Abstract
Leptospirosis has recently been reported as an emerging disease worldwide, and a seroprevalence study was undertaken in American Samoa to better understand the drivers of transmission. Antibodies indicative of previous exposure to leptospirosis were found in 15.5% of 807 participants, predominantly against three serovars that were not previously known to occur in American Samoa. Questionnaires and geographic information systems data were used to assess behavioral factors and environmental determinants of disease transmission, and logistic regression was used to identify factors associated with infection. Many statistically significant factors were consistent with previous studies, but we also showed a significant association with living at lower altitudes (odds ratio [OR] = 1.53, 95% confidence interval [CI]: 1.03–2.28), and having higher numbers of piggeries around the home (OR = 2.63, 95% CI: 1.52–4.40). Our findings support a multifaceted approach to combating the emergence of leptospirosis, including modification of individual behavior, but importantly also managing the evolving environmental drivers of risk.
Introduction
Leptospirosis has recently been reported as an emerging infectious disease around the world, including the Pacific region.1–9 The environmental determinants of disease transmission vary between places, and include climate change, extreme weather, land use, international trade, animal reservoirs, and farming practices.4,10,11 It has become increasingly apparent that rainfall, cyclones, flooding, urbanization, and recreation are important emerging risk factors,4,12–15 that biodiversity might be protective,16,17 and ecological changes can influence serovar emergence.9 Spatial epidemiology has recently been used to explore the complex transmission dynamics driving leptospirosis emergence, particularly in relation to environmental hazards.18–20
American Samoa (AS), a group of Pacific Islands with a population of ∼67,000,21 had identified its first laboratory-confirmed case of leptospirosis in 2003. In response to a number of cases and deaths,22 the Centers for Disease Control and Prevention USA (CDC) conducted a seroprevalence study in 2004.23 Of 341 adults surveyed, 17% had leptospiral antibodies indicating previous infection. Significant exposure risks included male gender, contact with animals, bathing in streams, and low income. Observational assessment identified likely peri-domestic contamination by dogs and rodents, and contamination of streams by piggeries. Currently, there is no leptospirosis vaccination program for animals in AS, and the paucity of information about infecting serovars makes it difficult to identify an appropriate vaccine and assess the potential benefits of vaccination.
Our field study aims to identify risk factors for leptospirosis in AS. It builds and expands upon the CDC’s findings by employing geographic information system (GIS) data to link environmental exposures around the home with the risk of leptospirosis infection; quantifying the relative importance of behavioral and environmental exposures; and identifying risk factors to facilitate public health interventions.
Materials and Methods
Study location and population.
A cross-sectional seroprevalence study was conducted from May to July 2010. Adults were recruited from the main island of Tutuila (where over 95% of the population live), the adjacent island of Aunu’u, and the remote Manu’a Islands (Ta’u, Ofu, and Olosega).
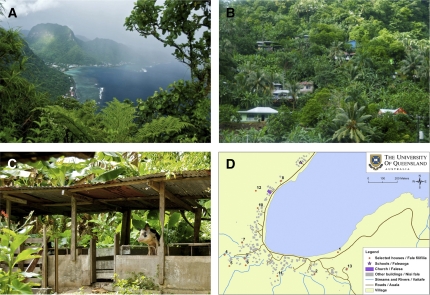
American Samoa is one of the wettest inhabited places in the world, with an average annual rainfall of more than 3,000 mm.24 Tutuila has mountains, valleys, tropical forests, wetlands, and fringing reefs and lagoons. Villages are mostly located on beaches, but some are inland at altitudes of over 450 m (Figure 1A and B). There are large numbers of small backyard piggeries located mostly behind homes (Figure 1C).
Figure 1.
Leptospirosis field study site: American Samoa. (A) Rainmaker Mountain and coastal villages on Tutuila, (B) steep hillside village, (C) backyard piggery, and (D) sample village map used in fieldwork.
Informed consent and ethics approvals.
Verbal and written information (available on request) on the study were given in Samoan and/or English according to the participant’s preference, and informed consent was obtained. Ethics approvals were obtained from the American Samoa Institutional Review Board, the Medical Research Ethics Committee of The University of Queensland (2010000114), and Queensland Health Forensic and Scientific Services Human Ethics Committee (HREC/10/QFSS/1). Permission was also sought from the Department of Samoan Affairs and village chiefs before village visits.
Sampling design.
The GIS spatial datasets on the locations of houses, rivers, and other landmarks were obtained from the AS Geographic Information Systems User Group (GISUG).25 Maps of Tutuila and Aunu’u were created and overlaid with a randomly generated grid, and houses closest to grid nodes were selected for sampling (primary sample). The dimensions of the grid were designed to select ∼1,000 houses, the maximum number that could potentially be sampled with the time and resources available. The grid was used to ensure that the samples had maximum spatial dispersion over the study area to facilitate future spatial prediction using geostatistical analysis. If a selected house could not be sampled (e.g., the house was destroyed or no longer inhabited) the closest available house was chosen. For each household, one adult was requested to volunteer for the study.
The Manu’a Islands are sparsely populated with tiny villages, and inhabitants are often away from home and “off island.” The previous sampling design was therefore impractical, and participants in Manu’a were all volunteers (i.e., a non-random convenience sample was used).
During village visits, some residents who were not part of the randomly selected primary sample wished to volunteer as participants. To maximize our sample size and the geographical spread of our sample in Tutuila, volunteers were also sought from the community college and health center. These participants were included in the study and noted as volunteers (i.e., non-random convenience samples).
All participants were geo-located to their primary place of residence.
Data collection.
Before village visits, community awareness of the study was raised through television and radio broadcasts, and presentations to village chiefs and mayors. Written information in Samoan and English were distributed to all village leaders. Village visits and data collection were carried out in close collaboration with village leaders and the AS Department of Health to ensure that procedures and activities were cultural appropriate and acceptable.
Mayors were given detailed maps of their villages showing the houses selected for sampling (Figure 1D), and asked to gather participants for village visits. At each village, the project was explained in Samoan and English, and informed consent obtained from each participant. A 5-mL blood sample was collected from each participant by a trained phlebotomist. Blood samples were centrifuged, and serum samples frozen and transported to Brisbane for laboratory analysis. Questionnaires were administered by one of four bilingual field assistants, either in Samoan or English according to the participant’s preference.
Questionnaire variables.
For all participants, standardized questionnaires were used to collect data on demographics; exposures at home, work, and during recreation; and knowledge about leptospirosis. The majority of questions had pre-specified categorical responses.
