Abstract
Yellow fever vaccine provides long-lasting immunity. Rare serious adverse events after vaccination include neurologic or viscerotropic syndromes or anaphylaxis. We conducted a systematic review of adverse events associated with yellow fever vaccination in vulnerable populations. Nine electronic bibliographic databases and reference lists of included articles were searched. Electronic databases identified 2,415 abstracts for review, and 32 abstracts were included in this review. We identified nine studies of adverse events in infants and children, eight studies of adverse events in pregnant women, nine studies of adverse events in human immunodeficiency virus-positive patients, five studies of adverse events in persons 60 years and older, and one study of adverse events in individuals taking immunosuppressive medications. Two case studies of maternal–neonate transmission resulted in serious adverse events, and the five passive surveillance databases identified very small numbers of cases of yellow fever vaccine-associated viscerotropic disease, yellow fever vaccine-associated neurotropic disease, and anaphylaxis in persons ≥ 60 years. No other serious adverse events were identified in the other studies of vulnerable groups.
Background
Yellow fever is endemic in the tropical areas of 45 African and Latin America countries with a total population of over 900 million. In jungles and forests in Africa, yellow fever virus is transmitted by Aedes africanus mosquitoes; in South America, it is transmitted by Haemagogus and Sabethes species, with monkeys as the primary host and forest dwellers, loggers, and construction workers as the infected groups. In savannah areas in Africa, Aedes species transmit the virus to monkeys or humans as the intermediate hosts, and in urban areas, Ae. aegyptii transmits infection between humans. It is the urban cycle that is particularly capable of generating rapidly increasing epidemics.1
The majority of yellow fever virus infections are asymptomatic.1 Monath and others2 estimate that 15% of those people infected with yellow fever virus develop moderate or severe disease with jaundice, that reported case fatality rates vary widely, and in one series of West Africans with jaundice, that the fatality rate approximated 20%. The number of yellow fever cases proven by clinical examination and detailed laboratory investigations is small, and it certainly underenumerates the true number of cases.
An outbreak of yellow fever can go undetected, because the signs and symptoms of yellow fever are similar to viral hepatitis, malaria, leptospirosis, typhus, Ebola hemorrhagic fever, and other hemorrhagic fevers. It is difficult for health workers to make a definitive diagnosis based on the signs and symptoms alone. Additionally, mild cases may go undetected, because the patient is likely to be treated at home and not seek care in a health facility.3
In 1992, the World Health Organization (WHO) estimated that yellow fever virus caused 200,000 cases of clinical disease and 30,000 deaths annually,4 and the survey by Rogers and others5 of the literature in 2006 repeated and did not update these estimates.
Yellow fever vaccination very rarely causes severe adverse neurologic, multisystem, or anaphylaxis reactions. The neurologic syndromes (encephalitis, myelitis, or encephalomyelitis) can be difficult to distinguish from bacterial encephalitis/meningitis or malaria, and the viscerotropic syndrome (which can result in multiorgan failure of the liver, kidneys, heart, and circulation) can be difficult to distinguish from hemorrhagic fevers, viral hepatitis, and many other causes of multisystem failure.6 There are no effective medications for these adverse effects of vaccination, and treatment consists of supportive care, including the intensive care unit (ICU). The number of these serious adverse events attributable to yellow fever vaccination that have been proven by clinical examination and detailed laboratory investigations is very small.
17D vaccines using the substrains 17DD and 17D-204 have very low interlot variability and high genetic stability levels; they are highly immunogenic (91–100%)6,7 and provide an estimated more than 40 years of protection. More than 600 million doses of yellow fever (YF) vaccines (YFVs) have been distributed worldwide.
The WHO advises vaccination for all individuals ≥ 9 months living in countries or areas at risk, except pregnant females and breastfeeding mothers, who may be vaccinated during epidemics or if unavoidably traveling to high-risk areas. Similarly, the WHO advises that individuals 60 years and older are at risk for severe adverse events after vaccination, and the risk and benefits need to be weighed.8
The US Advisory Committee on Immunization Practices (ACIP) indicates that YF vaccine is contraindicated in those people with sensitivity to eggs or chicken, infants < 6 months, individuals with thymus disorders or who have had a thymectomy, individuals with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS), and individuals on immunosuppressive therapies. ACIP advises precaution in vaccinating infants 6–8 months, individuals ≥ 60 years, individuals with asymptomatic HIV infection and moderate immune suppression (CD4 count = 200–499/mm3 for persons ≥ 6 years or 15–24% of total lymphocytes for children < 6 years), pregnant women, and breastfeeding women. The ACIP notes the limited database for these recommendations.1
The most complete description of the etiology, pathology, and vaccines for YF is in the work by Monath and others.2 A description of the symptoms of YFV-associated neurotropic disease (YEL-AND) and YFV-associated viscerotropic disease (YEL-AVD) and the Centers for Disease Control and Prevention (CDC) Working Group’s definitions are provided in the work by Staples and others.1 The Brighton Collaboration’s case definitions and guidelines for encephalitis, myelitis, and acute disseminated encephalomyelitis are presented in the work by Sejvar and others,9 and the Brighton Collaboration’s case definitions and guidelines for anaphylaxis are presented in the work by Rüggeberg and others.10 The Brighton Collaboration website (accessed October 10, 2011) indicates that the viscerotropic case definitions and guidelines are in progress. The mild reactions in vaccine-naïve subjects are usually low-grade fever, mild headache, arthralgias, and myalgias, and 15–20% of vaccinees may experience these reactions.11
We wished to ascertain the rate of serious adverse events in vulnerable populations which may be at greater risk of experiencing a serious YFV-related adverse events (AEs). These vulnerable populations include children, pregnant women, individuals with HIV, cancer, transplants, or immunosuppressive medication regimens, and older patients
Materials
We conducted a systematic review of AEs associated with YF vaccination. Serious AEs (SAEs) included neurotropic disease, viscerotropic disease, anaphylaxis/hypersensitivity, and other life-threatening events. Life-threatening events were defined as medical conditions that could, in theory, result in death or severe disability affecting a person’s autonomy, even if the affected individual does not suffer any of the outcomes during the course of their illness.12
We searched these electronic databases for reports of single studies and systematic reviews: the Cochrane Library (including the Cochrane CENTRAL Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the UK National Health Service (NHS) Database of Abstracts of Reviews of Effects), MEDLINE, EMBASE, BIOSIS Previews, Global Health, CAB Abstracts (a bibliographic database compiled by CAB International), and the Lilacs Database of Latin American and Caribbean literature. The searches included all languages, and no date limits were applied. Reference lists of included papers were scanned to identify additional studies of relevance.
Methods
All abstracts and full text articles were independently read by two reviewers to determine relevance, with any disagreements resolved by a third reviewer. The methods are reported in detail in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (Table 1). Results for studies using active surveillance are presented separately from results using passive surveillance: well-designed and conducted RCTs (randomized controlled trials) using active surveillance will provide more complete data but are likely to be too small to detect rare AEs, whereas passive surveillance studies (if they use a large database) may be able to detect rare sentinel events.
Table 1.
