Abstract
In this study, we aimed to estimate the effect that environmental, demographic, and socioeconomic factors have on dengue mortality in Latin America and the Caribbean. To that end, we conducted an observational ecological study, analyzing data collected between 1995 and 2009. Dengue mortality rates were highest in the Caribbean (Spanish-speaking and non-Spanish-speaking). Multivariate analysis through Poisson regression revealed that the following factors were independently associated with dengue mortality: time since identification of endemicity (adjusted rate ratio [aRR] = 3.2 [for each 10 years]); annual rainfall (aRR = 1.5 [for each 103 L/m2]); population density (aRR = 2.1 and 3.2 for 20–120 inhabitants/km2 and > 120 inhabitants/km2, respectively); Human Development Index > 0.83 (aRR = 0.4); and circulation of the dengue 2 serotype (aRR = 1.7). These results highlight the important role that environmental, demographic, socioeconomic, and biological factors have played in increasing the severity of dengue in recent decades.
Introduction
Worldwide, the incidence of dengue is greater than any other arbovirus infection.1,2 According to the World Health Organization (WHO), nearly 100 million new cases of dengue are reported every year, and this has a major social and economic impact, especially in tropical and subtropical regions.1–5 The dengue virus is transmitted by mosquitoes, primarily Aedes aegypti, which is typically found around human dwellings.6,7
Infection with any of the four dengue serotypes can be asymptomatic in 65–90% of cases.8–10 However, when such infections result in clinically apparent disease, symptoms can range from mild undifferentiated febrile illness to severe dengue, which can include fatal complications such as major bleeding and shock caused by abnormal capillary permeability with plasma leakage.3,11
Recent reports have shown that the incidence of dengue is increasing in the Americas.12–14 There has been a dramatic increase in the number of reported cases in Latin America and the Caribbean, a region in which the number of high-incidence countries (with > 100 cases/105 population) increased from 5 to 15 in the last three decades.14 Likewise, the annual number of dengue-related deaths has increased in the region—from 242 in the 1980s to 2,068 in the 2000s.14,15
This worrisome trend justifies the study of the macro-determinants of dengue mortality, which, in addition to biological and environmental factors, include demographic and socioeconomic aspects. Therefore, this study aims to estimate the effect that some of these determinants have on dengue mortality rates in Latin America and the Caribbean.
Methods
This was an ecological study restricted to Latin America and the Caribbean during the 1995–2009 period. We evaluated the data by calendar year and by country, presenting the results by subregion: Central America and Mexico; Andean (Bolivia, Colombia, Ecuador, Peru, and Venezuela); Southern Cone (Argentina, Chile, and Paraguay); Brazil; the Spanish-speaking Caribbean; and the non-Spanish-speaking Caribbean. These subregions (except Brazil) are the Pan American Health Organization (PAHO) classification used in epidemiological reports.14,15 Brazil was analyzed separately in consequence of its large territory and high numbers of clinical and fatal cases. We excluded countries with no confirmed cases of dengue reported to the PAHO during the study period (Bermuda, Uruguay, and the United States Virgin Islands), and years in which cases were reported for fewer than 16 epidemiological weeks or for which there were inconsistent data, such as the identification of the dengue serotype without any reported cases.
Data related to clinical cases (which included suspected and laboratory-confirmed cases) and to deaths from dengue fever, and to the circulating serotypes of the dengue virus, were obtained from the records of the PAHO.14 Deaths included all fatal cases detected among reported suspected dengue cases and not just deaths among laboratory-positive dengue cases.
For each country, we evaluated population density (inhabitants/km2; data obtained from the Population Division of the U.S. Census Bureau), and annual rainfall (103 L/m2; data obtained from the Food and Agriculture Organization of the United Nations), as well as the Human Development Index (HDI) and per capita government expenditure on health (GEH), both of which were extracted from the United Nations Development Program Human Development Reports. The HDI ranks countries by their relative progress toward development goals and is based on the evaluation of three variables: life expectancy, educational attainment, and income. A lower HDI indicates a lower level of development.16
We also evaluated the time since the identification of endemicity (hereafter referred to as “age of endemicity”), defined as the interval between the year in which there was emergence or the most recent reemergence of dengue (followed by endemic-epidemic transmission of the virus) and the calendar year corresponding to the data set analyzed (between 1995 and 2009). On the basis of epidemiological surveillance records of the PAHO,15,17,18 the following years were defined as the dengue endemicity starting points: 1969 for the Caribbean; 1978 for Venezuela, Colombia, Guyana, Suriname, Honduras, El Salvador, Guatemala, Belize, and Mexico; 1981 for Brazil; 1985 for Nicaragua; 1987 for Bolivia; 1988 for Ecuador, Peru, and Paraguay; 1993 for Costa Rica and Panama; 1998 for Argentina; and 2002 for Chile.