Occupations were classified into indoor, outdoor, mixed indoor/outdoor, fish cleaners (local tuna cannery is the major non-government employer in AS), and unemployed.
Environmental variables.
For Tutuila, additional household environmental variables were generated for each sampled house using geo-referenced environmental data from AS GISUG25 and the National Oceanic and Atmospheric Administration (NOAA).26 This was not done for smaller islands as there were insufficient environmental data, and populations were too small and localized for environmental analyses to be meaningful. Using the geographic location of sampled houses, the environmental variables explored included:
-
(1)
Relative elevation in the village.
A digital elevation model of AS26 was used to determine the altitude of all buildings. For each village, houses were categorized into quartiles based on altitude;
-
(2)
Distance to the closest stream;
-
(3)
Flooding risk as determined by a flood insurance risk map;
-
(4)
Average annual rainfall;
-
(5)
Piggery density within 250 m.
The American Samoa Environmental Protection Agency (ASEPA) provided data on the location of piggeries in 2008. To explore the effect of environmental contamination by piggery waste flowing downhill, aggregate variables were generated by combining data on piggery density and altitude to calculate “total piggeries within 250 m”, “piggeries within 250 m and above house”, and “piggeries within 250 m and below house”;
-
(6)
Population density within 250 m.
Piggeries were mostly located behind houses, and variables reflecting piggery density and population density were therefore expected to share similar environmental characteristics. The association between population density and infection was explored to determine whether piggery density had an additional effect on seroprevalence.
All data were collated, stored, mapped, and analyzed using ArcMap v 10.0 software (Environmental Systems Research Institute [ESRI], Redlands, CA).
Serological analysis.
Microscopic agglutination tests (MAT) were used to detect anti-Leptospira antibodies, and sera are expected to remain reactive for ∼3 years after an acute infection.4,27,28 Based on the known epidemiology of leptospiral serovars in the Pacific region, the WHO/FAO/OIE Collaborating Center for Reference and Research on Leptospirosis in Brisbane, Australia selected a MAT panel of 23 pathogenic serovars (Appendix), including two novel serovars cultured from samples from other Pacific islands. MAT titers of ≥ 1:50 were considered reactive or “sero-positive,”, and indicative of previous or current exposure to pathogenic leptospires. Although “serogroups” are no longer officially used in Leptospira nomenclature and classification, cross-reactions of MATs are known to occur between serovars within a serogroup. If a sample reacted to multiple serovars within a serogroup, the serovar with the highest titer was recorded as the reacting serovar. If a sample reacted to serovars in more than one serogroup, it was recorded as reacting to multiple serovars.
Statistical analysis.
The outcome measure used was sero-positive reactions to any serovars. Analyses were initially performed separately for participants from the primary (originally selected) sample of houses and volunteers, and all data were combined for the final analyses. STATA v 11.1 software (StataCorp, College Station, TX) was used, and P values of < 0.05 were taken to indicate statistical significance.
“Questionnaire variables” were assessed for participants from all islands. The χ2 tests or Fisher exact tests were used to compare groups for categorical outcomes, and variables with P < 0.1 were selected for further analyses using logistic regression. For each outcome measure, univariate logistic regression analysis was performed and odds ratios (ORs) were calculated for “questionnaire variables.” All variables with P < 0.1 on univariate analyses were entered into a multivariable model, and for those with P < 0.05, biological plausibility and collinearity between variables were taken into account in the selection of variables that were retained. Variables found to be statistically significant on univariate and multivariable analyses were reported.
The analysis of “environmental variables” was performed as described previously, but only included participants from Tutuila. Significant exposure variables on univariate analyses are reported. For multivariable analyses, questionnaire variables were combined with environmental variables to provide a more complete picture. Odds ratios were reported for statistically significant environmental variables on multivariable analyses, and questionnaire variables included in models are noted.
Maps.
Using data from AS GISUG,25 the following maps were generated: 1) Population distribution surfaces and point locations of sampled households, and 2) point locations of sero-positive participants.
The point locations of all houses in AS were represented as distribution surfaces using the kernel density interpolation method, available in ESRI’s Spatial Analyst. Density interpolation is a technique that allows the representation of discrete points as areal density values over a surface. This is done to aid visual interpretation of spatial patterns. However, a simple density method that divides the number of cases by the area of the search radius often produces a discontinuous surface when the search radii of adjoining cells overlap, and the kernel density method was used to produce a smoother interpolation. Kernel smoothing works by using a Gaussian volume centered on the cell value being estimated and tailing off to zero at the search radius. There are two variables to be specified in the use of kernel density interpolation: the resolution element of the surface (or grid cell size) and the search radius of the kernel, which is used to find all cases within a specified distance. The kernel shape is circular and although several radii were explored, we found a 1,000 m radius to provide a good regional representation of the spatial variation in population density29; a default grid resolution of 64 m is used to generate the surfaces. The primary implication of finer cell resolution is increased computational time, and because visualization is the primary purpose of the generated surfaces this cell size was deemed appropriate.
Results
Population sampled.
Blood samples were collected from 807 people in 659 households. Ages of participants ranged from 17 to 87 years of age (mean 40 years), 52% were males, 97% were of Samoan ethnicity, and 96% had lived in their current home for more than 3 years. For Tutuila and Aunu’u, 58.8% of 737 participants were from the primary selected sample of houses, and 41.2% were volunteers. The response rate from the originally selected households on Tutuila and Aunu’u was 43.3%. All participants from the Manu’a Islands were volunteers.
No statistically significant differences in exposure variables or outcome measures were found between participants from the primary sample of selected houses and volunteers. All data were therefore combined for the final analyses.
Seroprevalence.
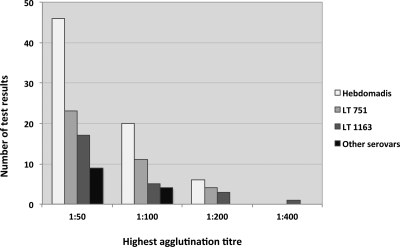
The overall seroprevalence was 15.5% (95% confidence interval 13.1% to 18.2%), with 125 samples having reactive MATs (titer ≥ 1:50) for one or more serovars. Fourteen samples were reactive for two serovars, and five were reactive for three serovars. Three predominant serovars accounted for 91.2% of reactive MATs: Leptospira interrogans serovars 1) Hebdomadis (48.3%), 2) LT 751 (25.5%), and 3) LT 1163 (17.4%). For the 72 samples reactive to serovar Hebdomadis, it was the sole reacting serovar in 82% of cases, and no other serovars in the serogroup were included in the MAT panel. For the 38 positive reactions to LT 751, 55% did not react to any other serovars, and 82% were the sole reacting serovar in the Australis serogroup (others in the MAT panel were serovars Australis and Bratislava). For the 26 positive reactions to serovar LT 1163, 54% did not react to any other serovars, and 73% were the sole reacting serovar in the Pyrogenes serogroup (also included in the MAT panel was serovar Pyrogenes). The distribution of the highest agglutinating MAT titers for the three most common serovars is shown in Figure 2 (results for other serovars are available on request). Table 1 shows the number of villages visited, households and people sampled, and seroprevalence for each island.