The safety of YFV 17D or 17DD in children, pregnant women, HIV+ individuals, or older persons: systematic review
| Section/topic | No. | Checklist item |
|---|---|---|
| Abstract | ||
| Structured summary | 2 | Background: Rare serious adverse events after vaccination include neurologic syndromes (encephalitis, myelitis, or ADEM = Acute disseminated encephalomyeliitis) or viscerotropic syndrome (multiorgan failure of the liver, kidneys, heart, and circulation). Objectives: To assess the rates of both serious and non-serious YFV AEs in vulnerable populations including children, pregnant women, older patients, or individuals with HIV, cancer, transplants, or immunosuppressive medication regimens. Data sources: The Cochrane Library (Cochrane CENTRAL Register of Controlled Trials, Cochrane Database of Systematic Reviews, NHS Database of Abstracts of Reviews of Effects [DARE], MEDLINE, EMBASE, BIOSIS Previews, Global Health, CAB Abstracts, and Lilacs Database of Latin America and Caribbean literature). Study eligibility criteria: Any research design. No language or date limits were applied. Participants: Children, pregnant women, older patients, or individuals with HIV, cancer, transplants, or immunosuppressive medication regimens who had received YFV. Interventions: YF vaccination 17D or 17DD. Study appraisal and synthesis methods: Independent assessment by two reviewers of all titles and abstracts as well as full text articles. Independent data entry into Excel database. Insufficient studies were identified for each group of participants to permit meta-analysis. Results: 2,415 abstracts were identified for review. Of these, 472 were selected for full text retrieval, and 32 were included in this review, which includes the above population groups of interest. We identified nine studies of infants and children (active surveillance; N = 1,866), two case-reports of maternal–neonate transmission, eight studies of pregnant women (active surveillance; N = 1,381), eight studies of HIV+ patients (active surveillance; N = 102), and five passive surveillance databases that reported data for all age groups and permitted extraction of data for older persons. Individual studies reported active, passive, or mixed active–passive surveillance techniques used to identify AEs. Two cases of maternal–neonate transmission causing serious adverse events were identified; otherwise, there were no adverse events in children, pregnant females, or HIV+ individuals. The five national-level passive surveillance databases identified very small numbers of cases of YEL-AVD and YEL-AND in the age groups 60 years and older, and the numbers are so small that comparing rates for decade-wide age groups would be inappropriate. Limitations: Small n values in datasets and passive surveillance missing many cases. Conclusions and implications of key findings: Two cases of maternal–neonate transmission causing SAEs and very small numbers of cases of YEL-AVD and YEL-AND were identified in the five national-level databases, including in individuals 60 years and older. |
| Introduction | ||
| Rationale | 3 | There is no existing review of the AEs of YF vaccination in these populations. |
| Objectives | 4 | Participants: Children, pregnant women, HIV+ individuals, older persons, and individuals with cancer, transplants, and immunosuppressive medication regimens. Intervention: YF vaccination 17D or 17DD. Comparisons: AEs in studies using active vs. passive surveillance. Outcomes: SAEs of YF vaccination. Study design: RCTs or cohort studies. |
| Methods | ||
| Protocol and registration | 5 | A systematic review of YF vaccination-associated SAEs. Protocol: Revised version March 3, 2010; submitted to WHO. |
| Eligibility criteria | 6 | All studies addressing the PICOS (Population, Intervention, Comparison, Outcomes, Study Design) criteria in any language with no date limits. |
| Information sources | 7 | The Cochrane Library (Cochrane CENTRAL Register of Controlled Trials, Cochrane Database of Systematic Reviews, NHS Database of Abstracts of Reviews of Effects [DARE], MEDLINE, EMBASE, BIOSIS Previews, Global Health, CAB Abstracts, and Lilacs Database of Latin America and Caribbean literature) was searched to identify relevant studies. Reference lists of included articles were scanned to identify additional studies. Last search was December 15, 2010. |
| Search | 8 | MEDLINE (OVID 1950 to December 2010) |
| Cochrane CENTRAL Register of Controlled Trials (OVID 4th Quarter, 2010) | ||
|
||
| Study selection | 9 | Populations: Children, pregnant women, HIV+ individuals, older persons, or individuals with cancer, transplants, or immunosuppressive medication regimens. Intervention: Received 17D or 17DD YFV. Study design: Any study design. |
| Data collection process | 10 | Independent extraction of data into Excel and ACCESS databases using standardized forms. |
| Data items | 11 | Population: Children, pregnant women, HIV+ individuals, older persons, or individuals with cancer, transplants, or immunosuppressive medication regimens. Intervention: YFV 17D or 17DD. Outcomes: YFV SAEs: neurologic syndromes (encephalitis, myelitis, or ADEM) or viscerotropic syndrome. |
| Risk of bias in individual studies | 12 | Cochrane Collaboration criteria for risk of bias were applied to the RCTs identified. |
| Summary measures | 13 | Numbers of SAEs and SAEs per million vaccinations. |
| Synthesis of results | 14 | It was planned to cumulate numbers of serious events for each population group of interest and report results as SAEs per million vaccinations. |
| Risk of bias across studies | 15 | Of the four studies of children, none described the method of randomization other than to say that the children “were randomized,” two described concealment of the allocation from the researchers and blinding of participants, and two experienced attrition. |
| Additional analyses | 16 | There were insufficient data for sensitivity or subgroup analyses within the population groups of interest. |
| Results | ||
| Study selection | 17 | 2,415 abstracts were identified for review. Of these, 472 were selected for full text retrieval, and 32 were included in this review, because they included the populations of interest. |
| Study characteristics | 18 | Study size, population, intervention (17D or 17DD vaccine), and outcomes (neurologic syndromes [encephalitis, myelitis, or ADEM] or viscerotropic syndrome). All studies were from 1930 to present. |
| Risk of bias within studies | 19 | Of the four RCTs of children, none described the method of randomization other than to say that the children “were randomized,” two described concealment of the allocation from the researchers and blinding of participants, and two experienced attrition. For the cohort studies of children and the other groups of interest, no study presented complete data on possible confounders or differential diagnoses. |
| Results of individual studies | 20 | We identified four RCTs in children that used active surveillance for adverse events (N = 1,866) and five studies that used passive surveillance. We identified four studies of pregnant women that used active surveillance (N = 1,381) and four studies that used passive surveillance. We identified one study of HIV+ individuals that used active surveillance (N = 102), six studies that used passive surveillance, and a review. We identified five national-level passive surveillance databases that analyzed rates of adverse events for older persons and reported very small numbers of cases of SAEs, YEL-AVD, YEL-AND, and anaphylaxis/hypersensitivity for all age groups, including those individuals 60 years and older; it would be inappropriate to compare rates for decade-wide age groups based on such small numbers. |
| Synthesis of results | 21 | Two cases of maternal–neonate transmission causing SAEs and very small numbers of cases of YEL-AVD and YEL-AND were identified in national passive surveillance databases for all age groups, including individuals 60 years and older. |
| Risk of bias across studies | 22 | With the exception of the two recent case reports of maternal–neonate transmission, no other study was designed to include definitive techniques (e.g., PCR amplicon analysis) to show replication of YFV in tissue from the individual with an SAE after YF vaccination or definitively test for likely differential diagnoses (e.g., bacterial encephalitis). |
| Additional analysis | 23 | No sensitivity or subgroup analyses or metaregression were performed. |
| Discussion | ||
| Summary of evidence | 24 | Two cases of maternal–neonate transmission resulting in SAEs were identified, and small numbers of cases of YEL-AVD, YEL-AND, and anaphylaxis in the five national-level passive surveillance databases for all age groups, including individuals 60 years and older, were identified. |
| Limitations | 25 | Small n values in datasets; passive surveillance is likely to miss many SAEs, particularly in remote populations or areas with insufficient medical resources to make definitive diagnoses. |
| Conclusions | 26 | Larger datasets are required using active surveillance to provide definitive estimates |
| Funding | ||
| Funding | 27 | The Global Advisory Committee on Vaccine Safety (GAVCS) requested that the WHO commission an independent systematic review of the safety of YFV. The systematic review was funded by The Global Alliance for Vaccines and Immunization (GAVI). The scientific independence of the researchers was at all times maintained. The sponsors did not participate in the collection, analysis, or interpretation of data or the writing of the report. |
Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. For more information, visit www.prisma-statement.org.
Confidence intervals were calculated using the Poisson approximation to the binomial distribution.13 The studies that used active surveillance identified no SAEs in infants/children or pregnant females. Accordingly, the convention is to report the upper limit to the 95% confidence interval and the point estimate is zero. We have computed this value for the four RCTs of children and the four studies of pregnant females that used active surveillance (Tables 3–5). Marked caution should be used in interpreting risk estimates computed for rare events based on small numbers of patients assessed.
Table 3.