Dengue mortality was selected as an indicator of disease burden. Although most of the economic burden of dengue is associated with nonfatal cases (outpatients and inpatients),19–21 we believe that the number of fatalities is a more reliable indicator of trends and determinants of the disease and its outcomes. This assumption is based on the fact that fatal cases are more likely to have been studied in depth to determine the etiology of the illness.22 In fact, various endemic countries, such as Colombia and Brazil, have specific protocols for the etiological study of all fatal cases of dengue by testing serum samples or samples of other types of tissue.23,24 In contrast, diagnostic tests are recommended in only a small proportion (< 10%) of cases of uncomplicated dengue.23 In addition, we believe that deaths are less susceptible to underreporting. This last assumption is based on observations suggesting that, in general, severe cases are less susceptible to underreporting than are less severe cases. In Puerto Rico, for example, for every case of dengue fever reported to the passive dengue surveillance system, 10–27 cases go unreported; and for every case of dengue hemorrhagic fever reported, about three cases go unreported.25,26 Therefore, by extension, we believe that fatal cases of dengue are less susceptible to underreporting than are nonfatal cases.
Data analysis.
Initially, we estimated mortality rates for each of the subregions, by period: 1995–1999; 2000–2004; and 2005–2009. Because data were not always available for full years, populations were weighted by the time of reporting. Univariate and multivariate analyses were performed to identify factors associated with dengue mortality (dependent variable). The continuous independent variables were evaluated at quantiles to determine whether their relationship with the dependent variable was linear or nonlinear. Brazil was used as a reference, because it has the largest exposed population and the highest number of deaths from dengue. We used Poisson regression analysis, calculating the rate ratio (RR) as a measure of association and the pseudo R2 as a measure of the proportion of variation explained by the variables and models.
Given that mortality is directly determined by incidence and case fatality rate (CFR), the number of deaths is conditioned not only by the factors that facilitate transmission but also by those that influence the severity of the disease and the ease of access to health care. Therefore, the factors associated with mortality were evaluated in two (secondary) multivariate models: one to predict the rate of clinical cases of dengue reported in the exposed population (incidence model); and another to predict the number of deaths among the reported clinical cases (CFR model). These two models were designed to identify the effect of a given determinant on dengue incidence and CFR, respectively.
Additional models, adjusted for previously identified determinants, were constructed to estimate the effect that circulating serotypes have on dengue mortality and the CFR. In this final analysis, we considered only the periods in which at least one dengue serotype was identified. Because of the lack of biological plausibility, serotype was not considered for a model of incidence.
Results
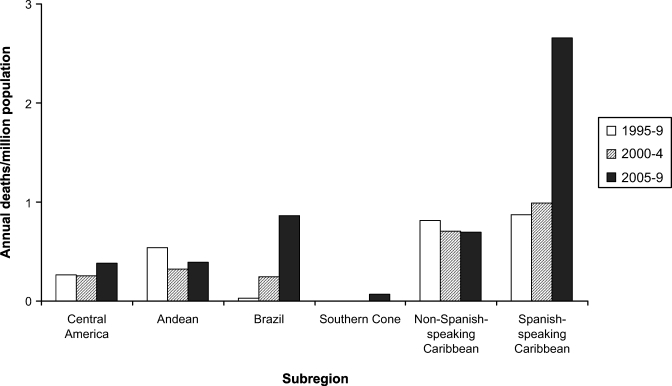
During the 15-year study period, 8.88 million clinical cases of dengue were reported and 2,870 deaths were attributed to the disease. Dengue mortality rates were highest in the Caribbean (Spanish-speaking and non-Spanish-speaking), followed by the Andean, Brazil, and Central America/Mexico. The mortality rate was lowest in the Southern Cone, where no dengue-related deaths had been reported before 2005. Over the course of the study period, dengue mortality rates increased in most of the subregions evaluated, the increase being particularly pronounced in Brazil and the Spanish-speaking Caribbean (Figure 1).
Figure 1.
Dengue-related mortality, which included suspected and laboratory-confirmed cases, in Latin America and the Caribbean, by subregion; 1995–2009.