Figure 2.
Distribution of microscopic agglutination test (MAT) titres for the most common serovars.
Table 1.
Sampling distribution and leptospirosis seroprevalence in the islands of American Samoa
| Island | Total population 2000 est. (30) | Number of households sampled | Number of people sampled | Number of positive MATs | Seroprevalence of most common serovars (%) | Overall seroprevalence (%) | ||
|---|---|---|---|---|---|---|---|---|
| Hebdomadis | LT 751 | LT 1163 | ||||||
| Tutuila | 55,876 | 592 | 721 | 117 | 10 | 4.3 | 3.5 | 16.2 |
| Aunu’u | 476 | 12 | 16 | 0 | 0 | 0 | 0 | 0 |
| Ta’u | 380 | 35 | 45 | 6 | 0 | 11.1 | 2.2 | 13.3 |
| Ofu | 289 | 9 | 11 | 1 | 0 | 9.1 | 0 | 9.1 |
| Olosega | 216 | 11 | 14 | 1 | 0 | 7.1 | 0 | 7.1 |
| Total | 57,291 | 659 | 807 | 125 | 8.9 | 4.7 | 3.2 | 15.5 |
MAT = microscopic agglutination tests.
Maps.
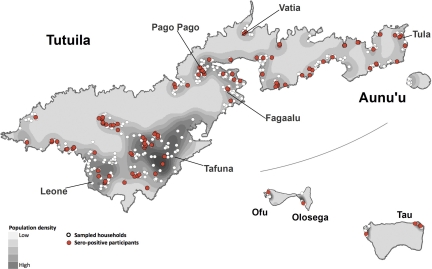
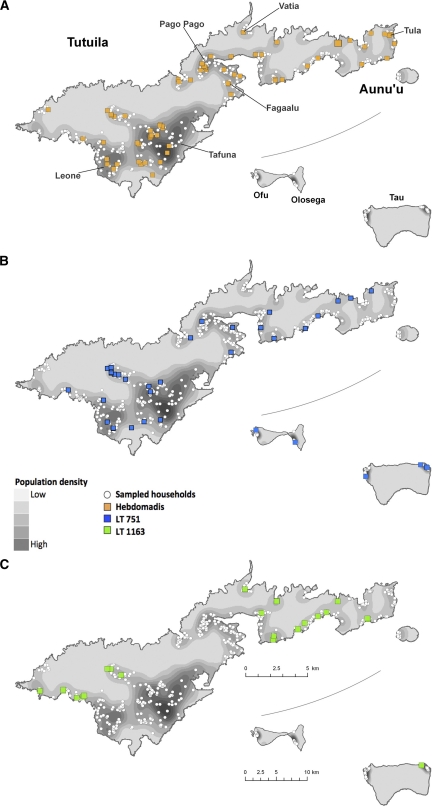
Figure 3 shows the distributions of the population of AS, sampled households, and sero-positive participants. Figure 4 shows the distribution of each of the three most common serovars.
Figure 3.
Distribution of population of American Samoa, sampled households for the study, and participants who were sero-positive for Leptospira antibodies.
Figure 4.
Distribution of population of American Samoa, sampled households for the study, and participants who were sero-positive for each of the three most common leptospiral serovars.
Risk factors for infection.
Odds ratios for statistically significant factors in the univariate and multivariable analyses for all serovars are shown in Table 2.
Table 2.
Variables significantly associated with positive serology for Leptospira spp. on univariate and multivariable analyses for all serovars*
| Risk factors | No. in category | No. with antibodies | Seroprevalence (%) | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|---|
| Questionnaire variables† | |||||
| Total sampled | 807 | 125 | 15.5% | ||
| Male | 423 | 93 | 22.0% | 3.06 (2.00–4.71) | 3.40 (2.18–5.33) |
| Heard of leptospirosis | 293 | 34 | 11.6% | 0.61 (0.40–0.93) | |
| Occupational groups | |||||
| Indoor | 192 | 20 | 10.4% | 1 | 1 |
| Outdoor (including fish cleaners) | 135 | 37 | 27.4% | 3.25 (1.79–5.90) | 2.69 (1.43–5.06) |
| Mixed Indoor/outdoor | 72 | 10 | 13.9% | 1.39 (0.62–3.13) | 0.82 (0.36–1.91) |
| Unemployed | 382 | 57 | 14.9% | 1.51 (0.88–2.59) | 1.62 (0.92–2.86) |
| Annual household income (USD) | |||||
| > 30,000 | 64 | 5 | 7.8% | 1 | 1 |
| 20,000–30,000 | 61 | 13 | 21.3% | 3.20 (1.06–9.60) | 3.67 (1.18–11.40) |
| 10,000–20,000 | 230 | 34 | 14.8% | 2.05 (0.77–5.47) | 1.97 (0.72–5.41) |
| < 10,000 | 324 | 61 | 18.8% | 2.74 (1.05–7.11) | 2.63 (0.98–7.05) |
| Did not declare | 128 | 12 | 9.4% | 1.22 (0.41–3.63) | 1.15 (0.37–3.57) |
| Swimming at beach | |||||
| Never | 269 | 32 | 11.9% | 1 | |
| Occasional (< once a week) | 295 | 41 | 13.9% | 1.20 (0.73–1.96) | |
| Frequent (> once a week) | 230 | 49 | 21.3% | 2.01 (1.23–3.26) | |
| Swimming or walking in rain puddles | |||||
| Never | 343 | 47 | 13.7% | 1 | |
| Occasional (< once a week) | 154 | 18 | 11.7% | 0.83 (0.47–1.49) | |
| Frequent (> once a week) | 298 | 58 | 19.5% | 1.52 (1.00–2.32) | |
| Fishing | |||||
| Never | 542 | 72 | 13.3% | 1 | |
| Occasional (< once a week) | 106 | 19 | 17.9% | 1.43 (0.82–2.48) | |
| Frequent (> once a week) | 145 | 31 | 21.4% | 1.78 (1.11–2.83) | |
| Environmental variablesठ| |||||
| Total sampled | 721 | 117 | 16.2% | ||
| House below median altitude of village | |||||
| No | 409 | 56 | 13.7% | 1 | |
| Yes | 312 | 61 | 19.6% | 1.53 (1.03–2.28) | |
| Piggeries within 250 m and above house | |||||
| 0–2 piggeries | 370 | 47 | 12.7% | 1 | 1 |
| 3–5 piggeries | 239 | 39 | 16.3% | 1.34 (0.85–2.12) | 1.44 (0.90–2.33) |
| > 5 piggeries | 112 | 31 | 27.7% | 2.63 (1.52–4.40) | 2.66 (1.55–4.57) |
Statistically significant odds ratios (ORs) are shown in bold.