Studies of children, pregnant females, HIV+ individuals, patients taking immunosuppressive medications, and older persons
| Study location, date, and reference | N, vaccine administered, ages, and seronegatives | Exclusions | N minor reactions | N adverse reactions |
|---|---|---|---|---|
| Children: active surveillance | ||||
| Peru, 200518 | 738 17D Arilvax; 369 17D YF-VAX; (9 months to 10 years); 36 immune at baseline | 0 | ARILVAX (N = 738): pyrexia 27%, pharyngitis 17%, diarrhea NOS (Not Otherwise Specified) 12%, nasopharyngitis 9%, cough 7%, pharyngotonsillitis 6%, headache 6%, vomiting 6% NOS 6%. YF-VAX (N = 369): pyrexia 27%, pharyngitis 15%, diarrhea 11%, nasopharyngitis 9%, decreased appetite NOS 11%, malaise 7%, cough 5%, pharyngotonsillitis 5%, headache 7%, vomiting NOS 3% | 0 |
| Ivory Coast, 198919 | 74 17D (66 seronegative at baseline); 85 17D and measles (69 seronegative at baseline) | 0 | 0 | 0 |
| Ghana, 200120 | 6 months N = 220; 9 months N = 164; 17D; (all seronegative at baseline) | 0 | Age 6 months: fever 51 (24%); cough 21 (10%); rash 13 (7%); running nose 5 (2%); diarrhea 9 (5%); vomiting 3 (1.5%); injection site redness 36 (18%); or swelling 1 (0.5%) Age 9 months: fever 42 (26%); cough 13 (8%); rash 8 (5%); running nose 11 (7%); diarrhea 12 (7%); vomiting 0; injection site redness 29 (18%); or swelling 0 | 0 |
| Mali, 199121 | Ages 4–8 months: n 17D = 71, 17D + measles vaccine N = 58; Ages 12–24 months: n 17 D = 44; n 17D + measles = 43* | 0 | Those who received only 17D: (1) N = 71 (4–8 months): fever ≥ 38°C 20%; induration 3%; eruption 1%, conjunctivitis 11% (2) N = 44 (12–24 months): fever 23%; induration 2%, eruption 2%, conjunctivitis 0%. Those who received 17D and measles vaccine: (1) N = 58 (4–8 months): fever ≥ 38°C 21%; induration 2%; eruption 2%, conjunctivitis 5% (2) N = 43 (12–24 months): fever 30%; induration 2%, eruption 0, conjunctivitis 2% | 0 |
| Children: passive surveillance | ||||
| Cameroon, 199022 | 139 17D (126 serognegative at baseline); (6–10 months) | 0 | 0 | 0 |
| Nigeria, 195523 | 35,000 (“many infants”) 17D, | 0 | 0 | 0 |
| 201026 | 3 breastfed neonates 10 days, 23 days and 5 weeks (mothers received 17D during first month of infant’s life) | 0 | 0 | 3 (encephalitis) |
| Brazil, 200924 | One neonate (15 days), (mother received 17DD) | 0 | 0 | 1 (convulsions) |
| 201125 | One neonate (10 days), (mother received 17D and typhoid) | 0 | 0 | 1 (meningoencephalitis) |
| Pregnant women: active surveillance | ||||
| Brazil, 200727 | 312 females who received 17DD and their infants (no serological tests during YFV campaign, so % seronegative not assessed) | Women vaccinated > 15 days before last menstrual period; 8 mother-infant pairs not followed up | No assessment | 10 malformations |
| Nigeria, 199328 | 101 17D (15–50 years) (22 had IgM antibodies before and 31 after YFV; 18 neutralizing antibodies before and 50 after YFV) | 0 | “no clinical symptoms resembling YF” after YF vaccination | 0 maternal deaths, 0 perinatal deaths, 0 growth retardation |
| Brazil, 200129 | 488 females received 17DD during campaign (no serological tests during YFV campaign, so % seronegative not assessed) | 19% minor symptoms “mostly headache, fever, myalgias.” | 0 | |
| Brazil, 200630 | 480 females who received 17DD during YFV campaign, 441 followed up, 304 evaluated by a geneticist (no serological tests during YFV campaign, so % seronegative not assessed) | 39 not followed up | headache 10.6%, other (not stated) 9% | 7 malformations |
| Pregnant women: passive surveillance | ||||
| Switzerland, 200831 | 6 females who attended a travel clinic, 17D, | Of 101 travel clinic attendees, 24 not contactable, 4 did not return questionnaires, 49 not pregnant | 0 | 0 |
| Brazil, 199832 | 39 females, 17D, during YFV campaign, with spontaneous abortion | 0 | 0 | 0 |
| France, Germany, Holland, and Israel, 199933 | 58 females reported by Pasteur Merieux Connaught and by Teratology Information services in Berlin, Bilthoven, Jerusalem, and 2 in Lyon (46 live births) | 18 incomplete information | 0 | 3–4% major malformations, 7 spontaneous abortions |
| Trinidad & Tobago, 199334 | 35 females, 17DD or 17 D, vaccinated in YFV campaign | survey of hospitals suggested 100–200 other pregnant females vaccinated | 0 | 0 |
| HIV+ individuals: active surveillance | ||||
| Four centers in Swiss HIV cohort study, 200935 | 102 received 17D after HIV diagnosis; median CD4 count 512/mm3 (range 368–664) | 2648 reported in 7 centers as having traveled to tropical country, 4 included centers reported 868, no information 172/868 travellers | 0 | 0 |
| HIV+ individuals: passive surveillance | ||||
| France, 199536 | 44 patients (all CD4 count > 200/mm3), Hôpital de l'Institut Pasteur, Paris, 17D | patients with CD4 counts < 200 | Not assessed | 0 |
| Brazil, 200842 | 7 (5 with CD4 counts > 200/mm3), University of São Paulo, YFV vaccine type not stated | Of 3598 patients at HIV clinic, random chart review of 144 | Not assessed | 0 |
| France, 200837 | 103, Hôpital Saint Antoine, Paris, 17D | Not assessed | 0 | |
| France, 2007 and 201040,41 | 23 (22 with CD4 count > 200/mm3), center hospitalier universitaire, Bordeaux, 17D | 5 (no follow-up) | 4 (fever 1, headache 1, asthenia 2) | 0 |
| France, 200038 | 2 (CD4 counts 674 and 1000/mm3), Bordeaux, 17D | 0 | Not assessed | 0 |
| France, 200439 | 12 (average CD4 count 485/mm3), university hospital, Rennes, 17D | 0 | Not assessed | 0 |
| Individuals taking immunosuppressive medications | ||||
| Brazil, 200945 | 70, University Hospital and a private hospital, Brasília (50 rheumatoid arthritis, 9 systemic lupus, 2 systemic sclerosis, 2 rheumatoid arthritis + lupus) | 0 | 1 rash; 2 fever; 3 myalgia; 1 arthralgia; 1 headache; 1 headache + diarrhea; 1 headache + myalgia/arthralgia; 1 myalgia + fever; 1 local pain, myalgia, arthralgia; 1 headache + myalgia; 1 arthralgia and fever (but all patients had a rheumatological disease) | 0 |
| Older Persons: passive surveillance in pharmacovigilance databases | ||||
| United States, 200512 | VAERS (Vaccine Adverse Event Reporting System) database 1990–2002; n 60–69 = 188,870; N ≥ 70 = 93,565 | 0 | Not assessed | SAEs* ages 60–69 = 8; age ≥ 70 = 7; YEL-AVD ages 60–69 = 2; age ≥ 70 = 3; YEL-AND ages 60–69 = 3; age ≥ 70 = 1; |
| United States, 200814 | VAERS database 2000−2006; n 60–69 = 191,025; N ≥ 70 = 87,177 | 0 | For all 660 reporting adverse events: fever 23%, pain 15%, pruritus 15%, headache 15%, injection site erythema 13%, urticaria 12%, rash 11%, nausea 9%, dizziness 8%, dyspnea 7% (total = 841 events) | SAEs* ages 60–69 = 12; age ≥ 70 = 11; YEL-AVD ages 60–69 = 2; age ≥ 70 = 2; YEL-AND ages 60–69 = 3; age ≥ 70 = 2; Anaphylaxis ages 60–69 = 0; age ≥ 70 = 0; |
| United States, 200116 | VAERS database 1990−1998; n 45–64 = 442,605; n 65–74 = 86,222; N ≥ 75 = 22,085 | 0 | Not assessed | SAEs** ages 44–64 = 12; age 65–74 = 5; ≥ 75 = 4 |
| Australia, 200415 | 1993–2002; n 45–64 = 48,697, N ≥ 65 = 8,984 | 0 | Not assessed | SAEs*** ages 45–64 = 4; age ≥ 65 = 2; YEL-AND = 0 ; YEL-AVD = 0 |
| United Kingdom, 200517 | 1995–1999; n’s are extrapolated from n of vaccinations for same age groups given by GP’s: n 45–64 = 270,420 (GP’s gave 3,272); n 65–74 = 34,960 (GP’s gave 423); N > 75 = 8,595 (GP’s gave 104) | 0 | Not assessed | SAEs**** ages 45–64 = 15; age 65–74 = 3; ≥ 75 = 0 |
NOS = not otherwise specified.
