education, sex of the head of household may also influence child health outcomes,50 likely because of lower access to financial resources.51 Because our study was conducted within the context of a CT program where transfers were given to the most senior woman of each household, this variable was not included in our analysis. The risk of chronic diarrhea was also higher in younger children, an observation consistent with a longitudinal study in Brazil that recorded a peak burden of diarrhea in children 7–12 months of age after which the number of episodes/year decreased.52 In contrast to diarrhea, we found that younger children were more likely to never have had a protozoan infection during the study period. This finding may have been caused by the fact that exclusively breastfed children had a lower exposure to food or water-borne pathogens or greater protection through breast milk. However, because the frequency of exclusively breastfed children was low in our study population (personal observation), we did not include it in our analysis. In Peru, children more than two years of age were more likely to be infected with protozoans,53 whereas no relationship was detected between age and persistent Giardia spp. infections (lasting ≥ 14 days) in studies in Brazil of urban preschool children54 or peri-urban children < 1–12 years of age (mean age = 3.4 years).40
Chronic infection and diarrhea were also higher in children with poor hygiene and from households with low water quality. Yard defecation, which was practiced by nearly one-third of children, increased the risk of chronic diarrhea and the likelihood of having had a protozoan infection over the 16-month study period. Open defecation is a recognized source of fecal contamination, especially among young children.55 Although it has been identified as a risk factor for gastrointestinal infection,56 it is often overlooked in sanitation research.57 Our study also supported previous findings that hand washing before eating reduces soil-transmitted infections in children,58,59 especially when soap is used.60 Furthermore, poor household water quality (measured by E. coli counts in household water samples) was identified as a risk factor for chronicity of protozoan infection and diarrhea. These results were not surprising given that fecal coliforms were present in water samples from virtually all households, and that pathogens causing protozoan infections and diarrhea can be spread through contaminated drinking water35 and prevented through drinking water quality interventions.61
Finally, we detected interactions between household density and wealth with regard to chronic protozoan infection and chronic diarrhea. Population density is an extremely important determinant of risk of transmission of pathogens.14,15 Enteric infections have been associated with the increased living densities in urban areas20 or crowding within a household.16,17 However, to our knowledge, only one other study has investigated this relationship in rural communities. A study in rural Ecuador found that diarrheal disease prevalence was higher in more densely populated communities and also highlighted the importance of social connections in reducing the risk of diarrhea.18 In our study, chronicity of diarrhea was independent of household density, which may have been caused in part by low sensitivity of our binary clinical diagnosis of diarrhea or non-infectious causes of diarrhea such as food-borne pathogens. In addition to household density, we also examined the influence of household crowding on chronic infection status. In contrast to the household density measure, the number of persons per room was not significantly related to CPI or CDI by univariate or multivariate analyses.
Our data show that the negative impact of household density on chronic protozoan infection is mitigated by household wealth. Densely spaced households were more likely to have a latrine and aqueduct and also to have a more complex set of durable goods, including stoves. Although none of the households had potable water, the level of fecal contamination was considerably lower in households with aqueducts than in those without aqueducts, which suggested a lower pathogen exposure and helped to explain the lower chronicity of protozoan infection and diarrhea in households with higher HWIs. High values of HWI were most strongly influenced not only by presence of a latrine and an aqueduct, but also by ownership of a stove. Perhaps households with a stove are more likely to boil drinking water, thus reducing exposure to water-borne pathogens. In contrast, one of the strongest negative weighting scores in the HWI was the presence of dirt floors, again a factor that would increase the likelihood of transmission of soil-borne pathogens. Our results complement those of a recent study40 that examined the association between Giardia spp. and an asset-based household wealth index in a peri-urban region of Peru. Despite the greater affluence in the population in Peru than in our rural region of extreme poverty, and despite their measure of short-term Giardia spp. infection persisting for more than 14 days compared with our measure of chronic infection over 16 months, the study in Peru also demonstrated that Giardia spp. were more common in households with a lower HWI. Thus, in the dense households in which protozoan infection is greater, household wealth plays an important role in reducing chronicity of infection and consequently improving child growth.