Participants from all islands.
Participants from Tutuila only.
Variables included in multivariable model: male, heard of leptospirosis, occupation, and piggeries within 250 m and above house.
CI = confidence interval; USD = United States dollar.
Piggery density below houses was not statistically significantly associated with infection, although density above houses was found to be more strongly associated with infection than total piggery density within 250 m. “Piggeries within 250 m and above house” was therefore chosen as the best indicator of exposure, and divided into approximate tertiles for cases: 0–2, 3–5, > 5 piggeries.
Fish cleaners were found to have similar seroprevalence to outdoor workers, and the two groups were combined for final analyses.
Univariate analysis showed that infection was associated with male gender, outdoor occupations, low income, swimming at beaches, contact with rain puddles, fishing, living below median altitude of village, and having more “piggeries within 250 m and above house.” Knowledge about leptospirosis was associated with lower risk of infection. Statistically significant exposures on multivariable analysis were male gender, outdoor occupations, low income, and “piggeries within 250 m and above house.”
No statistically significant association was found with age, owning dogs and pigs, or sighting rats and mice. For environmental variables, rainfall, and flooding risk were not associated with infection. Although population density was highly correlated with piggery density, it was not associated with infection. (ORs estimates and confidence intervals from univariate analysis of all variables are available on request.)
Infections with each of the three main serovars were associated with different behavioral and environmental exposures. Statistically significant ORs for univariate and multivariable analyses for serovars Hebdomadis, LT 751, and LT 1163 are shown in Tables 3–5, respectively.
Table 3.
Variables significantly associated with positive serology for Leptospira spp. on univariate and multivariable analyses for serovar Hebdomadis*
| Risk factors | No. in category | No. with antibodies | Seroprevalence (%) | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|---|
| Questionnaire variables† | |||||
| Total sampled | 807 | 72 | 8.9% | ||
| Male | 423 | 52 | 12.3% | 2.52 (1.48–4.31) | 2.71 (1.56–4.71) |
| Drink village water | 139 | 6 | 4.3% | 0.41 (0.18–0.98) | |
| Chickens on home property | 327 | 37 | 11.3% | 1.64 (1.01–2.67) | |
| Heard of leptospirosis | 293 | 18 | 6.1% | 0.56 (0.32–0.97) | |
| Sewage system | |||||
| Septic tank | 553 | 41 | 7.4% | 1 | |
| Mains sewage | 248 | 30 | 12.1% | 1.71 (1.04–2.82) | 1.85 (1.11–3.07) |
| Occupational groups | |||||
| Indoor | 192 | 9 | 4.7% | 1 | 1 |
| Outdoor (including fish cleaners) | 135 | 18 | 13.3% | 3.13 (1.36–7.20) | 2.88 (1.24–6.71) |
| Mixed indoor/outdoor | 72 | 6 | 8.3% | 1.85 (0.63–5.39) | 1.08 (0.34–3.42) |
| Unemployed | 382 | 39 | 10.2% | 2.31 (1.10–4.88) | 2.31 (1.09–4.91) |
| Work with animals | 88 | 13 | 14.8% | 1.90 (1.00–3.63) | |
| Environmental variablesठ| |||||
| Total sampled | 721 | 72 | 10.0% | ||
| Piggeries within 250 m and above house | |||||
| 0–2 piggeries | 370 | 27 | 7.3% | 1 | 1 |
| 3–5 piggeries | 239 | 29 | 12.1% | 1.75 (1.01–3.05) | 1.76 (1.00–3.11) |
| > 5 piggeries | 112 | 16 | 14.3% | 2.12 (1.10–4.09) | 2.35 (1.18–4.66) |
Statistically significant odds ratios (ORs) are shown in bold.
Participants from all islands.
Participants from Tutuila only.
Variables included in multivariable model: male, mains sewage, occupation, and piggeries within 250 m and above house.
CI = confidence interval.
Table 5.
Variables significantly associated with positive serology for Leptospira spp. on univariate and multivariable analyses for serovar LT 1163*
| Risk factors | No. in category | No. with antibodies | Seroprevalence (%) | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|---|
| Questionnaire variables† | |||||
| Total sampled | 807 | 26 | 3.2% | ||
| Male | 423 | 19 | 4.5% | 2.51 (1.04–6.03) | |
| Drink village water | 139 | 8 | 5.8% | 2.18 (0.93–5.13) | 2.68 (1.06–6.76) |
| Cats on home property | 404 | 18 | 4.5% | 2.27 (0.97–5.28) | 2.52 (1.03–6.21) |
| Flooding | |||||
| Flooding at work | 72 | 8 | 11.1% | 4.6 (1.45–14.57) | 3.76 (1.07–13.25) |
| Flooding on home property | 333 | 16 | 4.8% | 2.33 (1.04–5.20) | |
| Number of floods on home property: | |||||
| Nil | 472 | 10 | 2.1% | 1 | 1 |
| 1 to 2 times | 123 | 10 | 8.1% | 4.09 (1.66–10.06) | 4.01 (1.51–10.61) |
| 3 to 5 times | 58 | 3 | 5.2% | 2.52 (0.67–9.43) | 1.97 (0.50–7.87) |
| More than 5 times | 147 | 3 | 2.0% | 0.96 (0.26–3.54) | 0.55 (0.14–2.22) |
| Swimming at beach | |||||
| Never | 269 | 3 | 1.1% | 1 | 1 |
| Occasional (< once a week) | 295 | 8 | 2.7% | 2.47 (0.65–9.41) | 2.50 (0.64–9.81) |
| Frequent (> once a week) | 230 | 14 | 6.1% | 5.75 (1.63–20.26) | 5.32 (1.47–19.31) |
| Swimming in river | |||||
| Never | 680 | 20 | 2.9% | 1 | |
| Occasional (< once a week) | 82 | 1 | 1.2% | 0.41 (0.05–3.08) | |
| Frequent (> once a week) | 30 | 4 | 13.3% | 5.08 (1.62–15.92) | |
| Kayaking | |||||
| Never | 742 | 34 | 4.6% | 1 | |
| Occasional (< once a week) | 27 | 0 | 0.0% | no cases | |
| Frequent (> once a week) | 24 | 3 | 12.5% | 4.68 (1.30–16.85) | |
| Camping | |||||
| Never | 704 | 21 | 3.0% | 1 | |
| Occasional (< once a week) | 62 | 1 | 1.6% | 0.53 (0.07–4.03) | |
| Frequent (> once a week) | 27 | 3 | 11.1% | 4.07 (1.13–14.57) | |
| Environmental variablesठ| |||||
| Total sampled | 721 | 25 | 3.5% | ||
| House less than 50 m from stream | 260 | 14 | 5.4% | 2.32 (1.04–5.21) | |
| Piggeries within 250 m and above house | |||||
| 0–2 piggeries | 370 | 9 | 2.4% | 1 | 1 |
| 3–5 piggeries | 239 | 4 | 1.7% | 0.68 (0.21–2.24) | 0.67 (0.20–2.24) |
| > 5 piggeries | 112 | 12 | 10.7% | 4.81 (1.97–11.75) | 3.32 (1.29–8.54) |
| House in lowest 25th centile of altitude in village | |||||
| No | 606 | 16 | 2.6% | 1 | 1 |
| Yes | 115 | 9 | 7.8% | 3.13 (1.34–7.27) | 2.80 (1.12–6.97) |
Statistically significant odds ratios (ORs) are shown in bold.