The numbers in the serological study are smaller than in the clinical study, and thus, the number of seronegatives at baseline in the clinical study is not clear.
Table 5.
95% upper confidence limits for the zero relative risks identified in studies of infants and females
| References | Total n | Estimated risk | 95% Confidence interval upper limit |
|---|---|---|---|
| Children | |||
| 18–21 | 1,866 | 0 | 2.0 events/1,000 doses |
| Pregnant females | |||
| 27–30 | 1,381 | 0 | 2.7 events/1,000 doses |
Results and Discussion
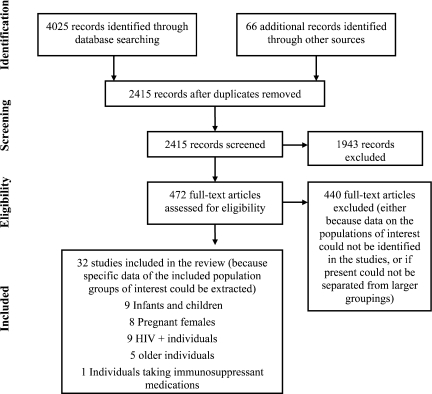
Electronic database and other searching identified 4,025 abstracts for review, and 66 abstracts were identified from other sources (2,415 after removal of duplicates). Of these abstracts, 472 were selected for full text retrieval, because they were relevant to YF and AEs; 441 were excluded (because data on the populations of interest could not be identified in the studies or if present, could not be separated from larger groupings). Thirty-two studies were included in the review, because specific data on the included population groups of interest could be extracted: nine studies of infants and children, eight studies of pregnant females, nine studies of HIV+ individuals, five studies of pharmacovigilance databases for older individuals, and one study of individuals taking immunosuppressant medications (Figure 1). Included studies reported active, mixed active and passive, and passive surveillance techniques used to identify AEs.
Figure 1.
PRISMA flow diagram.
A SAE was defined in detail only by studies reporting on pharmacovigilance databases.12,14–17 Anaphylaxis/hypersensitivity was defined in detail only in the work by Lindsey and others14 (Table 2), and YEL-AND and YEL-AVD were not explicitly defined. Although the works by Lawrence and others15 and Monath and others17 based their definitions on the definitions in the work by Martin and others,16 the lists of symptoms on which they based their analyses do not entirely correspond (Table 2).
Table 2.
Definitions of SAEs after YF vaccination
| Refs. | Definition of SAE or anaphylaxis |
|---|---|
| 12 | Code of Federal regulations definition: “any adverse experience, occurring after administration of any vaccine dose that results in any of the following outcomes: death, life-threatening illness, inpatient hospitalization, prolongation of existing hospitalization, persistence of significant disability” (included cases of anaphylaxis/hypersensitivity in the non-serious category). |
| 14 | Systemic AEs: onset within 2 weeks of vaccination. Neurological: new onset seizures, encephalitis, myelitis, altered mental status, focal cranial or peripheral neurological deficits, paresthesia, and vertigo with or without headache. Multisystemic: myalgia, arthralgia, impaired hepatic function, respiratory distress, nausea, vomiting, and impaired renal function with or without fever.* |
| 16 | Reports of death, hospitalization, disability, or life-threatening illness requiring an emergency room or doctor visit. Onset < 2 weeks after vaccination, and duration > 72 hours. Neurologic: Guillain–Barré syndrome, new onset seizures, encephalitis, myelitis, altered mental status, focal cranial or peripheral neurologic deficits, paresthesias, vertigo, and headaches (headaches alone are not sufficient for neurologic diagnosis). Multisystemic: myalgias, arthralgias, rhabdomyolysis, elevated transaminases, respiratory distress, nausea, vomiting, diarrhea, nephropathy, and disseminated intravascular coagulation with or without fever. |
| 17 | Systemic AEs: onset < 2 weeks after vaccination, and duration > 72 hours. Neurologic: Guillain–Barré syndrome, new onset seizures, encephalitis, myelitis, altered mental status, focal cranial or peripheral neurologic deficits, paresthesias, vertigo, and headaches (headaches alone are not sufficient for neurologic diagnosis). Multisystemic: myalgias, arthralgias, elevated transaminases, respiratory distress, nausea, vomiting, diarrhea, nephropathy, and disseminated intravascular coagulation with or without fever.* |
| 15 | If reaction occurred within 4 hours of vaccination and at least one dermatologic symptom (urticaria, flushing, angioedema, pruritis, or rash) and at least one respiratory symptom (dyspnea, bronchospasm, pharyngeal edema, wheezing, throat tightness, or dysphonia).† |
Infants and children
Nine studies investigated YFV AEs in infants and children.18–26 We identified four RCTs that used active surveillance for AEs.18–21 Two of the RCTs18,20 involving children are at low risk of bias, and two RCTs19,21 are at moderate risk of bias. None described the method of randomization other than to say that the children were randomized (i.e., they did not describe a strong method of randomization), and two studies described concealment of the allocation from the researchers and blinding of participants.18,21 Not surprisingly in studies of vaccinating children, two of four studies experienced attrition of participants.20,21
Active surveillance
There were four studies of infants and children that used active surveillance methods to detect adverse reactions. The most carefully conducted study (by Belmusto-Worn and others18) was a randomized, double blind, non-inferiority phase III trial in children 9 months to 10 years in Peru: 738 children received 17D (Arilvax, Sanofi-Pasteur, Lyon, France) and 369 children received 17D (YF-VAX, Evans Vaccines, Liverpool, UK). A power computation was conducted, and 144 children were required per treatment group for a power of 0.80 with α = 0.05 (one-sided) to detect a 97% conversion rate. An upper bound of 0.004 was required for the 95% confidence interval (95% CI) for severe adverse reactions in the ARILVAX group, and an upper bound of 0.009 was required for the 95% CI for severe adverse reactions for the YF-VAX group. Active surveillance at every clinic visit included a structured interview about AEs, a review of diary cards, and an assessment of AEs and their possible relationship to YFV. Children who did not attend scheduled visits were visited at home, and parents/guardians were asked to bring the child to the clinic if there was a fever or they were concerned with their child’s health during the follow-up period (days 1–31). Children who developed a generalized febrile illness within the first 10 days post-vaccination were examined by the study's on-site investigator, and if there was no plausible explanation such as a respiratory infection, liver function and viremia tests were performed. No SAEs attributable to YFV were reported, and about one-half of the subjects reported minor adverse reactions, mostly fever and upper respiratory symptoms (Tables 3–5). No local reactions at the injection site were reported, which casts doubt about the completeness of the assessments.
Lhuillier and others19 gave 74 infants 6–9 months old in the Ivory Coast 17D vaccine and 85 infants 6–9 months old 17D and measles vaccine. Active surveillance consisted of questioning the mother during the post-vaccination sampling period, and passive surveillance was analyzing the reasons that the infants were brought to sector dispensaries after vaccination. There was no assessment of the completeness of the assessment, data quality, or how the data were analyzed. No SAEs or minor adverse reactions were reported, which does not seem credible.
Osei-Kwasi and others20 gave 384 infants in Ghana 17D vaccine at either 6 or 9 months. Active surveillance was described as instructing mothers to visit the clinic if the child became unwell after immunization and also, to return for a routine check on day 10 after immunization (non-attenders were visited at home on days 11 or 12). The infants were examined, axillary temperature was measured, and adverse reactions were recorded on a questionnaire. No serious adverse reactions were noted, and the non-serious adverse reactions were mostly fever and upper respiratory symptoms, with no significant differences between the age groups in rate or type of symptoms (Tables 3–5).