Our use of non-validated indices of chronic protozoan infection and diarrhea differs from the duration and frequency of symptomatic episodes in child health literature. However, our easily compiled indices based on MINSA field diagnostic procedures proved valuable in assessing the developmental impact of the frequency of illness over a 16-month period. Further limitations of our study related to the inclusion criteria in the recruitment process. Specifically, a selection bias may have been introduced by working exclusively with households that were part of the CT program. However, it is believed that the study population is representative of the region because more than 90% of households were eligible to receive the CT program in the study area. Unfortunately, we were not able to compare our sample population to the overall population because the larger census, used to identify eligible households, did not record information from those without preschool children or who had not participated in the CT program. Furthermore, the inclusion of multiple children per household could have inflated the influence of household level variables in our statistical models. However, our total sample regression models were supported by the virtually identical results obtained from the index child models. Finally, our estimates of household density were limited to participant households, thus reflecting the density only of households with children less than five years of age. Although this factor led to an underestimation of the actual density of households, we believe it is of specific health relevance because the proximity of preschool children has implications for child-to-child transmission. On the basis of our intriguing results, we suggest that further research on interactions between household assets and household spacing be conducted with regard to stunting and protozoan infection in preschool children to determine whether our findings can be generalized to other regions of extreme poverty.
Our investigation in the comarca Ngäbe–Buglé in western Panama has highlighted the link between chronic infection and child growth and furthermore has demonstrated the importance of household density and asset-based household wealth for child growth in an impoverished, rural area. Despite better access to physical infrastructure, children from more spatially dense households had a greater chronicity of protozoan infection, which was associated with stunting. Thus, it is believed that the physical infrastructure available to dense households was insufficient to meet the health needs of the population. Finally, we showed that greater chronicity of infection associated with increased population density was mitigated by higher household wealth, which suggested that health interventions are most urgent for children from the poorest households in the more densely populated regions.
ACKNOWLEDGMENTS
We thank our collaborators in the Panamanian Ministry of Health and the Instituto ConmemorativoGorgas de Estudios de la Salud for logistical support and guidance during field work, and community interviewers and study participants for their contributions to the study.
Footnotes
Financial support: This study was supported by the Canadian International Development Research Centre, the Natural Science and Engineering Research Council of Canada, and the Secretaría Nacional de Ciencia, Tecnología e Innovación of Panamá. Research at the Institute of Parasitology is supported by a regroupement stratégique from the Fonds Québecois pour la Recherche sur la Nature et les Technologies.
Authors’ addresses: Carli M. Halpenny and Marilyn E. Scott, Institute of Parasitology and McGill School of Environment, Macdonald Campus of McGill University, Ste-Anne de Bellevue, Quebec, Canada, E-mails: carli.halpenny@mail.mcgill.ca and marilyn.scott@mcgill.ca. Kristine G. Koski, School of Dietetics and Human Nutrition, Macdonald Campus of McGill University, Ste-Anne de Bellevue, Quebec, Canada, E-mail: kris.koski@mcgill.ca. Victoria E. Valdés, Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad de Panamá, Panamá, City, Panamá, E-mail: victoriavalds@gmail.com.
Variability in anthropometry sample sizes was caused by an error in data recording (height n = 7, weight n = 4).
