Abstract
Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing β-cells in the pancreatic islets. There is an immediate need to restore both β-cell function and immune tolerance to control disease progression and ultimately cure T1D. Currently, there is no effective treatment strategy to restore glucose regulation in patients with T1D. FoxP3-expressing CD4+ regulatory T cells (Tregs) are potential candidates to control autoimmunity because they play a central role in maintaining self-tolerance. However, deficiencies in either naturally occurring Tregs (nTregs) themselves and/or their ability to control pathogenic effector T cells have been associated with T1D. Here, we hypothesize that nTregs can be replaced by FoxP3+ adaptive Tregs (aTregs), which are uniquely equipped to combat autoreactivity in T1D. Unlike nTregs, aTregs are stable and provide long-lived protection. In this review, we summarize the current understanding of aTregs and their potential for use as an immunological intervention to treat T1D.
Keywords: type 1 diabetes, adaptive regulatory T cells, autoimmunity, immunotherapy
Introduction
Type 1 diabetes (T1D) is an autoimmune disease wherein insulin-producing pancreatic islet β-cells are attacked and destroyed by the immune system (Stadinski et al., 2010). As a result, T1D patients require life-long insulin treatment and face high risks of medical complications that lead to kidney failure, blindness, heart disease, stroke, and amputation, which contribute to early mortality. Studies of patients and animal models, especially the non-obese diabetic (NOD) mouse model, have generated extensive insights into the pathology and immunology of T1D. However, the detailed mechanisms of how the autoimmune response is initiated remain unclear. Consequently, there is no means to prevent the events that initiate the destruction of pancreatic islet β-cells. Furthermore, there is no therapeutic mechanism to control the autoimmune response after the diagnosis of diabetes, which would be crucial to the success of strategies to restore β-cell function and physiological control of glucose metabolism. This dilemma is highlighted by the observation that patients who received islet transplantation and immunosuppression to prevent graft rejection developed recurrent T1D because of the underlying autoimmunity against β-cells (Vendrame et al., 2010). This outcome underscores the need for immune tolerance restoration before β-cell replacement or regeneration can be achieved successfully. Furthermore, proliferation of insulin-producing β-cells has recently been reported in pancreatic islets of long-term T1D patients (Keenan et al., 2010). Thus, at a minimum, it is possible that the initiation of lasting control of autoimmunity would protect the remaining β-cells, enabling them to survive and function to reduce insulin dependence and subsequent medical complications.
The greatest prospect for achieving control of autoimmunity is to exploit mechanisms that participate physiologically in the control of tolerance and immunity. The discovery that CD4+ regulatory T cells (Tregs) play an indispensible and central role in maintaining self-tolerance (Wing and Sakaguchi, 2010) has led to the prospect that these cells have great potential as a cell-based treatment to restore self-tolerance and to treat autoimmune diseases, such as T1D. In this review, we focus on the remarkable potential of adaptive Tregs (aTregs) as candidates for the treatment of T1D, since they provide immunological memory that can be exploited to restore self-tolerance indefinitely.
The underpinnings of T1D
Autoimmunity escalates silently over a prolonged period of time before diabetes is diagnosed. As with many other autoimmune diseases, T1D is thought to be initiated by unclear environmental factors in genetically predisposed individuals. T1D is a disease with multiple genetic associations, the strongest of which is the HLA class II (DR3/DR4 and DQ2/DQ8). In addition, many immune response-related genes such as IL-2, CD25, CTLA4, and PTPN22 are highly associated with T1D (Todd et al., 2007). The involvement of these and many other genes or loci in T1D suggests that the disease progression involves multiple steps and modifiers (Barrett et al., 2009). In addition, epidemiological studies in the last decade have shown that the incidence of T1D is increasing, with disease onset trending towards younger ages most likely because of environmental factors that undermine tolerance (Vehik and Dabelea, 2011; Ziegler et al., 2011). Despite extensive research, the mechanistic triggers for the autoimmune response against β-cells remain elusive.