Soula and others21 randomized two groups of children 4–8 and 12–24 months to 17D vaccine, measles vaccine, or both. Active surveillance on days 1, 2, 7, and 14 included axillary temperatures, enquiry about local (pain, induration, or abscess at the injection site) and general reactions (rash, cough, conjunctivitis, gastrointestinal troubles, convulsions, or general malaise), and any other signs discovered by the investigator. No SAEs were noted, and minor adverse reactions were fever, with low rates of reported upper respiratory or injection site symptoms. These reactions were similar both for the two age groups and the groups who received 17D vaccine and 17D and measles vaccine.
Thus, a total of 1,866 infants were vaccinated in four RCTs, and no SAEs were identified. For three of four studies, not all of the infants were seronegative at baseline (Tables 3–5), which possibly reduces the number who might have had adverse reactions.
Passive surveillance
We identified five studies that used passive surveillance. Mouchon and others22 vaccinated 139 infants in Cameroon with 17D vaccine. Passive surveillance merely consisted of checking each child’s file for any local and general reactions or symptoms mentioned by the mother on the 30th day after vaccination. There was no statement of how the quality of data was assessed. No serious or minor AEs were identified, which does not seem credible. No conclusions can be drawn from the brief note by Cannon,23 who stated that 35,000 vaccinations (“mostly infants”) had been given with Rockefeller or Burroughs Wellcome (London, currently owned by GlaxoSmithKline) 17D vaccine in Lagos with no serious adverse reactions reported. There was no statement about surveillance methods.
There are five reports of infant transmission after maternal vaccination with YFV, and two of these reports were well-documented. Couto24 reported that, in Brazil in 2009, a mother received 17DD vaccine 15 days after delivery, and the infant at 23 days of age developed upper extremity clonic convulsions. The white blood cell count (WBC) was 25,400/mm3, the CSF (cerebrospinal fluid) WBC was normal at 1/mm3, protein was elevated at 67 mg/dL, and the gram stain was negative. No specimens for bacterial or fungal cultures were obtained, but serum and CSF for dengue-specific immunoglobulin M (IgM), viral cultures for herpes simplex, cytomegalovirus, and varicella, reverse transcription–polymerase chain reaction (RT-PCR) for enteroviruses, and a chest X-ray were all negative.
Kuhn and others25 reported that the mother of a Canadian infant received 17D and inactivated typhoid vaccines when her infant was 10 days old, and at 40 days of age the child had seizures. The WBC was slightly elevated at 13.2×109/L, platelets were elevated at 740×109/L, and computed tomography (CT) of the head was normal; however, a later magnetic resonance imaging (MRI) study showed fronto-parietal meningeal enhancement consistent with meningoencephalitis. The CSF WBC was elevated at 128.9×106, with 30% neutrophils, 32% lymphocytes, 36% monocytes, and no red blood cells (RBCs), glucose was 2.2 mmol/L, and protein was elevated at 1.1 g/L. Bacterial cultures of blood, urine, and CSF, PCR of CSF for herpes simplex and enteroviruses, and nasopharyngeal nucleic acid sequencing for enteroviruses were all negative. Serum IgM enzyme-linked immunosorbent assay (ELISA) was positive for YF, a plaque reduction neutralization test (PRNT) was positive at 1:5,120, and the hemagglutination inhibition titer was positive at 1:160, but YF IgG was negative. CSF IgM ELISA was positive, but PCR was negative for YF virus. Serology for dengue, Western and Eastern equine encephalitis, Venezuelan equine encephalitis, St. Louis encephalitis, Powassan encephalitis, Mayaro, and West Nile viruses was all negative.
The Global Advisory Committee on Vaccine Safety,26 in a brief note without details of country or citations, stated that they had reviewed recent data suggesting that three neonates (aged 10 days, 23 days, and 5 weeks) developed encephalitis as a result of infection with YFV virus transmitted to them from recently vaccinated mothers. All three infants were being breastfed, but the mode of transmission was not established. All three mothers had received the vaccine for the first time during the infant’s first month of life.
Pregnant women
We identified four studies of pregnant women that used active surveillance to identify YFV AEs. All studies were of vaccination campaigns; three studies were in Brazil, and none specified how they assessed severe or minor adverse reactions. We identified four studies of passive surveillance.
Active surveillance
Cavalcanti and others27 studied 312 pregnant women who received 17D vaccine during a campaign in Brazil and then sought care at one hospital (a total of 2,070,000 individuals were vaccinated). Active surveillance consisted of examining 304 babies at birth and 1 month to 1 year of age and comparing them to 10,691 births that occurred in the same region and timeframe from 1997 to 1999. The number in this comparison cohort of 10,961 who had received YFV was not stated, and therefore, the work by Cavalcanti and others27 was comparing its cohort of 304 to a cohort with an unknown YFV rate. Ten malformations were noted (3.3%; 95% CI = 1.7–14.6%, P = 0.003), with no differences in seven types of major malformations except for Down's syndrome (three cases among babies exposed in utero to YFV; P = 0.003). No data were collected about any possible maternal reactions to YFV.
Nasidi and others28 studied 101 pregnant females in Nigeria aged 15–50 years who were vaccinated with 17D vaccine during a YF outbreak during 1986–1987. Active surveillance consisted of obtaining information about the pregnant woman or mother and child, physical examination at intervals of 2–8 weeks, with monitoring of newborns with growth and development indices for up to 4 years, and home visits by the social workers or hospital staff. Passive surveillance consisted of enquiries when pregnant women called at the hospital for routine medical visits. No child showed any physical or psychological abnormality or growth retardation. There was no statement about assessment forms used or assessment of data quality. There was no assessment of any clinical symptoms attributable to YFV. There was a poor response of the pregnant women to YFV (consistent with T- and B-cell function immunosuppression) (Tables 3–5), which may affect potential adverse responses to vaccine.
Papaiordanou and others,29 in a brief abstract, reported on 488 pregnant women who inadvertently received 17DD vaccine during a campaign in Brazil and were enrolled in a prospective surveillance cohort to evaluate AEs, seroconversion, perinatal results, and teratogenicity. There were no serious local or systemic AEs, and 19% reported minor symptoms. There was no statement of assessment methods or evaluation of data quality.
Suzano and others30 followed 480 pregnant women in Brazil inadvertently vaccinated with 17DD vaccine during a campaign. Active surveillance consisted of three or more antenatal visits using pre-coded forms. For a subsample of 86 babies born at a university hospital, a more detailed neonatal protocol was used, with examination of placental and umbilical cord blood by PCR, neonatal serology (IgM by IgM antibody capture ELISA [MAC-ELISA] and IgG by PRNT), transfontanel ultrasound, brainstem-evoked response audiometry, fundoscopy, clinical dysmorphologic and neurological exam, and visits at 3, 6, and 12 months for clinical and serological evaluation. For babies born in other maternity hospitals, site visits were scheduled at 3, 6, and 12 months for serology and one clinical dysmorphologic exam. For the sample of 304 evaluated by a geneticist, the 11 miscarriages, 7 malformations, 3 fetal deaths, 2 early neonatal deaths, and 7.8% premature delivery rate were similar to those occurrences in the general population. Mild AEs within 15 days of vaccination were reported by 19.6% of mothers.
Thus, a total of 1,381 pregnant females was studied, and rates of AEs above those AEs routinely expected in pregnancy were not found. Only a subsample of 86 in the study by Suzano and others30 received detailed serological examinations and imaging.
Passive surveillance
D'Acremont and others31 identified six pregnant women who had attended a Swiss travel clinic and received 17D and multiple other vaccines. There were no deleterious effects of vaccinations on child outcomes. Nishioka Sde and others32 conducted a study of pregnant women who had inadvertently received YFV during a campaign in Brazil during a dengue epidemic and compared 39 women who attended a university hospital with spontaneous abortions with a control group of 74 women seen at the antenatal clinic. Information was collected on possible risk factors for spontaneous abortion, The odds ratio (OR) for spontaneous abortion after YFV was 2.29 (95% CI = 0.65–8.03) after controlling for oral contraceptive use, hypertension, age, smoking, exanthems, and schooling. No sample results were reported for antibodies to YF or dengue.