References
- 1.Nations United. Rethinking Poverty: Report on the World Social Situation 2010. Geneva: United Nations; 2009. [Google Scholar]
- 2.Fiszbein A, Schady N. Conditional Cash Transfers: Reducing Present and Future Poverty. Washington, DC: World Bank; 2009. [Google Scholar]
- 3.Khandker SR, Bakht Z, Koolwal GB. The poverty impact of rural roads: evidence from Bangladesh. Econ Dev Cult Change. 2009;57:685–722. [Google Scholar]
- 4.Reardon T, Berdegué J, Escobar G. Rural nonfarm employment and incomes in Latin America: overview and policy implications. World Dev. 2001;29:395–409. [Google Scholar]
- 5.Escobal J, Ponce C. The Benefits of Rural Roads: Enhancing Income Opportunities for the Rural Poor. Lima, Peru: Grupo de Análisis para el Desarrollo (GRADE); 2004. [Google Scholar]
- 6.Brenneman A, Kerf M. Infrastructure and Poverty Linkages: A Literature Review. Washington, DC: The World Bank; 2002. [Google Scholar]
- 7.Ombok M, Adazu K, Odhiambo F, Bayoh N, Kiriinya R, Slutsker L, Hamel MJ, Williamson J, Hightower A, Laserson KF, Feikin DR. Geospatial distribution and determinants of child mortality in rural western Kenya 2002–2005. Trop Med Int Health. 2010;15:423–433. doi: 10.1111/j.1365-3156.2010.02467.x. [DOI] [PubMed] [Google Scholar]
- 8.Denno D. Global child health. Pediatr Rev. 2011;32:e25–e38. doi: 10.1542/pir.32-2-e25. [DOI] [PubMed] [Google Scholar]
- 9.Agee MD. Reducing child malnutrition in Nigeria: combined effects of income growth and provision of information about mothers' access to health care services. Soc Sci Med. 2010;71:1973–1980. doi: 10.1016/j.socscimed.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Bartram J, Cairncross S. Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med. 2010;7:e1000367. doi: 10.1371/journal.pmed.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins MW, Curtis V. Achieving the ‘good life’: why some people want latrines in rural Benin. Soc Sci Med. 2005;61:2446–2459. doi: 10.1016/j.socscimed.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins MW, Cairncross S. Modelling latrine diffusion in Benin: towards a community typology of demand for improved sanitation in developing countries. J Water Health. 2010;8:166–183. doi: 10.2166/wh.2009.111. [DOI] [PubMed] [Google Scholar]
- 13.Hunter PR, MacDonald AM, Carter RC. Water supply and health. PLoS Med. 2010;7:e1000361. doi: 10.1371/journal.pmed.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luque Fernández MA, Mason PR, Gray H, Bauernfeind A, Fesselet JF, Maes P. Descriptive spatial analysis of the cholera epidemic 2008–2009 in Harare, Zimbabwe: a secondary data analysis. Trans R Soc Trop Med Hyg. 2010;105:38–45. doi: 10.1016/j.trstmh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari MJ, Djibo A, Grais RF, Bharti N, Grenfell BT, Bjornstad ON. Rural-urban gradient in seasonal forcing of measles transmission in Niger. Proc Biol Sci. 2010;277:2775–2782. doi: 10.1098/rspb.2010.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younas M, Shah S, Talaat A. Frequency of Giardia lamblia infection in children with recurrent abdominal pain. J Pak Med Assoc. 2008;58:171–174. [PubMed] [Google Scholar]
- 17.Chacín-Bonilla L, Barrios F, Sanchez Y. Environmental risk factors for Cryptosporidium infection in an island from western Venezuela. Mem Inst Oswaldo Cruz. 2008;103:45–49. doi: 10.1590/s0074-02762008005000007. [DOI] [PubMed] [Google Scholar]
- 18.Bates SJ, Trostle J, Cevallos WT, Hubbard A, Eisenberg JNS. Relating diarrheal disease to social networks and the geographic configuration of communities in rural Ecuador. Am J Epidemiol. 2007;166:1088–1095. doi: 10.1093/aje/kwm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milroy CA, Borja PC, Barros FR, Barreto ML. Evaluating sanitary quality and classifying urban sectors according to environmental conditions. Environ Urban. 2001;13:235–255. [Google Scholar]
- 20.Garenne M. Urbanisation and child health in resource poor settings with special reference to under-five mortality in Africa. Arch Child Dis. 2010;95:464–468. doi: 10.1136/adc.2009.172585. [DOI] [PubMed] [Google Scholar]