In the NOD mouse model, it is thought that the physiological tissue remodeling of pancreatic islets during neonatal development initially releases β-cell antigen(s) that are processed and presented by dendritic cells (DCs) in pancreatic lymph nodes (PLNs) (Scaglia et al., 1997; Turley et al., 2003). Such a mechanism may also function in humans (Kassem et al., 2000). However, viral infections, particularly with enteroviruses such as Coxsackievirus, have been associated with the induction of anti-β-cell responses through direct islet cell damage and antigen release and/or molecular mimicry (Horwitz et al., 1998; Hiemstra et al., 2001; Wen et al., 2004).
Recent studies have shown that intestinal microbiota can modulate the spontaneous development of diabetes in NOD mice (Wen et al., 2008). Indeed, NOD mice that are housed in germ-free environments have higher diabetes incidence than in specific pathogen-free or conventional facilities (Todd, 1991). This is consistent with the ‘hygiene hypothesis’ that a decrease in infections would increase the risk of immune-mediated diseases, such as T1D (Bach, 2002). Nevertheless, some dietary factors have also been proposed to modulate T1D, such as cow's milk and vitamin D (Stene and Joner, 2003; Akerblom et al., 2005).
Extensive studies established that T cells, particularly CD4+ T cells, play a central role in autoimmune responses in T1D, whereas B cells mainly contribute to antigen processing and presentation (Dai et al., 2005). T cells are the major cell population found in islet infiltrates in NOD mice and in T1D patients (Gepts and Lecompte, 1981; Miyazaki et al., 1985). Furthermore, depletion of T cells by neonatal thymectomy can prevent diabetes in NOD mice and T cells from diabetic NOD mice are diabetogenic (Bendelac et al., 1987; Christianson et al., 1993). The initial T cell responses in T1D are likely to involve limited antigen specificities against islet β-cells. With increasing islet damage and escalating inflammation, more antigens are released and T cell responses spread to broader specificities. When β-cell loss reaches an extent where the production of insulin from remaining β-cells does not meet metabolic requirements, clinical diabetes occurs. However, at this end stage in the autoimmune process, the antigens involved are unknown and likely to be many, thwarting efforts to devise an antigen-specific treatment to downmodulate the autoimmune response.
Challenges to current immune interventions in T1D
For both economical and technical reasons, it is not practical to screen for T1D risk in the general population (Atkinson, 2005). Thus, when patients are diagnosed with T1D, autoimmunity has already developed to an advanced stage, with extensive β-cell destruction and compromised glucose regulation. This currently makes finding interventions that can preserve residual β-cells and restore tolerance a more urgent task than devising a strategy to ‘prevent’ diabetes. It was estimated that more than 400 agents or agent combinations have been investigated in preclinical T1D intervention studies using NOD mice (Shoda et al., 2005). However, the number of targets in ongoing clinical trials is very limited (Luo et al., 2010; Waldron-Lynch and Herold, 2011). In general, these agents broadly inhibit the immune system and raise concern that responses to infections could be inappropriately suppressed. Examples include cyclosporine, the first immunomodulatory agent used in clinical trials, depletion of individual immune cell populations such as with anti-CD3 antibody for T cells or anti-CD20 antibody for B cells, and blocking agents against cytokines that mediate/amplify inflammation, such as TNFα or IL-1 (Luo et al., 2010; Waldron-Lynch and Herold, 2011). The modified anti-CD3 monoclonal antibody has shown the best results in recent onset patients (Herold et al., 2002, 2005), but failed to achieve long-term protection, suggesting that the efficacy of a single agent is limited, and combinations of multiple agents may be needed.
While approaches that induce/restore islet antigen-specific tolerance would be the best for treatment of T1D, in the advanced stages of autoimmunity prior to diabetes onset, increases in the number of antigens recognized by responding T cells are likely. Therefore, targeting one or even a handful of antigens may not be sufficient to suppress the overall autoreactive response, and this probably explains the failures of some of the antigen-specific clinical trials such as administration of insulin or GAD65 (Waldron-Lynch and Herold, 2011; Wherrett et al., 2011). Ideally, a single dose treatment with long-lasting protection offers the best hope for clinical translation, as has been achieved for infectious diseases with immunization strategies. From our studies focusing on the regulation of T cell memory, we hypothesize that immunological memory, particularly in CD4+ T cells, can be exploited for this purpose in T1D.