Robert and others33 studied 58 pregnant females who had received 17D vaccine and had follow-up information in 5 of 11 European Network of Teratology Information Services. Among the 46 live births, the rate of major malformations was 3–4%, and there were seven spontaneous abortions (close to the expected percentage). No blood samples were reported for antibodies to YF. Tsai and others34 identified 35 pregnant females who received 17D or 17DD vaccine during a campaign in Trinidad and Tobago and recorded no SAEs. These four studies are small samples, with substantial risk of underenumeration and underinvestigation. The work by Nishioka Sde and others32 is the only study that found a statistically significant difference (in the spontaneous abortion rate) for those women exposed to YFV, but it used passive surveillance.
HIV+ individuals
We identified one study that used active surveillance, seven studies with passive surveillance of YFV AEs (six of which were reviews of patients seen in travel clinics in France and one was a review of patients in Brazil), and a review of serious adverse events following immunization (AEFIs) in HIV+ individuals after YF vaccination.
Active surveillance
Veit and others,35 from reports from four of seven Swiss HIV Cohort Study (SHCS) centers in 1996–2005, identified 102 HIV+ patients who received 17D vaccine. SHCS collects information with standardized criteria on structured forms at enrollment and 6-month follow-up visits, asks about visits to tropical countries, and collects plasma samples at each visit. Surveillance comprised consultations, phone calls, questionnaires, or chart reviews about tropical travel, vaccine certificates were checked for 17D and other vaccines, and charts of deceased patients and the Swiss vaccination surveillance system were checked. In total, 174 HIV+ individuals received 17D vaccine; details were collected for 102 individuals, and no SAEs were observed.
Passive surveillance
Five retrospective reviews were conducted of HIV+ patients who attended travel clinics in France and received 17D vaccine. Goujon and others36 reported on 44 HIV+ patients at the Hôpital de l’Institut Pasteur, Paris, France. Pacanowski and others37 reported on 103 patients at the Hôpital Saint Antoine, Paris, France. Receveur and others38 reported two case reports from a travel clinic in Bordeaux, France. Tattevin and others39 reported on 12 patients at Pontchaillou Hospital, Rennes, France. Pistone and others40,41 reported on 23 patients (for 12 patients, it was their second YF vaccination) at the Center Hospitalier Universitaire in Bordeaux, France. Only Pistone and others40,41 described any assessment of symptoms (patients filled out a questionnaire), and no study reported any serious AEFIs. Ho and others42 reported on seven patients at the University of São Paulo, Brazil. No serious AEFIs were recorded, and no method of assessing symptoms was reported. There was one fatal case of meningoencephalitis, occurring shortly after the receipt of 17D vaccine, reported in a Thai adult with a previously undiagnosed HIV infection and a CD4 cell count of 108 cells/mm3, but testing to prove causality was not available.43
The review by Veit and others44 included the above studies and also concluded that no SAEs had been identified.
Because of the small numbers of HIV+ individuals reported to have received YFV in these published articles, no conclusions can be drawn.
Patients taking immunosuppressants: passive surveillance
We identified one study of patients taking immunosuppressants45 that studied 70 consecutive patients in Brazil with rheumatological diseases who were taking immunosuppressant medications. Surveillance was partly active (questionnaires that enquired about the effect of YFV) but primarily passive in that patients with rheumatological symptoms were not being seen in any clinic; additionally, the rheumatological patients who did not consult between specific dates were excluded. No SAEs were recorded, and the few minor symptoms (fever or arthralgias) are difficult to interpret, because the patients already had a rheumatological illness. We did not find any articles reviewing datasets on patients with other autoimmune illnesses, cancer, or transplants.
Older persons: passive surveillance
We identified five passive surveillance reports that analyzed rates of AEs for older persons: three reports from the US VAERS (Vaccine Adverse Event Reporting System) database for partially overlapping periods, one report from the Australian ADRAC (Adverse Drug Reactions Advisory Commiteee) database, and one report that used the UK General Practice Research database. For the US VAERS database, Khromava and others12 and Lindsey and others14 reported cases of SAEs, YEL-AND, and YEL-AVD. Lindsey and others14 also reported cases of anaphylaxis/hypersensitivity, and Martin and others16 only reported SAEs. SAEs were reported for Australia by Lawrence and others,15 and SAEs were reported for the United Kingdom by Monath and others.17
Khromava and others12 reviewed VAERS reports from 1990 to 2002 of cases of SAEs, YEL-AND, and YEL-AVD (cases of hypersensitivity/anaphylaxis were excluded, because they were reported elsewhere). Telephone surveys with civilian healthcare providers indicated little or no wastage, and the number of doses distributed (2,230,760 for all age groups) was assumed to be a reasonable estimate of numbers vaccinated. One problem is that for the 722 reports of any AE, in 26% of these reports, only YFV was administered, and in 50% of reports, YF and other vaccines were administered (in the civilian cases, YFV was with typhoid, hepatitis A, or both, and in the military personnel, YFV was with anthrax, measles–mumps–rubella, tetanus–diptheria toxoid, influenza, and many other vaccines). However, there is no statement for the other 24%. There were 465 civilian reports of AEs, with 35 reports classified as serious (of these reports, 7 were classified as YEL-AVD, and 8 were classified as YEL-AND). There were 257 military reports, with 12 reports classified as serious (of these reports, 1 was classified as YEL-AVD and 2 were classified as YEL-AND). The YEL-AVD and YEL-AND cases certainly occurred only in those individuals who received YFV. There were very few cases of YEL-AND and YEL-AND in individuals ≥ 60 years, resulting in risk ratios (RRs) with wide CIs (Tables 3–5), and it would be inappropriate to compute rates per 100,000 vaccinees or compare rates for the individual age groups with any certainty.
Lindsey and others14 reviewed 660 civilian reports in the VAERS database for a shorter and more recent period (2000–2006); of these reports, 190 involved individuals who had received only YFV, and 470 had YF and other vaccines. Of the 660 events, 72 events were classified as serious (with overlapping classifications in Table 1 in the work by Lindsey and others14 of 4 deaths, 21 life-threatening illnesses, 58 hospitalizations, 5 prolongations of hospitalization, and 7 cases of permanent disability). The numbers in the work by Lindsey and others14 for those people aged 60–69 and ≥ 70 years, like those numbers in the work by Khromava and others,12 are small (Tables 3–5).
Martin and others16 reviewed the US VAERS database for a shorter period (1990–1998) within the period reviewed by Khromava and others12 (1990–2002), and they identified that 1,443,686 doses of YFV were administered to civilians of all age groups; 19 cases of serious adverse reactions in which YFV alone was administered were identified, and 16 cases of serious adverse reactions in which it was administered with other vaccines were identified. The work by Martin and others16 noted that, for the VAERS data, the estimated age distribution of travelers from GeoSentinel clinics receiving vaccine in 1998 was assumed to apply for the entire study (1990–1998).
Lawrence and others15 for the Australian ADRAC database from 1993 to 2002 identified 210,656 doses of 17D vaccine administered to civilians and 42 reports of any AEs (YF was the only suspected vaccine in 15 reports). The age distribution was estimated from a national network of travel clinics. Of the nine SAEs, four occurred in individuals aged 45–64 years, and two occurred in individuals aged ≥ 65 years. These are small numbers, and the 95% confidence limits for the RR computed by Lawrence and others15 are wide (Tables 3–5).
Monath and others17 in the UK General Practice Research Database for 1995–1999 identified 1,043,416 individuals who had received 17D vaccine; 36 SAEs were identified, of which 15 events were in the age group 45–64 years and 3 events were in the age group 65–74 years (Tables 3–5). Monath and others17 did not state if other vaccines were given.
Conclusions
We identified four RCTs of infants and children (N = 1,866), two of which were at low risk of bias18,20 and two of which were at moderate risk of bias,19,21 and no SAEs were identified. The main minor AEs were fever and upper respiratory tract symptoms, with similar rates across studies. There are two well-documented cases of breastfeeding mothers with SAEs caused by maternal–child transmission and three cases without published documentation.
We identified four studies of pregnant females (N = 1,351) with no SAEs reported, and rates of malformation were similar to rates in the general population. Because the vaccinations were given inadvertently during campaigns, there were no serological tests before vaccination in three of the studies; therefore, the proportion of individuals who already had antibodies is not known, and thus, the number of individuals who could potentially get severe adverse reactions may be smaller than the total number vaccinated. The numbers (138) assessed by studies that used passive surveillance are small, and no conclusions can be drawn.