Participants from all islands.
Participants from Tutuila only.
Variables included in multivariable model: drink village water, swim at beach, piggeries, and house below 25th centile of altitude.
CI = confidence interval.
Seroprevalence estimation chart.
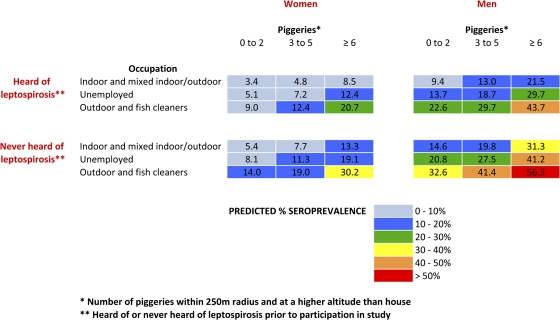
A seroprevalence estimation chart (Figure 5) was generated from the logistic regression model using the four important exposure variables: gender, occupation, knowledge about leptospirosis, and “piggeries within 250 m and above houses.” The numbers in each cell represent the predicted seroprevalence for each of the combinations of categories of exposure. The model fit was good (χ2 for goodness of fit = 18.08, degrees of freedom = 29, P = 0.94).
Figure 5.
Seroprevalence estimation chart based on four risk factors: gender, occupation, knowledge, and piggeries around the home.
The occupational categories of “indoor” and “mixed indoor/outdoor” were combined because there were small numbers in the latter group, and both groups had similar ORs from the regression analyses.
The seroprevalence estimation chart shows that although females who knew about leptospirosis, worked indoors, and had few “piggeries within 250 m and above house” had an estimated seroprevalence of 3.4%; males who had never heard of leptospirosis, worked outdoors, and had the most “piggeries within 250 m and above house” had an estimated seroprevalence of over 50%.
Discussion
Leptospirosis in AS is caused by three predominant serovars, with 15.5% of participants having serological evidence of being infected. The seroprevalence found in our study is similar to the 17% found in the 2004 CDC study,23 although it is difficult to make direct comparisons because of differences in sampling methods, sampling locations, and serovars used in MAT panels.
The epidemiology of leptospirosis varied between islands, and the three main serovars have not previously been identified in AS. This finding might reflect failure of ascertainment in previous studies, or the emergence of new serovars. Hebdomadis and related serovars have been reported in many animal reservoirs including rodents, pigs, dogs, cattle, horses, bats, and chickens.10,30 Serovar LT 751 was isolated from rodent samples from Micronesia,31 and little is known about serovar LT 1163, which was isolated from a human serum sample during an infection acquired in Samoa.
Both behavioral and household environmental exposures were important in disease transmission, and environmental drivers of disease emergence that have been documented elsewhere (flooding, recreational exposure, and animal husbandry) also operate in AS. Although some statistically significant exposures identified in our study also corroborate the findings in other studies worldwide (e.g., male and occupational risks), other exposures were specific to the environmental and cultural setting in AS, such as the proximity of piggeries. Although fish cleaning is performed indoors, seroprevalence in this occupational group was similar to outdoor workers. A possible explanation is that the nature of work activities for fish cleaners is similar to outdoor work because workers are likely to have cuts, wounds, and waterlogged skin; the working environment is constantly wet; and fish waste can attract mice and rats.
Although sighting rats or mice at home was not associated with infection, it is possible that the pervasive nature of this exposure (65% of participants) rendered it difficult to detect an association even if it truly existed. Similarly, owning dogs (67% of participants) was not associated with infection, but the majority of the population are exposed to the large numbers of unrestrained dogs in AS. Furthermore, 75% of participants reported bats around their homes. Further studies are required to fully understand the role animals play as potential reservoirs and in subsequent disease transmission.
Each of the three predominant serovars was associated with different exposures, suggesting varying ecological mechanisms of transmission. Serovar Hebdomadis was particularly associated with animal contact, and having chickens at home (chicken feed and waste can attract rodents). Septic tanks and drinking village water were associated with a lower OR, but these exposures might reflect living in smaller villages, rather than real risks. However, mains sewage could potentially be associated with infection if it is inadequately maintained. Recreational water exposures were associated with serovars LT 751 and LT 1163, but not Hebdomadis.
Serovar LT 751 was associated with living further from streams, whereas serovar LT 1163 was associated with living closer to streams, living at lower altitudes, and exposure to flooding. Possible explanations are that each serovar is carried by animal reservoirs living in different ecological zones (e.g., bats, pigs, and rodents), or that serovars might have different optimal conditions for survival in the environment. These hypotheses are supported by the differences in geographic distribution of serovars.