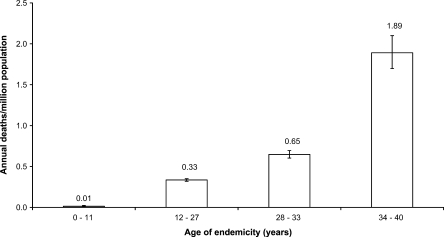
Univariate analysis indicated that the variable calendar year was significantly associated with mortality, which increased annually by 11% during the study period (Table 1). However, the calendar year explained < 9% of the variation in mortality. In contrast, the age of endemicity explained > 25% of the variation and showed a relationship consistent with linearity (Figure 2), the RR per each 10 years of endemicity being 3.2 (95% confidence interval [CI] = 3.02–3.39).
Table 1.
Univariate analysis of variables associated with dengue mortality in Latin America and the Caribbean, 1995–2009
| Variable | P-Y | Deaths | Crude RR (95% CI) | P value | Pseudo R2 (%) |
|---|---|---|---|---|---|
| Region | 7.14 | 2,870 | 14.67 | ||
| Brazil | Reference | ||||
| Andean subregion | 1.09 (0.99–1.20) | 0.09 | |||
| Central America/Mexico | 0.89 (0.80–0.98) | 0.02 | |||
| Non-Spanish-speaking Caribbean | 2.31 (1.83–2.90) | < 0.001 | |||
| Southern Cone | 0.09 (0.06–0.14) | < 0.001 | |||
| Spanish-speaking Caribbean | 4.22 (3.77–4.73) | < 0.001 | |||
| Calendar year | 7.14 | 2,870 | 1.11 (1.10–1.12) | < 0.001 | 8.51 |
| Age of endemicity (per each 10 years) | 7.14 | 2,870 | 3.2 (3.02–3.39) | < 0.001 | 25.6 |
| Population density | 7.14 | 2,870 | 19.11 | ||
| < 20 inhabitants/km2 | Reference | ||||
| 20–120 inhabitants/km2 | 6.17 (4.94–7.71) | < 0.001 | |||
| > 120 inhabitants/km2 | 24.13 (19.09–30.48) | < 0.001 | |||
| Annual rainfall (per 103 L/m2) | 7.14 | 2,855 | 1.90 (1.78–2.02) | < 0.001 | 6.45 |
| HDI ≥ 0.83 | 7.07 | 2,775 | 0.30 (0.25–0.34) | < 0.001 | 5.73 |
| GEH (per US$ 100) | 7.07 | 2,775 | 0.87 (0.84–0.89) | < 0.001 | 1.90 |
| Circulating serotypes | |||||
| Dengue 1 | 6.40 | 2,783 | 0.73 (0.66–0.82) | < 0.001 | 0.53 |
| Dengue 2 | 6.40 | 2,783 | 2.49 (2.07–2.99) | < 0.001 | 2.17 |
| Dengue 3 | 6.40 | 2,783 | 1.05 (0.97–1.14) | 0.22 | 0.03 |
| Dengue 4 | 6.40 | 2,783 | 1.49 (1.38–1.60) | < 0.001 | 1.75 |
P-Y = population-year (×109); RR = rate ratio; HDI = Human Development Index; GEH = government expenditure on health (in US dollars per capita).
Figure 2.
Dengue mortality rate (with 95% confidence interval), by age of endemicity. Deaths included suspected and laboratory-confirmed cases.
Other variables associated with dengue mortality were rainfall (RR = 1.9 [per 103 L/m2]; 95% CI = 1.78–2.02) and population density. However, the association between dengue mortality and population density appeared to be nonlinear, and the cutoff values were set at 20 inhabitants/km2 and 120 inhabitants/km2 (RR = 6.17 and 24.13, respectively; Table 1). Dengue mortality was significantly lower only in the highest HDI quintile, and the HDI cutoff value was set at 0.83, the RR was 0.3 (95% CI = 0.25–0.34). The GEH was associated with a reduction in mortality (RR = 0.87 [for each US$ 100]; 95% CI = 0.84–0.89). However, this variable explained only 1.9% of the variation in dengue mortality.
In a multivariate analysis model, age of endemicity, population density, rainfall, and HDI were independently associated with dengue mortality (Table 2), collectively accounting for 37.16% of the variation. The variables “calendar year” and GEH were strongly correlated with age of endemicity and HDI, respectively (Pearson's r = 0.56 and 0.70). Therefore, to avoid problems of collinearity, calendar year and GEH were not considered in the model.
Table 2.