We identified only one study of HIV+ individuals35 that used active surveillance, with no reported SAEs, but the number assessed was small (N = 102). There were seven retrospective studies of travel clinics that used passive surveillance (six in France and one in Brazil) with a small number assessed (191): no SAEs were reported, and minor AEs were assessed only by Pistone and others.40,41
The pharmacovigilance databases reviewed large numbers of individuals vaccinated with YFV. For the US VAERS database, Khromava and others12 reviewed cases from 1990 to 2002 and identified 188,870 individuals aged 60–69 years and 93,565 individuals aged ≥ 70 years. For the slightly overlapping period of 2000–2006, Lindsey and others14 identified 191,025 individuals aged 60–69 years and 87,177 individuals aged ≥ 70 years. One problem for the database by Khromava and others12 is that for the 722 AEs, in only 26% of cases was only YFV administered; in 50% of cases, other known vaccines were administered, and in 24% of cases, the vaccine is not stated. Similarly, for the database by Lindsey and others14 for the AEs, only 190 involved YFV alone, and 470 involved other vaccines. The numbers of SAEs for the age groups ≥ 60 years are small. For those individuals aged 60–69 years in the database by Khromava and others,12 SAEs = 8, YEL-AVD = 2, and YEL-AND = 3; in the database by Lindsey and others,14 SAEs = 12, YEL-AVD = 2, and YEL-AND = 3. For the age group ≥ 70 years, the numbers in the work by Khromava and others12 are SAEs = 7, YEL-AVD = 3, and YEL-AND = 1, and the numbers in the work by Lindsey and others14 are SAEs = 11, YEL-AVD = 2, and YEL-AND = 2. The database by Khromava and others12 spans 13 years, and the database by Lindsey and others14 spans 7 years. It is inappropriate to compute rates per 100,000 patients or compare rates for age groups with such small numbers, although trends may be perceived. The assessments by Monath and others17 are based on extrapolating for the number of 1,043,416 doses of vaccine given in the United Kingdom from 1995 to 1999 the age structure from the much smaller UK General Practice Database. For the age group 65–74 years, this assessment involved extrapolating that 34,960 YF vaccinations were given to the entire age group from the 423 doses recorded in the UK General Practice Database, and for the age group > 75 years, this assessment involved extrapolating that 8,595 YF vaccinations were given to that entire age group from the 104 doses recorded in the UK General Practice Database.
This review has not shown any change in the current understanding of the risk of SAEs because of YFV, and it shows that studies and databases to date have not resulted in sufficient data at low risk of bias to provide any recommendations for contraindications or precautions more than those recommendations of ACIP or CDC.
Table 4.
Comparisons for pharmacovigilance databases
| Reference | Age group (years) | SAEs | YEL-AND | YEL-AVD | |||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| 12 | 60–69 | 5.9 | 1.6–22.2 | N/A | 0.6–21.1 | 4.4 | 0.4–48.7 |
| 15 | 65–74 | 3.7 | 1.3–10.7 1.49–53.5 | N/A | N/A | N/A | N/A |
| 16 | ≥ 65 | 8.95 | 0.81–9.81 | N/A | N/A | N/A | N/A |
| 17 | 65–74 | 2.82 | 3.5 | N/A | N/A | N/A | N/A |
| 12 | ≥ 70 | 10.4 | 2.7–40.2 | 13.4 | 1.4–128.5 | 2.4 | 0.2–26.2 |
| 16 | ≥ 75 | 11.6 | 3.7–7.36 | N/A | N/A | N/A | N/A |
| 17 | > 75 | 0 | Undefined | 0 | Undefined | 0 | Undefined |
ACKNOWLEDGMENTS
The Global Advisory Committee on Vaccine Safety (GAVCS) requested that the World Health Organization (WHO) commission complete an independent systematic review of the safety of yellow fever vaccine. This independent commissioned systematic review was prepared for the WHO and GACVS by a research team at the University of Calgary headed by Roger E. Thomas. The focal contact person for the WHO was Alejandro Costa with Dr. Rosamund Lewis. There was extensive correspondence with the WHO focal person and Dr. Rosamund Lewis, with additional correspondence with Dr. Sergio Yactayo.
Disclaimer: The scientific independence of the researchers was at all times maintained. The sponsors did not participate in the collection, analysis, or interpretation of data or the writing of the report.
Footnotes
Financial support: The systematic review was funded by The Global Alliance for Vaccines and Immunization (GAVI).
Authors’ addresses: Roger E. Thomas, Department of Family Medicine, University of Calgary, Calgary, Alberta, Canada, E-mail: rthomas@ucalgary.ca. Diane L. Lorenzetti and Tyler Williamson, Department of Community Health Sciences, Faculty of Medicine, University of Calgary, Calgary, Alberta, Canada, E-mails: dllorenz@ucalgary.ca and tswillia@ucalgary.ca. Wendy Spragins and Dave Jackson, Independent Research Consultants, Calgary, Alberta, Canada, E-mails: spragins@ucalgary.ca and djackson@ucalgary.ca.
References
- 1.Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep Recommendations. 2010:1–27. [PubMed] [Google Scholar]
- 2.SA Plotkin, WA Orenstein, PA Offit., editors. Vaccines. 5th ed. Philadelphia, PA: W.B. Saunders; 2008. pp. 959–1055. (Yellow fever vaccine). [Google Scholar]
- 3.WHO Emerging and Other Communicable Diseases District Guidelines for Yellow Fever Surveillance. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 4.WHO Division of Epidemiological Surveillance and Health Situation Trend Assessment Global Health Situation and Projections—Estimates. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 5.Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv Parasitol. 2006;62:181–220. doi: 10.1016/S0065-308X(05)62006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on Medicine and Health in the Tropics, Marseille, France, Monday 12 September 2005. Vaccine. 2007;25:2758–2765. doi: 10.1016/j.vaccine.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Pugachev KV, Guirakhoo F, Ocran SW, Mitchell F, Parsons M, Penal C, Girakhoo S, Pougatcheva SO, Arroyo J, Trent DW, Monath TP. High fidelity of yellow fever virus RNA polymerase. J Virol. 2004;78:1032–1038. doi: 10.1128/JVI.78.2.1032-1038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization International Travel and Health. 2011. http://www.who.int/ith/chapters/en/index.html Available at. Accessed October 9, 2011.