nTreg failure in T1D
Compelling evidence indicates that loss of control of the autoimmune response by FoxP3+ Tregs is a major contributing factor to T1D development. FoxP3+ Tregs mainly comprise naturally occurring Tregs (nTregs) developed in the thymus and aTregs differentiated in the periphery under certain conditions (Bluestone and Abbas, 2003). nTregs have received the most intense scrutiny as a potential cell-based therapy, because of their potency in controlling generalized autoimmunity. An initial analysis of Tregs in T1D patients, using CD4 and CD25 as markers, showed reduced numbers in the circulation (Kukreja et al., 2002). Later studies, especially those using FOXP3 expression as the identifier, showed no difference in overall Treg number and distribution in T1D patients when compared with those in healthy controls (Brusko et al., 2007; Long et al., 2010). Confounding these studies is the findings that CD4+ effector T cells can share the expression of FOXP3, and can also exhibit low expression of IL-7Rα, an additional trait associated with nTregs. Functionally, defective nTregs from T1D patients have been reported (Brusko et al., 2005; Lindley et al., 2005; Putnam et al., 2005); effector T cells from established T1D patients also displayed resistance to nTreg suppression (Lawson et al., 2008; Schneider et al., 2008). Thus, ineffective nTregs and more potent effector T cells may both contribute to the loss of control of the autoimmune response in T1D.
At the heart of the issue of defective nTreg function in T1D is that nTregs are intrinsically dependent on IL-2 for their homeostasis and function (Setoguchi et al., 2005; Josefowicz and Rudensky, 2009). Genetic studies have now shown a clear link between T1D and defects in the IL-2 pathway in both T1D patients and NOD mice (Wicker et al., 2005; Dendrou and Wicker, 2008). Indeed, due to diminished IL-2 levels, nTreg numbers are reduced in the pancreata of NOD mice, which may result from impaired survival and/or loss of regulatory function. Furthermore, nTregs can convert into diabetogenic effector cells (Zhou et al., 2009), which may be a crucial factor in the final stages of the inflammatory processes that lead to diabetes onset. This may in part explain why few nTregs are detected in pancreatic islets of recent onset T1D patients (Willcox et al., 2009). Importantly, in NOD mice, administration of exogenous IL-2 can correct defective nTreg function (Tang et al., 2008). In parallel, a defect in IL-2R signaling in Tregs from T1D patients resulted in diminished maintenance of FOXP3 expression and function (Long et al., 2010). In non-autoimmune mice, reduced IL-2 signaling can also reduce nTreg functionality, with the development of autoimmune sequelae (Cheng et al., 2011).
There are further challenges for clinical translation of nTregs. First, it has been difficult to unequivocally identify and isolate nTregs from human patients because of the lack of unique marker(s). Second, since nTregs represent a minor subset of the total CD4+ T cell population, extensive expansion is necessary to achieve the requisite numbers for clinical treatment, further increasing the concern that contaminating pathogenic cells might be simultaneously expanded. Additionally, nTregs exhibit considerable plasticity, and those from autoimmune patients can acquire pathogenic effector phenotype, such as the production of proinflammatory cytokines. Moreover, T1D patients have higher percentages of nTregs expressing IFNγ, an effector cytokine produced by Th1 cells that are strongly associated with the development of T1D (McClymont et al., 2011). These findings reveal that, for T1D in particular, administration of nTregs would likely not provide a long-term benefit to patients once homeostasis has returned.
aTregs: generation and function in T1D
aTregs have received less attention as a possible disease intervention because they represent a minor and more diverse population than nTregs. Because of limited phenotypic characterization, aTregs that arise physiologically are often indistinguishable from nTregs. In addition, they may be more important in downmodulating immune responses when infections are resolved than in maintaining self-tolerance. Although spontaneous development of pancreatic antigen-specific aTregs does not appear to occur during the progression to diabetes (Wong et al., 2007), we and others have demonstrated that they can be effective regulators of T1D. In general, it has been considered that weak T-cell receptor (TCR) stimulation (Bresson et al., 2006; Kretschmer et al., 2006) or TCR stimulation under tolerogenic conditions, such as with immature DCs, promotes the induction of aTregs (Luo et al., 2007; Maldonado and von Andrian, 2010). β-cell antigen-pulsed immature DCs have also been shown to protect prediabetic NOD recipients from developing diabetes, probably through the in vivo induction of Tregs (Lo et al., 2006). These findings confirm that aTregs can control the autoimmune response in T1D. The fact that these cells differentiate from naïve CD4+ T cells in the periphery highlights their potential to be developed under controlled conditions in vitro.