Human leptospirosis is generally associated with freshwater rather than seawater exposure because of the poor survival of leptospires in salt water. However, our study found that swimming at the beach and fishing (predominantly in the ocean) were associated with exposure. A possible explanation is that most people in AS swim and fish in reef-fringed lagoons during low tide. The combination of frequent heavy rainfall washing bacteria into the sea and diluting the relatively stagnant lagoon water during low tide could potentially result in sufficient concentration and survival of leptospires to pose a risk.
Piggeries were significantly associated with overall seroprevalence as well as serovars Hebdomadis and LT 1163. Piggery density and population density were highly correlated and therefore likely to share concomitant environmental attributes (e.g., rats, dogs, cats, sewage, garbage, and environmental degradation). However, population density per se was not associated with infection, supporting the hypothesis that piggeries are more likely to be the true source of exposure. In the analysis of piggery densities around houses, buffer distances other than 250 m were also explored, and increasing buffer distances were associated with a decreasing association with seroprevalence. A buffer of 250 m was chosen for final analyses because houses did not have many piggeries closer than 250 m, and larger distances sometimes included piggeries in other villages and watersheds.
The CDC study in 200423 made an observational assessment that streams contaminated by piggery waste were a likely source of infection. In response, the ASEPA established an ongoing Piggeries Compliance Program32 to promote proper waste management, approve piggery designs, and ensure that piggeries are built away from streams. The program has resulted in a significant reduction in piggery numbers from more than 1,000 (housing over 8,000 pigs) in 2006 to 430 (housing 3,500 pigs) in 2010 (ASEPA, personal communication). Our study found that “piggeries within 250 m and above house” was associated with infection, which supports the CDC’s observational findings and the interventional activities undertaken by the ASEPA.
Our study did not show any statistically significant association between infection and living in a high-risk flood zone. However, the flood risk map used to obtain these data was produced to identify areas susceptible to severe damage for insurance purposes. Results from questionnaire data showed that 68.6% of 86 people living in flood zones (according to the insurance risk map) reported flooding around their home in the past three years, but 42.5% of 633 people not living in flood zones also reported flooding. The insurance risk map is therefore an inaccurate indicator of flooding, and questionnaire responses and relative elevation of houses might be better indicators. There was no statistically significant association between infection and rainfall in our study, but with the exceptionally high average annual rainfall (range 2,370–5,475 mm for our sampling sites), it is possible that all areas are at risk in this extremely wet tropical environment.
The geo-referenced environmental data were valuable for investigating environmental exposures that could not be ascertained from questionnaires. For example, the exposure “owning pigs” obtained from questionnaires was not associated with infection. However, the high density of piggeries indicates that most people are exposed to piggeries regardless of whether they own pigs. The GIS-based environmental analysis allowed a link to be identified between piggeries and infection.
The seroprevalence estimation chart (Figure 5) incorporates locally relevant exposures to show the combined effects of factors in estimating overall prevalence of infection. Three of the four variables used (gender, occupation, and “piggeries within 250 m and above houses”) were significantly associated with seroprevalence on multivariable analyses, whereas knowledge about leptospirosis was associated with seroprevalence on univariate analysis. The four variables were chosen because they were most likely to be of practical use for identifying those at risk, and for directing potential public health interventions such as occupational safety, public education and warnings, and piggery management. Although income was significantly associated with seroprevalence on multivariable analyses, it was not chosen for inclusion in the seroprevalence prediction chart because 1) it was not a readily modifiable variable, and therefore not easily amenable to public health interventions; and 2) the majority of the study participants (reflective of the population of AS) fall into the high-risk income groups earning less than $30,000/year, and income was therefore not very useful for identifying those at risk. The four chosen variables represent different types of exposure (gender, behavior, knowledge, and environment), and were useful for demonstrating the relative importance of exposures and the value of multifaceted approaches for control strategies. The multivariable model provides a better estimate of seroprevalence than do single exposures alone or a simple count of multiple exposures, and can be useful for informing community level interventions.
Support and enthusiasm from the AS Department of Health, Department of Samoan Affairs, and the fieldwork team were crucial to the success of the project.
There are limitations to our study findings and the interpretation of results. Sources of bias include the use of volunteers and randomly selected participants; variation in sampling techniques between islands caused by logistical considerations; multiple participants from some households (although this only applied to 16% of participants); and the exclusion of children. Inaccuracies in GIS data are possible because of changes between the time of data collection and our study period. Piggery numbers change with time, and pig numbers were not taken into account although the majority is backyard piggeries with small numbers of pigs. A more complete investigation would include other animal reservoirs, particularly rodents, dogs, cats, and bats. Accuracy of serovar-specific disease maps is limited by the low prevalence of serovars LT 751 and LT 1163. Data were geo-located to the primary place of residence, and some participants had a secondary residence in a different village as a result of family connections. In addition, infections could also occur at work and during recreation.
There are limitations in serological tests available for leptospirosis. In this study MAT titers of ≥ 1:50 were considered reactive or sero-positive, and used to indicate exposure to pathogenic leptospires. At the World Health Organization (WHO) Collaborating Center for Reference and Research on Leptospirosis in Brisbane, MAT titers of ≥ 1:50 are used as the standard cutoff in seroprevalence studies to indicate exposure in asymptomatic persons, and higher cutoff titers are used for the diagnosis of acute infections. There is currently no international consensus on cutoff titers, and the selection of a different cutoff point would have produced different seroprevalence results. However, if higher cutoff titers were used to define seropositivity in this study, the relative importance of serovars would remain unchanged (Figure 2) and the geographic distribution of serovars would be similar (data not shown). Although MATs are currently the WHO “gold standard” serological test for leptospirosis, cross-reactions are known to occur between serovars. Careful consideration was given when selecting the most appropriate panel of serovars for the Pacific region, but it is possible that reactions do not reflect the actual infecting serovar. For definitive serovar identification, isolates of leptospires are required.
To reduce leptospirosis disease burden, it is important to address both behavioral and environmental exposures. Knowledge of leptospirosis was associated with lower risk of infection, and illustrates the importance of public health education.
Our study shows that environmental data can provide valuable information on leptospirosis epidemiology, allowing the exploration of spatial patterns of infection and environmental drivers of transmission. The ability to quantify the relative importance of behavioral and environmental exposures is essential for directing evidence-based and cost-effective public health interventions for leptospirosis.
For individuals, recommendations include using personal protection during high-risk activities such as working outdoors, cleaning fish, or working with animals (e.g., wearing gloves, boots, and covering open wounds); avoiding contact with rain puddles, swimming in polluted rivers and beaches; keeping homes clean to reduce attraction of rodents; avoiding direct contact with rodents; improving community awareness and knowledge; and placing warning signs in high-risk areas.