- 21.Lutter CK, Chaparro CM. Malnutrition in Infants and Young Children in Latin America and the Caribbean: Achieving the Millenium Development Goals. Washington, DC: Pan American Health Organization; 2008. [Google Scholar]
- 22.Golden MH. Specific deficiencies versus growth failure: type I and type II. J Nutr Environ Med. 1996;6:301. [PubMed] [Google Scholar]
- 23.Falkingham J, Namazie C. Measuring Health and Poverty: A Review of Approaches to Identifying the Poor. London: United Kingdom Department for International Development; 2002. [Google Scholar]
- 24.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data - or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 25.Rutstein S, Johnson K. The DHS Wealth Index. Calverton, MD: ORC Macro, MEASURE DHS+; 2004. [Google Scholar]
- 26.Ministerio de Economía y Finanzas Encuesta de Niveles de Vida 2003. Panama City, Panama: Ministerio de Economía y Finanzas; 2003. [Google Scholar]
- 27.Payne L, Koski KG, Ortega-Barria E, Scott ME. Benefit of vitamin A supplementation on Ascaris re-infection is less evident in stunted children. J Nutr. 2007;137:1455–1459. doi: 10.1093/jn/137.6.1455. [DOI] [PubMed] [Google Scholar]
- 28.Ministerio de Economía y Finanzas La Distribución del Ingreso en Los Hogares de Panamá: Encuesta de Niveles de Vida 2008. Panama City, Panama: Ministerio de Economía y Finanzas; 2010. [Google Scholar]
- 29.World Health Organization (WHO) WHO Child Growth Standards: Methods and Development: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. WHO Multicentre Growth Reference Study Group; World Health Organization: 2006. [Google Scholar]
- 30.Hamilton WP, Kim M, Thackston EL. Comparison of commercially available Escherichia coli enumeration tests: implications for attaining water quality standards. Water Res. 2005;39:4869–4878. doi: 10.1016/j.watres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 31.World Bank Panama Poverty Assessment: Priorities and Strategies for Poverty Reduction. Washington, DC: Human Development Department, World Bank; 1999. [Google Scholar]
- 32.UNICEF The State of the World's Children 2009: Maternal and Newborn Health. New York: UNICEF; 2008. [Google Scholar]
- 33.Victora CG. The association between wasting and stunting: an international perspective. J Nutr. 1992;122:1105–1110. doi: 10.1093/jn/122.5.1105. [DOI] [PubMed] [Google Scholar]
- 34.Fotso JC, Kuate-Defo B. Socioeconomic inequalities in early childhood malnutrition and morbidity: modification of the household-level effects by the community SES. Health Place. 2005;11:205–225. doi: 10.1016/j.healthplace.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO) Guidelines for Drinking Water Quality. Geneva: World Health Organization; 2008. [Google Scholar]
- 36.Bateman OM, Smith S. A Comparison of the Health Effects of Water Supply and Sanitation in Urban and Rural Guatemala. Washington, DC: U.S. Agency for International Development; 1991. [Google Scholar]
- 37.Van De Poel E, Hosseinpoor AR, Speybroeck N, Van Ourti T, Vega J. Socioeconomic inequality in malnutrition in developing countries. Bull World Health Organ. 2008;86:282–291. doi: 10.2471/BLT.07.044800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong R, Mishra V. Effect of wealth inequality on chronic under-nutrition in Cambodian children. J Health Popul Nutr. 2006;24:89–99. [PubMed] [Google Scholar]
- 39.Raso G, Utzinger J, Silué KD, Ouattara M, Yapi A, Toty A, Matthys B, Vounatsou P, Tanner M, N’Goran EK. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Côte d’Ivoire. Trop Med Int Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 40.Nundy S, Gilman RH, Xiao L, Cabrera L, Cama R, Ortega YR, Kahn G, Cama VA. Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. Am J Trop Med Hyg. 2011;84:38–42. doi: 10.4269/ajtmh.2011.10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gwatkin D, Wagstaff A, Yazbeck A. Reaching the Poor with Health Nutrition and Population Services: What Works, What Doesn't and Why. Washington, DC: World Bank; 2005. [Google Scholar]