Multivariate analysis of variables associated with dengue mortality in Latin America and the Caribbean*
| Variable | Adjusted RR (95% CI) | P value |
|---|---|---|
| Region | ||
| Brazil | Reference | |
| Andean subregion | 0.90 (0.80–1.01) | 0.08 |
| Central America | 1.07 (0.95–1.20) | 0.29 |
| Non-Spanish-speaking Caribbean | 0.3 (0.21–0.42) | < 0.001 |
| Southern Cone | 2.28 (1.41–3.69) | 0.001 |
| Spanish-speaking Caribbean | 1.04 (0.84–1.28) | 0.75 |
| Age of endemicity (per each 10 years) | 3.20 (2.92–3.51) | < 0.001 |
| Population density | ||
| < 20 inhabitants/km2 | Reference | |
| 20–120 inhabitants/km2 | 2.13 (1.64–2.78) | < 0.001 |
| > 120 inhabitants/km2 | 3.25 (2.37–4.45) | < 0.001 |
| Annual rainfall (per 103 L/m2) | 1.54 (1.38–1.72) | < 0.001 |
| HDI ≥ 0.83 | 0.37 (0.31–0.45) | < 0.001 |
Poisson regression was used in this analysis, and the rate ratios (RRs) in this model were adjusted for all variables in the table. In the multivariate analysis, data were available for 7.07×109 population-years and 2,775 deaths. The model exhibited a pseudo R2 of 37.16% in predicting the mortality rate.
RR = rate ratio; CI = confidence interval; HDI = Human Development Index.
When evaluating the factors independently associated with mortality in secondary models (which evaluated factors associated with incidence and CFR), we noted that the rate at which cases of dengue were reported was significantly higher in Brazil than in all of the other subregions (the adjusted rate ratio [aRR] for incidence was lower than 1.0 for all), because the CFR of dengue was lower in Brazil (the aRR for case fatality was higher than 1.0 for all other subregions, Table 3).
Table 3.
Secondary models to predict the incidence of reported cases and case fatality rate of dengue. Latin America and the Caribbean, 1995–2009*
| Variable | Incidence model | Case fatality rate model | ||
|---|---|---|---|---|
| (Pseudo R2 49.87%) | (Pseudo R2 48.3%) | |||
| Adjusted RR (95% CI) | P value | Adjusted RR (95% CI) | P value | |
| Region | ||||
| Brazil | Reference | Reference | ||
| Central America | 0.32 (0.32–0.32) | < 0.001 | 2.78 (2.48–3.11) | < 0.001 |
| Andean subregion | 0.37 (0.37–0.37) | < 0.001 | 3.41 (3.06–3.81) | < 0.001 |
| Non-Spanish-speaking Caribbean | 0.15 (0.15–0.15) | < 0.001 | 3.75 (2.66–5.29) | < 0.001 |
| Southern Cone | 0.25 (0.25–0.25) | < 0.001 | 6.03 (3.65–9.96) | < 0.001 |
| Spanish-speaking Caribbean | 0.13 (0.13–0.13) | < 0.001 | 13.01 (10.19–16.6) | < 0.001 |
| Age of endemicity (each 10 years) | 1.54 (1.53–1.54) | < 0.001 | 1.99 (1.82–2.17) | < 0.001 |
| Population density | ||||
| < 20 inhabitants/km2 | Reference | Reference | ||
| 20–120 inhabitants/km2 | 1.24 (1.24–1.24) | < 0.001 | 2.56 (1.93–3.40) | < 0.001 |
| > 120 inhabitants/km2 | 1.00 (0.99–1.01) | 0.91 | 2.50 (1.75–3.56) | < 0.001 |
| Annual rainfall (per 103 L/m2) | 2.11 (2.10–2.11) | < 0.001 | 0.95 (0.85–1.06) | 0.35 |
| HDI ≥ 0.83 | 1.26 (1.26–1.27) | < 0.001 | 0.27 (0.23–0.33) | < 0.001 |
This table presents two separate multivariate models: one to predict the dengue incidence rate and another to predict the case fatality rate. In the multivariate analysis, data were available for 7.07×109 population-years, 8,637,372 clinical cases, and 2,775 deaths.
RR = rate ratio; CI = confidence interval; HDI = Human Development Index.
According to the models, every 10 years of endemicity resulted in a 54% increase in the rate of reported cases and a 99% increase in the CFR. However, population density was mainly associated with the CFR, whereas rainfall was independently associated only with the incidence of clinical cases. Finally, the fact that HDI was found to be negatively and independently associated with mortality was validated by our finding of lower CFRs in countries with HDIs ≥ 0.83 (aRR = 0.27; 95% CI = 0.23–0.33; Table 3).