For communities, environmental exposures can be reduced by ensuring proper drainage of piggeries; building piggeries further away and downhill from houses; minimizing flooding risk (improving drainage systems, keeping drains and streams clear of garbage and debris); keeping streams and beaches clean; controlling rodent populations; and consideration of animal vaccination programs.
At the regional level, our findings are likely to apply to other Pacific Islands with similar climate, culture, lifestyle, and animals. With global climate change, predictions of increasing frequency and severity of cyclones in the Pacific can potentially worsen flooding risk, and exacerbate the disease burden from leptospirosis. Communities should prepare for the need to manage such rapidly evolving environmental drivers of disease transmission.
Table 4.
Variables significantly associated with positive serology for Leptospira spp. on univariate and multivariable analyses for serovar LT 751*
| Risk factors | No. in category | No. with antibodies | Seroprevalence (%) | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|---|
| Questionnaire variables† | |||||
| Total sampled | 807 | 38 | 4.7% | ||
| Male | 423 | 33 | 7.8% | 6.35 (2.45–16.43) | 5.75 (2.21–14.99) |
| Touched rats | 112 | 11 | 9.8% | 2.62 (1.26–5.45) | |
| Heard of leptospirosis | 293 | 8 | 2.7% | 0.45 (0.20–1.00) | |
| Sewage system | |||||
| Septic tank | 553 | 32 | 5.8% | 1 | |
| Mains sewage | 248 | 6 | 2.4% | 0.40 (0.17–0.98) | |
| Occupational groups | |||||
| Indoor | 192 | 7 | 3.7% | 1 | |
| Outdoor (including fish cleaners) | 135 | 11 | 8.2% | 2.34 (0.88–6.21) | |
| Mixed indoor/outdoor | 72 | 1 | 1.4% | 0.37 (0.04–3.08) | |
| Unemployed | 382 | 18 | 4.7% | 1.31 (0.54–3.19) | |
| Swimming at beach | |||||
| Never | 269 | 5 | 1.9% | 1 | |
| Occasional (< once a week) | 295 | 17 | 5.8% | 3.23 (1.17–8.88) | |
| Frequent (> once a week) | 230 | 15 | 6.5% | 3.68 (1.32–10.30) | |
| Swimming in river | |||||
| Never | 680 | 29 | 4.3% | 1 | |
| Occasional (< once a week) | 82 | 4 | 4.9% | 1.15 (0.39–3.36) | |
| Frequent (> once a week) | 30 | 4 | 13.3% | 3.45 (1.13–10.55) | |
| Swimming or walking in rain puddles | |||||
| Never | 343 | 6 | 1.7% | 1 | 1 |
| Occasional (< once a week) | 154 | 8 | 5.2% | 3.08 (1.05–9.03) | 3.12 (2.21–14.99) |
| Frequent (> once a week) | 298 | 23 | 7.7% | 4.70 (1.89–11.70) | 4.24 (1.69–10.64) |
| Environmental variablesठ| |||||
| Total sampled | 721 | 31 | 4.3% | ||
| House more than 100 m from stream* | 262 | 19 | 7.3% | 2.59 (1.24–5.42) | 2.36 (1.10–5.07) |
Statistically significant odds ratios (ORs) are shown in bold.
Participants from all islands.
Participants from Tutuila only.
Variables included in multivariable model: male, swimming or walking in rain puddles, house more than 100 m from stream.
CI = confidence interval.
Appendix.
Panel of Leptospira serovars used in MAT
| Serovar | Serogroup* |
|---|---|
| Australis | Australis |
| Bratislava | Australis |
| LT 751 | Australis |
| Autumnalis | Autumnalis |
| Ballum | Ballum |
| Bataviae | Bataviae |
| Canicola | Canicola |
| Celledoni | Celledoni |
| Cynopteri | Cynopteri |
| Djasiman | Djasiman |
| Grippotyphosa | Grippotyphosa |
| Hebdomadis | Hebdomadis |
| Copenhageni | Icterohaemorrhagiae |
| Javanica | Javanica |
| Manhao | Manhao |
| Mini | Mini |
| Panama | Panama |
| Pomona | Pomona |
| LT 1163 | Pyrogenes |
| Pyrogenes | Pyrogenes |
| Shermani | Santarosai |
| Hardjo | Sejroe |
| Tarassovi | Tarassovi |
Serogroup designation is no longer used in the official classification and nomenclature of Leptospira serovars, but is provided here as a reference.
MAT = microscopic agglutination tests.
ACKNOWLEDGMENTS
We thank the following people for generously providing assistance with our field study: Tele Hill (American Samoa Department of Health); John DePasquale (Lyndon Baines Johnson Tropical Medical Centre); our field assistants Paeae Sakalaia, Tapakea Tufono, Fui Mei Lin, and Iris Hirata; the Department of Samoan Affairs; the village chiefs and mayors of American Samoa; and Don Vargo and Mark Schmaedick (American Samoa Community College). We also thank Michael Dohnt and Mary-Anne Burns (WHO/OIE/FAO Collaborating Centre for Reference and Research on Leptospirosis, Queensland Health Forensic and Scientific Services, Brisbane, Australia) for providing technical expertise, microbiological advice, and laboratory support for the study; and David Dickeson (Centre for Infectious Diseases and Microbiology, Westmead, Australia) for providing the LT 1163 isolate.
Footnotes
Financial support: This study was funded by the School Population Health, The University of Queensland; a Graduate School Research Travel Grant from The University of Queensland; and the WHO/OIE/FAO Collaborating Centre for Reference and Research on Leptospirosis, Queensland Health Forensic and Scientific Services, Brisbane, Australia.
Disclaimer: The authors do not have any conflicts of interest to declare.
Authors’ addresses: Colleen L. Lau, Annette J. Dobson, Emily J. Fearnley, Chris Skelly, and Archie C. A. Clements, School of Population Health, The University of Queensland, Herston, Queensland, Australia, E-mails: colleen.lau@uqconnect.edu.au, a.dobson@sph.uq.edu.au, emilyjfearnley@gmail.com, Wchris.skelly@gmail.com, and a.clements@sph.uq.edu.au. Lee D. Smythe and Scott B. Craig, WHO/OIE/FAO Collaborating Centre for Reference and Research on Leptospirosis, Queensland Health Forensic and Scientific Services, Brisbane, Queensland, Australia, E-mails: Lee_Smythe@health.qld.gov.au, and Scott_Craig@health.qld.gov.au. Saipale D. Fuimaono, American Samoa Department of Health, Pago Pago, American Samoa, E-mail: sfuima6@yahoo.com. Philip Weinstein, Barbara Hardy Institute, University of South Australia, Adelaide, South Australia, Australia, E-mail: philip.weinstein@unisa.edu.au.