Our studies focusing on the regulation of memory in CD4+ T cells led us to investigate the role of immunological memory in T cells during the escalation of autoimmunity leading to T1D. In an effort to drive the differentiation of islet-specific effector cells and the development of memory in these cells, we used an in vitro approach that was thought to elicit memory CD4+ T cells (Weinberg et al., 1992), combining TGF-β1 and IL-2 with an optimal level of immobilized anti-CD3. Based on our observations that withdrawal of stimulation through TCR signals and differentiating cytokines resulted in a transition of CD4+ effector T cells to a resting memory phenotype (Harbertson et al., 2002), we analyzed the capacity of activated versus rested effectors to elicit diabetes in an adoptive transfer model. We showed that activated or rested populations were capable of persisting as memory cells after transfer (Weber et al., 2006; Godebu et al., 2008). However, instead of being pathogenic, they were protective against the development of spontaneous and immunologically accelerated diabetes. Furthermore, these aTregs restored normoglycemia to recent onset diabetic mice with a treatment efficacy of 50%–80%.
Studies from many other groups also showed that aTregs could be induced from naïve CD4+ T cells by TCR stimulation in the presence of IL-2 and TGF-β (Zheng et al., 2002; Chen et al., 2003; Fantini et al., 2004; Davidson et al., 2007). In the NOD mouse model, stimulation of islet antigen-specific TCR transgenic CD4+ T cells with a combination of splenic DCs, TGF-β1, and a mimotope peptide elicited FoxP3+ aTregs with the ability to prevent the development of diabetes in an accelerated diabetes model and to protect syngeneic islet grafts (Luo et al., 2007). TGF-β can elicit expression of FoxP3 through Smad3 signaling (Tone et al., 2008). Since FoxP3 is required for the regulatory function of nTregs, FoxP3-expressing aTregs that are induced in the presence of TGF-β have been considered to more closely resemble nTregs or aTregs that arise in vivo than other populations of Tregs, such as IL-10- or vitamin D3-induced Tr1 cells (Cobbold et al., 2003). In addition, the transcription factor Runx1, which complexes with FoxP3, is essential for the regulatory function of both nTregs and aTregs (Wong et al., 2011).
Although both populations can exhibit considerable heterogeneity in their genetic signatures (Feuerer et al., 2010), activated TGF-β-induced aTregs share many additional characteristics with nTregs, including the expression of CD25, CTLA-4, and GITR, and the secretion of TGF-β and IL-10, which contribute to their regulatory functions (Weber et al., 2006; Li et al., 2011). Since TGF-β-induced aTregs can be easily grown in vitro from abundant precursors, they are attractive candidates for the treatment of autoimmune diseases. Indeed, our studies using the NOD mouse model show that over 90% of TGF-β-induced aTregs express high levels of FoxP3, as has been reported in other models.
In vivo, TGF-β promotes aTreg development in gut mucosal tissues via mechanisms involving all-trans retinoic acid (ATRA) (Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007), which enhances Smad3 expression and activation (Xiao et al., 2008). It has been reported that aTregs can also be induced in vivo in NOD mice by administration of a variety of agents (Bruder et al., 2005; You et al., 2007; Kerkvliet et al., 2009; Zaccone et al., 2010). For instance, administration of chemicals that bind the transcription factor aryl hydrocarbon receptor to NOD mice induced FoxP3-expressing Treg-like cells and protected the mice from diabetes (Kerkvliet et al., 2009). However, diabetes developed after the termination of treatment. These results suggest that aTregs induced by this approach did not survive long after treatment and/or did not develop into memory cells.