- 9.Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, Gidudu J, Katikaneni L, Khuri-Bulos N, Oleske J, Tapianen T, Wiznitzer M. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 10.Rüggeberg JU, Gold MS, Bayas J-M, Blum MD, Bonhoeffer J, Friedlander S, de Souza Brito G, Heininger U, Imoukhuede B, Khamesipour A, Erlewyn-Lajeunesse M, Martin S, Mäkela M, Nell P, Pool V, Simpson N. Brighton Collaboration Analphylaxis Working Group. Anaphylaxis: Case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25:5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 11.Bae HG, Domingo C, Tenorio A, de Ory F, Munoz J, Weber P, Teuwen DE, Niedrig M. Immune response during adverse events after 17D-derived yellow fever vaccination in Europe. J Infect Dis. 2008;197:1577–1584. doi: 10.1086/587844. [DOI] [PubMed] [Google Scholar]
- 12.Khromava AY, Eidex RB, Weld LH, Kohl KS, Bradshaw RD, Chen RT, Cetron MS. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005;23:3256–3263. doi: 10.1016/j.vaccine.2005.01.089. [DOI] [PubMed] [Google Scholar]
- 13.Rosner BA. Fundamentals of Biostatistics. 3rd ed. Boston, MA: PWS-KENT; 1990. pp. 94–95. [Google Scholar]
- 14.Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, Slade BA, Barnett ED, Brunette GW, Horan K, Staples JE, Kozarsky PE, Hayes EB. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–6082. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence GL, Burgess MA, Kass RB. Age-related risk of adverse events following yellow fever vaccination in Australia. Commun Dis Intell. 2004;28:244–248. [PubMed] [Google Scholar]
- 16.Martin M, Weld LH, Tsai TF, Mootrey GT, Chen RT, Niu M, Cetron MS. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg Infect Dis. 2001;7:945–951. doi: 10.3201/eid0706.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monath TP, Cetron MS, McCarthy K, Nichols R, Archambault WT, Weld L, Bedford P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum Vaccin. 2005;1:207–214. doi: 10.4161/hv.1.5.2221. [DOI] [PubMed] [Google Scholar]
- 18.Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, Pastor-Cauna G, Echevarria C, Laguna-Torres VA, Samame BK, Baldeon ME, Burans JP, Olson JG, Bedford P, Kitchener S, Monath TP. Randomized, double-blind, phase III, pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (Arilvax and YF-VAX) in healthy infants and children in Peru. Am J Trop Med. 2005;72:189–197. [PubMed] [Google Scholar]
- 19.Lhuillier M, Mazzariol MJ, Zadi S, Le CN, Bentejac MC, Adamowicz L, Marie FN, Fritzell B. Study of combined vaccination against yellow fever and measles in infants from six to nine months. J Biol Stand. 1989;17:9–15. doi: 10.1016/0092-1157(89)90023-1. [DOI] [PubMed] [Google Scholar]
- 20.Osei-Kwasi M, Dunyo SK, Koram KA, Afari EA, Odoom JK, Nkrumah FK. Antibody response to 17D yellow fever vaccine in Ghanaian infants. Bull World Health Organ. 2001;79:1056–1059. [PMC free article] [PubMed] [Google Scholar]
- 21.Soula G, Sylla A, Pichard E, Kodio B, Bentejac MC, Teulieres L. Saliou P, 1991. Ėtude d'un nouveau vaccin combiné contre la fièvre jaune et la rougeole chez les enfants agés de 6 au 24 mois au Mali. Bull Soc Pathol Exot. 84:885–897. [PubMed] [Google Scholar]
- 22.Mouchon D, Pignon D, Vicens R, Tu HT, Tekaia F, Teulieres L. Garrigue G, 1990. Ėtude de la vaccination combinée rougeole-fièvre jaune chez l'enfant africain agé de 6 à 10 mois. Bull Soc Pathol Exot. 83:537–551. [PubMed] [Google Scholar]
- 23.Cannon D. Encephalitis after yellow fever vaccination. BMJ. 1955;1:1090. [Google Scholar]
- 24.Couto AM. Transmission of yellow fever vaccine virus through breast feeding—Brazil 2009. MMWR Morb Mortal Wkly Rep. 2010;59:130–132. [PubMed] [Google Scholar]
- 25.Kuhn S, Twele-Montecinos L, MacDonald J, Webster P, Law B. Case report: probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. CMAJ. 2011;183:E243–E245. doi: 10.1503/cmaj.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Advisory Committee on Vaccine Safety Wkly Epidemiol Rec. 2010;85:285–291. [PubMed] [Google Scholar]
- 27.Cavalcanti DP, Salomão MA, Lopez-Camelo J, Pessoto MA. Early exposure to yellow fever vaccine during pregnancy. Trop Med Int Health. 2007;12:833–837. doi: 10.1111/j.1365-3156.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 28.Nasidi A, Monath TP, Vandenberg J, Tomori O, Calisher CH, Hurtgen X, Munube GR, Sorungbe AO, Okafor GC, Wali S. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87:337–339. doi: 10.1016/0035-9203(93)90156-k. [DOI] [PubMed] [Google Scholar]
- 29.Papaiordanou P, Sato H, Freire M, Yamamura A, Gouveia F, Amaral E. Yellow fever vaccination during pregnancy. Abs Intersci Conf Antimicrob Agents Chemother. 2001;41 [Google Scholar]
- 30.Suzano CE, Amaral E, Sato HK, Papaiordanou PM. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24:1421–1426. doi: 10.1016/j.vaccine.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 31.D'Acremont V, Tremblay S, Genton B. Impact of vaccines given during pregnancy on the offspring of women consulting a travel clinic: a longitudinal study. J Travel Med. 2008;15:77–81. doi: 10.1111/j.1708-8305.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishioka Sde A, Nunes-Araujo FR, Pires WP, Silva FA, Costa HL. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health. 1998;3:29–33. doi: 10.1046/j.1365-3156.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 33.Robert E, Vial T, Schaefer C, Arnon J, Reuvers M. Exposure to yellow fever vaccine in early pregnancy. Vaccine. 1999;17:283–285. doi: 10.1016/s0264-410x(98)00051-6. [DOI] [PubMed] [Google Scholar]
- 34.Tsai TF, Paul R, Lynberg MC, Letson GW. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis. 1993;168:1520–1523. doi: 10.1093/infdis/168.6.1520. [DOI] [PubMed] [Google Scholar]
- 35.Veit O, Niedrig M, Chapuis-Taillard C, Cavassini M, Mossdorf E, Schmid P, Bae HG, Litzba N, Staub T, Hatz C, Furrer H. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin Infect Dis. 2009;48:659–666. doi: 10.1086/597006. [DOI] [PubMed] [Google Scholar]
- 36.Goujon C, Tohr M, Feuillie V, Coulaud P, Dupont B, Sansonetti P. Good tolerance and efficacy of yellow fever vaccine among carriers of human immunodeficiency virus. J Travel Med. 1995;2:145. [Google Scholar]
- 37.Pacanowski J, Campa P, Dabrowska M, Lacombe K. Antibody response and safety of yellow fever vaccination in HIV infected patients. Proceedings of the 48th Annual International Conference on Antimicrobial Agents and Chemotherapy, October 25–28. Washington: DC; 2008. p. 2008. [Google Scholar]
- 38.Receveur MC, Thiébaut R, Vedy S, Malvy D, Mercié P, Le Bras M. Yellow fever vaccination of human immunodeficiency virus-infected patients: report of 2 cases. Clin Infect Dis. 2000;31:E7–E8. doi: 10.1086/314031. [DOI] [PubMed] [Google Scholar]
- 39.Tattevin P, Depatureaux AG, Chapplain JM, Dupont M, Souala F, Arvieux C, Poved JD, Michelet C. Yellow fever vaccine is safe and effective in HIV-infected patients. AIDS. 2004;18:825–827. doi: 10.1097/00002030-200403260-00020. [DOI] [PubMed] [Google Scholar]
- 40.Pistone T, Verdière C-H, Receveur M-C, Ezzedine K, Lafon ME, Malvy D. Immunogénicité et tolérance du vaccin amaril chez le voyageur vivant avec le VIH, France, 2005. Bull Epidemiologique Hebdomadaire. 2007;25/26:238–240. [Google Scholar]
- 41.Pistone T, Verdière C-H, Receveur M-C, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerability of yellow fever vaccination in 23 French HIV-infected patients. Curr HIV Res. 2010;8:461–466. doi: 10.2174/157016210793499277. [DOI] [PubMed] [Google Scholar]
- 42.Ho YL, Enohata T, Lopes MH, De Sousa Dos Santos S. Vaccination in Brazilian HIV-infected adults: a cross-sectional study. AIDS Patient Care STDS. 2008;22:65–70. doi: 10.1089/apc.2007.0059. [DOI] [PubMed] [Google Scholar]
- 43.Kengsakul K, Sathirapongsasuti K, Punyagupta S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J Med Assoc Thai. 2002;85:131–134. [PubMed] [Google Scholar]
- 44.Veit O, Hatz C, Niedrig M, Furrer H. Yellow fever vaccination in HIV-infected patients. HIV Ther. 2010;4:17–26. [Google Scholar]
- 45.da Mota LMH, Oliveira ACV, Lima RAC, dos Santos-Neto LL, Tauil PL. Vacinação contra febre amarela em pacientes com diagnósticos de doenças reumáticas, em uso de immunosuppressores. Rev Soc Bras Med Trop. 2009;42:23–27. doi: 10.1590/s0037-86822009000100006. [DOI] [PubMed] [Google Scholar]
- 46.Kelso JM, Mootrey GT, Tsai TF. Anaphylaxis from yellow fever vaccine. J Allergy Clin Immunol. 1999;1999:698–701. doi: 10.1016/s0091-6749(99)70245-9. [DOI] [PubMed] [Google Scholar]