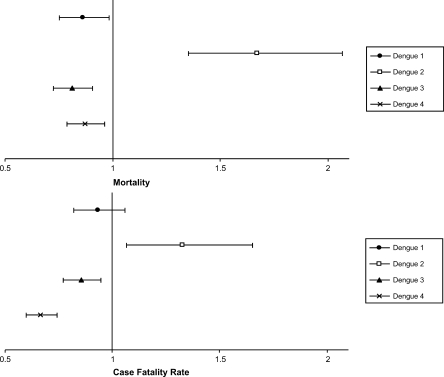
In the univariate analysis, circulation of the dengue 2 and dengue 4 serotypes was associated with greater mortality (Table 1). However, in multivariate models specific for assessing serotype as a biological determinant, only the dengue 2 serotype was independently associated with a significant increase in dengue mortality (aRR = 1.67; 95% CI = 1.35–2.07) and in the CFR of the disease (aRR = 1.33; 95% CI = 1.07–1.65, Figure 3).
Figure 3.
Rate ratios (with 95% confidence interval) for dengue mortality and dengue case fatality rate, associated with circulating serotypes. Estimates were adjusted for subregion, age of endemicity, rainfall, population density, and Human Development Index.
Discussion
The results of this study indicate that there was a marked increase in deaths from dengue in Latin America and the Caribbean over the 15-year period evaluated. This increase was most dramatic in Brazil and in the Spanish-speaking Caribbean. The Spanish-speaking Caribbean has had a history of being hyperendemic for dengue, and the 1981 epidemic of dengue hemorrhagic fever in Cuba was a hallmark of the recent reemergence of dengue in the Americas.12,27 In this region, consisting of Cuba, Dominican Republic, and Puerto Rico, the significant increase in mortality might be caused by multiple factors, including interactions between and among social and environmental determinants. These factors could explain why, despite the preventive measures taken, this region has not achieved the desired goal of reducing dengue mortality in recent years.28
However, regardless of the subregion, the variable that best explained the variation in dengue mortality was the age of endemicity (Table 1). Our results indicate that mortality rates tripled over every 10 years of endemicity. This association can be explained by several mechanisms. First, the fact that the simple spread of the disease has been unchecked by preventive measures, which have been poor or insufficient, has resulted in a greater incidence and consequently a higher number of dengue-related deaths. In addition, an increase in the age of endemicity increases the likelihood that individuals exposed to a first infection will develop a secondary infection with another serotype, the latter being the leading recognized risk factor for severe forms of the disease.29–34 Furthermore, longer time since the first infection increases the likelihood that a secondary infection will be more severe. For example, some epidemiological studies have suggested that a long interval between infections (which can only occur in regions with a long history of endemicity) is an additional risk factor for hospitalization and death.33 Moreover, phenomena such as virus-vector-host interactions could eventually lead to changes in the immunity of the population, and to the selection of viral genotypes with higher transmissibility and virulence.35,36 In view of these facts, it is not surprising that, in this study, the age of endemicity was the main predictor of death from dengue, maintaining a relationship with incidence and CFR (Table 3).
An alternative explanation for the relationship between the duration of the endemic period and dengue mortality is that surveillance improves over time. Surveillance systems are adapted on the basis of their experience with dengue. Surveillance activities are often stepped up when more cases are reported to the point that there is probably substantial over-reporting of dengue cases during outbreaks. However, because fatalities are typically studied in greater detail,22 we believe that this bias is less likely to affect mortality indicators (although it could affect the incidence rates of clinical cases).
Our analysis highlighted the importance of population density. Historically, population growth and uncontrolled urbanization have been linked to an increase in the incidence of dengue.12 The potential importance of this variable is evidenced by estimates that a community must have a population of at least 10,000 to sustain dengue transmission.11 Indeed, viral transmission to humans might be favored in urban areas, where there is close contact between vectors and the vertebrate hosts, as well as larval habitats, refuges, and appropriate microclimates that promote the survival of mosquito populations.37
Nevertheless, in our secondary models, population density was apparently more closely related to the CFR than to incidence (Table 3). Therefore, we hypothesize that health care in disorderly urbanized areas and in highly populated cities is inadequate or less effective, leading to higher CFRs. Another possibility is that, in areas of high population density, it is more common for severe cases to be prioritized,38,39 resulting in the underreporting of mild cases, which is a common bias of epidemiological surveillance.39,40 Therefore, in highly populated regions, it is likely that the incidence of dengue is underestimated and that the dengue CFR is overestimated.