References
- 1.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008;12:351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Leptospirosis: an emerging public health problem. Wkly Epidemiol Rec. 2011;86:45–50. [PubMed] [Google Scholar]
- 4.Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 5.Victoriano AF, Smythe LD, Gloriani-Barzaga N, Cavinta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulombe CA, Yanagihara Y, Yoshida SI, Adler B. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulsiani SM, Lau CL, Graham GC, Van Den Hurk AF, Jansen CC, Smythe LD, McKay DB, Craig SB. Emerging tropical diseases in Australia. Part 1. Leptospirosis. Ann Trop Med Parasitol. 2010;104:543–556. doi: 10.1179/136485910X12851868779867. [DOI] [PubMed] [Google Scholar]
- 7.Berlioz-Arthaud A, Kiedrzynski T, Singh N, Yvon JF, Roualen G, Coudert C, Uluiviti V. Multicentre survey of incidence and public health impact of leptospirosis in the Western Pacific. Trans R Soc Trop Med Hyg. 2007;101:714–721. doi: 10.1016/j.trstmh.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Goarant C, Laumond-Barny S, Perez J, Vernel-Pauillac F, Chanteau S, Guigon A. Outbreak of leptospirosis in New Caledonia: diagnosis issues and burden of disease. Trop Med Int Health. 2009;14:926–929. doi: 10.1111/j.1365-3156.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 9.Katz AR, Buchholz E, Hinson K, Park SY, Effler PV. Leptospirosis in Hawaii, USA, 1999–2008. Emerg Infect Dis. 2011;17:221–226. doi: 10.3201/eid1702.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desvars A, Cardinale E, Michault A. Animal leptospirosis in small tropical areas. Epidemiol Infect. 2010;139:167–188. doi: 10.1017/S0950268810002074. [DOI] [PubMed] [Google Scholar]
- 11.Cascio A, Bosilkovski M, Rodriguez-Morales AJ, Pappas G. The socio-ecology of zoonotic infections. Clin Microbiol Infect. 2011;17:336–342. doi: 10.1111/j.1469-0691.2010.03451.x. [DOI] [PubMed] [Google Scholar]
- 12.Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanization and leptospirosis: fueling the fire? Trans R Soc Trop Med Hyg. 2010;104:631–638. doi: 10.1016/j.trstmh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Smythe L, Weinstein P. Leptospirosis: an emerging disease in travelers. Travel Med Infect Dis. 2010;8:33–39. doi: 10.1016/j.tmaid.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MA, Smith H, Joeph P, Gilman RH, Bautista CT, Campos KJ, Cespedes M, Klatsky P, Vidal C, Terry H, Calderon MM, Coral C, Cabrera L, Parmar PS, Vinetz JM. Environmental exposure and leptospirosis, Peru. Emerg Infect Dis. 2004;10:1016–1022. doi: 10.3201/eid1006.030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derne BT, Fearnley EJ, Lau CL, Paynter S, Weinstein P. Biodiversity and leptospirosis risk: a case of pathogen regulation? Med Hypotheses. 2011;77:339–344. doi: 10.1016/j.mehy.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogish T, Ostfeld RS. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis RB, Ribeiro GS, Felzemburgh RD, Santana FS, Mohr S, Melendez AX, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis. 2008;2:e228. doi: 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellos C, Sabroza PC. Socio-environmental determinants of the leptospirosis outbreak of 1996 in western Rio de Janeiro: a geographical approach. Int J Environ Health Res. 2000;10:301–313. doi: 10.1080/0960312002001500. [DOI] [PubMed] [Google Scholar]
- 20.Barcellos C, Sabroza PC. The place behind the case: leptospirosis risks and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad Saude Publica. 2001;17((Suppl)):59–67. doi: 10.1590/s0102-311x2001000700014. [DOI] [PubMed] [Google Scholar]
- 21.Central Intelligence Agency The World Factbook: American Samoa (Territory of the US) 2010. http://www.cia.gov/library/publications/the-world-factbook/geos/aq.html Available at. Accessed April 3, 2011.
- 22.ProMED-mail Leptospirosis, Fatal: USA (American Samoa) 2004. http://www.promedmail.org ProMED-mail; 19 Aug: 20040820.2307.Available at. Accessed March 28, 2011.
- 23.Winger K. Leptospirosis: A Seroprevalence Survey on American Samoa. 2004. http://www.botany.hawaii.edu/basch/uhnpscesu/pdfs/sam/Winger2004AS.pdf Available at. Accessed September 12, 2009.
- 24.National Oceanic and Atmospheric Administration, National Weather Service . Pago Pago, American Samoa. NOWData: NOAA Online Weather Data; 2011. 2011. http://www.weather.gov/climate/xmacis.php?wfo=samoa Available at. Accessed March 27. [Google Scholar]
- 25.American Samoa GIS User Group 2011. 2011. http://gis.doc.as/ Available at. Accessed March 21.
- 26.Lim E, Taylor LA, Eakins BW, Carignan KS, Grothe PR, Caldwell RJ, Friday DZ. Digital Elevation Models of Pago Pago, American Samoa: Procedures, Data Sources and Analysis. Boulder, CO: National Geophysical Data Center, Marine Geology and Geophysics Division; 2010. [Google Scholar]
- 27.WHO Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. 2003. http://www.who.int/zoonoses/resources/Leptospirosis/en/ Available at. Accessed August 8, 2009.
- 28.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. Second edition. Melbourne, Australia: MediSci; 1999. [Google Scholar]
- 29.Silverman BW. Density Estimation for Statistics and Data Analysis. New York: Chapman and Hall; 1986. [Google Scholar]
- 30.Everard CO, Fraser-Chanpong GM, James AC, Butcher LV. Serological studies on leptospirosis in livestock and chickens from Grenada and Trinidad. Trans R Soc Trop Med Hyg. 1985;79:859–864. doi: 10.1016/0035-9203(85)90138-5. [DOI] [PubMed] [Google Scholar]
- 31.Simms J. Animal leptospirosis in the Federated States of Micronesia. Pac Health Dialog. 1997;5:30–37. [Google Scholar]
- 32.American Samoa Environmental Protection Agency. Piggery Compliance. 2011. http://asepa.gov/piggery-compliance.asp Available at. Accessed March 2.