- 42.Adekanmbi VT, Kayode GA, Uthman OA. Individual and contextual factors associated with childhood stunting in Nigeria: a multilevel analysis. Matern Child Nutr. 2011 doi: 10.1111/j.1740-8709.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 44.Sackey ME, Weigel MM, Armijos RX. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J Trop Pediatr. 2003;49:17–23. doi: 10.1093/tropej/49.1.17. [DOI] [PubMed] [Google Scholar]
- 45.Boeke CE, Mora-Plazas M, Forero Y, Villamor E. Intestinal protozoan infections in relation to nutritional status and gastrointestinal morbidity in Colombian school children. J Trop Pediatr. 2010;56:299–306. doi: 10.1093/tropej/fmp136. [DOI] [PubMed] [Google Scholar]
- 46.Simsek Z, Zeyrek FY, Kurcer MA. Effect of Giardia infection on growth and psychomotor development of children aged 0–5 years. J Trop Pediatr. 2004;50:90–93. doi: 10.1093/tropej/50.2.90. [DOI] [PubMed] [Google Scholar]
- 47.Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363:641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- 48.LeVine RA, Rowe ML. Maternal literacy and child health in less-developed countries: evidence, processes, and limitations. J Dev Behav Pediatr. 2009;30:340–349. doi: 10.1097/DBP.0b013e3181b0eeff. [DOI] [PubMed] [Google Scholar]
- 49.Boyle MH, Racine Y, Georgiades K, Snelling D, Hong S, Omariba W, Hurley P, Rao-Melacini P. The influence of economic development level, household wealth and maternal education on child health in the developing world. Soc Sci Med. 2006;63:2242–2254. doi: 10.1016/j.socscimed.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Haidar J, Kogi-Makau W. Gender differences in the household-headship and nutritional status of pre-school children. East Afr Med J. 2009;86:69–73. doi: 10.4314/eamj.v86i2.46936. [DOI] [PubMed] [Google Scholar]
- 51.McIntyre L, Rondeau K, Kirkpatrick S, Hatfield J, Islam KS, Huda SN. Food provisioning experiences of ultra poor female heads of household living in Bangladesh. Soc Sci Med. 2011;72:969–976. doi: 10.1016/j.socscimed.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Genser B, Strina A, Teles CA, Prado MS, Barreto ML. Risk factors for childhood diarrhea incidence: dynamic analysis of a longitudinal study. Epidemiology. 2006;17:658–667. doi: 10.1097/01.ede.0000239728.75215.86. [DOI] [PubMed] [Google Scholar]
- 53.Silva RR, da Silva CAM, de Jesus Pereira CA, de Carvalho Nicolato RL, Negrão-Corrêa D, Lamounier JA, Carneiro M. Association between nutritional status, environmental and socio-economic factors and Giardia lamblia infections among children aged 6–71 months in Brazil. Trans R Soc Trop Med Hyg. 2009;103:512–519. doi: 10.1016/j.trstmh.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Teixeira JC, Heller L, Barreto ML. Giardia duodenalis infection: risk factors for children living in sub-standard settlements in Brazil. Cad Saude Publica. 2007;23:1489–1493. doi: 10.1590/s0102-311x2007000600024. [DOI] [PubMed] [Google Scholar]
- 55.Lanata C, Huttly SRA, Yeager BAC. Diarrhea: whose feces matter? Reflections from studies in a Peruvian shanty town. Pediatr Infect Dis J. 1998;17:7–9. doi: 10.1097/00006454-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, Morales G, Diaz-Camacho SP. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:225. doi: 10.1186/1471-2458-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mara D, Lane J, Scott B, Trouba D. Sanitation and health. PLoS Med. 2010;7:e1000363. doi: 10.1371/journal.pmed.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rinne S, Rodas EJ, Galer-Unti R, Glickman N, Glickman LT. Prevalence and risk factors for protozoan and nematode infections among children in an Ecuadorian highland community. Trans R Soc Trop Med Hyg. 2005;99:585–592. doi: 10.1016/j.trstmh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Hussein AS. Prevalence of intestinal parasites among school children in northern districts of West Bank-Palestine. Trop Med Int Health. 2011;16:240–244. doi: 10.1111/j.1365-3156.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 60.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–281. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 61.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]