Rainfall was another factor independently associated with dengue mortality. In the secondary models, this association was explained by the relationship between rainfall and incidence and not by that between rainfall and dengue CFR. This is consistent with studies conducted in endemic Latin American cities, in which it has been shown that rainfall correlates positively with the larval population density of the vector and with the incidence of dengue.41–43
Poverty has been identified as intrinsically related to the incidence of neglected tropical diseases such as dengue. Most of the countries with a low HDI and a greater burden of such diseases are located in tropical and subtropical regions.44 In this study, we observed an inverse association between HDI and dengue mortality. This association was almost entirely explained by the fact that the dengue CFR is lower (by 73%) in countries with HDIs ≥ 0.83. This could be attributable to an effect of poverty on access to or the quality of medical care for dengue. However, it is likely that the convergence of other biological factors, such as the coexistence of other neglected diseases, increases the risk of complications and death in developing countries.
We found that dengue mortality was nearly 2.5 times higher when the dengue 2 serotype is circulating. When adjusted for other factors, this association was weakened but remained statistically significant. This finding is consistent with those of epidemiological and clinical studies showing that the dengue 2 serotype, especially its Southeast Asian genotype is highly virulent. In vitro studies suggest that alterations in three genomic regions (5′- and 3′-untranslated regions, and E390) give a greater capacity for replication of this serotype, which has been associated with major epidemics in the Americas, with a high incidence of severe cases and mortality.11,31,32,45
One limitation of this study is that it was based on data from passive epidemiological surveillance systems whose ability to identify cases of dengue (sensitivity) is low. Other studies have suggested that there is underreporting of mild cases of dengue and that severe cases are misclassified.46–48 Because mortality statistics are less susceptible to such errors, we believe that mortality models are more reliable than are models of incidence or CFR, which can be skewed in opposing directions by factors that affect the reporting of clinical cases. This would explain our finding that fatality tended to be higher in regions in which the incidence was lower (Table 3).
Another major limitation of this study is that, as in other ecological studies, it was not possible to make individual inferences from aggregated data. In addition, we did not have the necessary data for an analysis adjusted for age and sex. This is because the mortality statistics, which are widely available, do not include distribution by age group for all countries and for all periods.14–16 This underscores the need to improve the systems of information of public health surveillance to facilitate trend analysis and comparisons between regions. However, despite the lack of individual data for a better adjustment of the estimates, we believe that this study provides information that is useful for hypothesis and for estimating the importance of some of the macro-determinants of dengue. In addition, ecological studies are extremely well suited to informing intersector public policies. Furthermore, the determinants identified highlight the importance of an interdisciplinary approach to study and reduce dengue-related mortality.
In summary, we have provided an estimate of the effect that age of endemicity has on dengue mortality. Our data also show how the confluence of factors related to environment (rainfall), demographics (population density), socioeconomics (HDI), and biology (circulating serotypes)—because of their effect on incidence, case fatality, or both—can contribute to the alarming increase in mortality and in the burden of dengue in Latin America and the Caribbean.
ACKNOWLEDGMENTS
We thank Ruth Aralí Martínez-Vega for her critiques and recommendations in reviewing the manuscript.
Footnotes
Financial support: This study was partially supported by a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development; grant no. 312131/2006-2) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education) through the Programa de Estudiante-Convênio de Pós-Graduação (PEC-PG, Graduate Student Support Program).
Authors' addresses: Fredi Alexander Díaz-Quijano, Departamento de Epidemiologia, Faculdade de Saúde Pública da Universidade de São Paulo, São Paulo, SP, Brazil, E-mail: frediazq@msn.com. Eliseu Alves Waldman, Faculdade de Saúde Pública da Universidade de São Paulo, São Paulo, SP, Brazil, E-mail: eawaldma@usp.br.
References
- 1.Nathan MB, Dayal-Drager R, Guzman M. Epidemiology, burden disease and transmission. World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR). Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition. Geneva: WHO Press; 2009. pp. 1–22. [Google Scholar]
- 2.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue hemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 4.Beauté J, Vong S. Cost and disease burden of dengue in Cambodia. BMC Public Health. 2010;10:521. doi: 10.1186/1471-2458-10-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapia-Conyer R, Méndez-Galván JF, Gallardo-Rincón H. The growing burden of dengue in Latin America. J Clin Virol. 2009;46((Suppl 2)):S3–S6. doi: 10.1016/S1386-6532(09)70286-0. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Scott TW, Amerasinghe PH, Morrison AC, Lorenz LH, Clark GG, Strickman JD, Kittayapong P, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J Med Entomol. 2000;37:89–101.. doi: 10.1603/0022-2585-37.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Porter KR, Beckett CG, Kosasih H, Tan RI, Alisiahbana B, Rudiman PI, Widjaja S, Listiyaningsih E, Ma'Roef CN, McArdle JL, Parwati Sudjana P, Jusuf H, Yuwond D, Wuryadi S. Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. Am J Trop Med Hyg. 2005;72:60–66. [PubMed] [Google Scholar]
- 9.Tan PC, Rajasingam G, Devi S, Omar SZ. Dengue infection in pregnancy: prevalence, vertical transmission, and pregnancy outcome. Obstet Gynecol. 2008;111:1111–1117. doi: 10.1097/AOG.0b013e31816a49fc. [DOI] [PubMed] [Google Scholar]
- 10.Waterman SH, Novak RJ, Sather GE, Bailey RE, Rios I, Gubler DJ. Dengue transmission in two Puerto Rican communities in 1982. Am J Trop Med Hyg. 1985;34:625–632. doi: 10.4269/ajtmh.1985.34.625. [DOI] [PubMed] [Google Scholar]
- 11.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 12.Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. doi: 10.1016/s1386-6532(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 13.Guzman A, Istúriz RE. Update on the global spread of dengue. Int J Antimicrob Agents. 2010;36((Supp 1)):S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 14.San Martín JL, Brathwaite O, Zambrano B, Solórzano JO, Bouckenooghe A, Dayan GH, Guzmán MG. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan American Health Organization (PAHO) Number of Reported Cases of Dengue and Dengue Hemorrhagic Fever (DHF) in the Americas, by Country from 1995 through 2009. 2009. [Google Scholar]
- 16.United Nations Development Programme . Human Development Report 1995. New York: Oxford University Press.; 1995. [Google Scholar]
- 17.Organización Panamericana de la Salud El resurgimiento del dengue [The resurgence of dengue] Boletín epidemiológico. 1997;18:1–16. [Google Scholar]
- 18.Siqueira JB, Jr, Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armien B, Suaya JA, Quiroz E, Sah BK, Bayard V, Marchena L, Campos C, Shepard DS. Clinical characteristics and national economic cost of the 2005 dengue epidemic in Panama. Am J Trop Med Hyg. 2008;79:364–371. [PubMed] [Google Scholar]
- 20.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, Tan LH, Kongsin S, Jiamton S, Garrido F, Montoya R, Huy R, Castillo L, Caraman M, Sah BK, Sughayyar R, Tyo KR, Halstead SB. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009;80:846–855. [PubMed] [Google Scholar]
- 21.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Araújo JM, Schatzmayr HG, de Filippis AM, Dos Santos FB, Cardoso MA, Britto C, Coelho JM, Noqueira RM. A retrospective survey of dengue virus infection in fatal cases from an epidemic in Brazil. J Virol Methods. 2009;155:34–38. doi: 10.1016/j.jviromet.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Grupo de Vigilancia y Control en Salud Pública. Instituto Nacional de Salud . Protocolo de Vigilancia y Control de Dengue [Protocol of Dengue Surveillance and Control] 2010. [Google Scholar]
- 24.Secretaria de Vigilância em Saúde. Ministério da Saúde . Protocolo de Investigação de Óbitos de Dengue. 2009. [Google Scholar]
- 25.Rigau-Perez JG. Surveillance for an emerging disease: dengue hemorrhagic fever in Puerto Rico, 1988–1997. Puerto Rico Association of Epidemiologists. P R Health Sci J. 1999;18:337–345. [PubMed] [Google Scholar]
- 26.Dechant EJ, Rigau-Perez JG. Hospitalizations for suspected dengue in Puerto Rico, 1991–1995: estimation by capture-recapture methods. The Puerto Rico Association of Epidemiologists. Am J Trop Med Hyg. 1999;61:574–578. doi: 10.4269/ajtmh.1999.61.574. [DOI] [PubMed] [Google Scholar]
- 27.Guzmán MG, Triana C, Bravo J, Kourí G. The estimation of the economic damages caused as a consequence of the epidemic of hemorrhagic dengue in Cuba in 1981. Rev Cubana Med Trop. 1992;44:13–17. [PubMed] [Google Scholar]
- 28.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infection in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel JM, Bonet M, Ibarra AM, Pagliccia N, Ouellette V, Yassi A. Social and environmental determinants of Aedes aegypti infestation in Central Havana: results of a case-control study nested in an integrated dengue surveillance programme in Cuba. Trop Med Int Health. 2007;12:503–510. doi: 10.1111/j.1365-3156.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 30.Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Erlin, Sutaryo, Porter KR, Halstead SB. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–419. doi: 10.4269/ajtmh.1999.61.412. [DOI] [PubMed] [Google Scholar]
- 31.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 32.Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aye KM, Aaskov K. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 33.Guzmán MG, Kourí G, Valdés L, Bravo J, Vázquez S, Halstead SB. Enhanced severity of secondary dengue-2 infections: death rates in 1981 and 1997 Cuban outbreaks. Rev Panam Salud Publica. 2002;11:223–227. doi: 10.1590/s1020-49892002000400003. [DOI] [PubMed] [Google Scholar]
- 34.Halstead SB, Rojanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983;32:154–156. doi: 10.4269/ajtmh.1983.32.154. [DOI] [PubMed] [Google Scholar]
- 35.Rico-Hesse R. Dengue virus virulence and transmission determinants. Curr Top Microbiol Immunol. 2010;338:45–55. doi: 10.1007/978-3-642-02215-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moncayo AC, Fernandez Z, Ortiz D, Diallo M, Sall A, Hartman S, Davis CT, Coffey L, Mathiot CC, Tesh RB, Weaver SC. Dengue emergence and adaptation to peridomestic mosquitoes. Emerg Infect Dis. 2004;10:1790–1796. doi: 10.3201/eid1010.030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gleiser RM, Zalazar LP. Distribution of mosquitoes in relation to urban landscape characteristics. Bull Entomol Res. 2010;100:153–158. doi: 10.1017/S0007485309006919. [DOI] [PubMed] [Google Scholar]
- 38.Kerr EA, Heisler M, Krein SL, Kabeto M, Langa KM, Weir D, Piette JD. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med. 2007;22:1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brabazon ED, O'Farrell A, Murray CA, Carton MW, Finnegan P. Under-reporting of notifiable infectious disease hospitalizations in a health board region in Ireland: room for improvement? Epidemiol Infect. 2008;136:241–247. doi: 10.1017/S0950268807008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rigau-Pérez JG. Clinical manifestations of dengue hemorrhagic fever in Puerto Rico, 1990–1991. Puerto Rico Association of Epidemiologists. Rev Panam Salud Publica. 1997;1:381–388. doi: 10.1590/s1020-49891997000500007. [DOI] [PubMed] [Google Scholar]
- 41.Díaz-Quijano FA, González-Rangel AL, Gómez-Capacho A, Espíndola-Gómez R, Martínez-Vega RA, Villar-Centeno LA. Rainfall and acute febrile syndrome in a dengue-endemic area. Rev Salud Publica (Bogota) 2008;10:250–259. doi: 10.1590/s0124-00642008000200005. [DOI] [PubMed] [Google Scholar]
- 42.Souza SS, Silva IG, Silva HH. Association between dengue incidence, rainfall and larval density of Aedes aegypti, in the State of Goiás. Rev Soc Bras Med Trop. 2010;43:152–155. doi: 10.1590/s0037-86822010000200009. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez L, Vanlerberghe V, Alfonso L, Marquetti Mdel C, Guzman MG, Bisset J, van der Stuyft P. Aedes aegypti larval indices and risk for dengue epidemics. Emerg Infect Dis. 2006;12:800–806. doi: 10.3201/eid1205.050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindoso JA, Lindoso AA. Neglected tropical diseases in Brazil. Rev Inst Med Trop Sao Paulo. 2009;51:247–253. doi: 10.1590/s0036-46652009000500003. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira MF, Galvao Araujo JM, Ferreira OC, Jr, Ferreira DF, Lima DB, Santos FB, Schatzmayr HG, Tanuri A, Ribeiro Noqueira RM. Two lineages of dengue virus type 2, Brazil. Emerg Infect Dis. 2010;16:576–578. doi: 10.3201/eid1603.090996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martínez-Vega RA, Díaz-Quijano FA, Villar-Centeno LA. Low concordance between early clinical suspicion of dengue and its serological confirmation. Rev Med Chil. 2006;134:1153–1160. [PubMed] [Google Scholar]
- 47.Balmaseda A, Hammond SN, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Harris E. Short report: assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. Am J Trop Med Hyg. 2005;73:1059–1062. [PubMed] [Google Scholar]
- 48.Camacho T, de la Hoz F, Cárdenas V, Sánchez C, de Calderón L, Pérez L, Bermúdez A. Incomplete surveillance of a dengue-2 epidemic in Ibagué, Colombia, 1995–1997. Biomedica. 2004;24:174–182. [PubMed] [Google Scholar]