Our studies of T1D show that adoptively transferred TGF-β-induced aTregs distribute throughout the lymphoid compartment and in the pancreas of recipient NOD mice. They can prevent the localization of pathogenic Th1 cells in the pancreas (Weber et al., 2006) and, like nTregs (Peng et al., 2004), they proliferate within the islets where they mediate local control of inflammation, depending on TGF-β (Li et al., 2011). Most other studies of aTregs in T1D have not addressed mechanisms by which the development of diabetes is controlled. Functionally, aTregs also share many characteristics with nTregs (Vignali et al., 2008) and have been shown to inhibit naïve T cell proliferation in vitro and in vivo. They can also inhibit the differentiation of other helper T cell subsets through the production of IL-10 and TGF-β, and can inhibit effector functions such as IFNγ production (Weiner, 2001; Barrat et al., 2002). Since we find that aTregs themselves can produce IL-2 (Weber et al., 2006), in addition to suppressing the autoimmune response of pathogenic effector cells, it is possible that they support the expansion of nTregs with IL-2 and help them to maintain their function in controlling β-cell destruction (Figure 1). Furthermore, aTregs could have the potential to promote the differentiation of endogenous aTregs from naïve CD4+ T cells within the PLNs by virtue of IL-2 and TGF-β production, as has been demonstrated in vitro (Zheng et al., 2002, 2004). Indeed, we did observe an increased frequency of endogenous FoxP3+CD4+ T cells in recipient NOD mice whose blood glucose had been reversed to normal levels after aTreg transfer (our unpublished data).
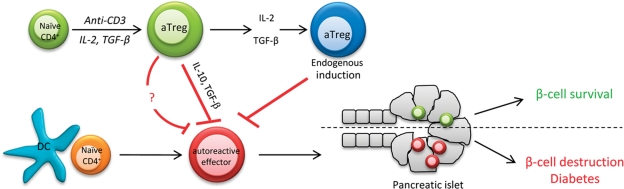
Figure 1.
Possible function of aTregs to control T1D. β-cell antigens are processed and presented by DCs in PLNs and initiate the activation of autoreactive effector T cells. If unchecked, these cells will migrate to the pancreas and mediate destruction of the β-cells. aTregs that are generated in vitro by stimulation with anti-CD3 in the presence of IL-2 and TGF-β, produce IL-10 and TGF-β, and are capable of suppressing autoreactive T effector cells and protect or reverse diabetes. In addition, aTregs may promote the differentiation of endogenous aTregs from naïve CD4+ T cells within the pancreatic LN by virtue of IL-2 and TGF-β production. Although the exact mechanism(s) of aTreg action are unclear, they play a crucial role in controlling β-cell destruction and T1D.
Stability of aTregs in T1D
Although it was shown that aTregs may lose FoxP3 when TGF-β is withdrawn (Floess et al., 2007; Selvaraj and Geiger, 2007), our studies, as well as those of others, indicate that after in vitro differentiation, the expression of FoxP3 is stable in the absence of TGF-β (Davidson et al., 2007; Li et al., 2011). One possibility for these differences in outcomes is that the conditions of stimulation can greatly affect the extent of differentiation status from naïve CD4+ T cells to effector T cells. Stable phenotypes are associated with epigenetic mechanisms regulating heritable patterns of gene expression (Ansel et al., 2003). Indeed, there is a substantial degree of plasticity during the initial differentiation of developing helper T cell subsets before the phenotypic stabilization over time with repeated stimulation under Th1 or Th2 polarizing conditions (O'Garra et al., 2011). Another key factor is antigen dose or the strength of TCR signaling. It was reported that high levels of TCR stimulation prevented the induction of FoxP3-expressing aTregs (Turner et al., 2009), whereas suboptimal TCR stimulation was found to favor aTreg differentiation (Oliveira et al., 2011). In our studies, we demonstrated that high doses of immobilized anti-CD3 in combination with IL-2 and TGF-β allow for both the optimal induction of FoxP3 and expansion of aTregs without a requirement for costimulation via CD28. Low-dose stimulation with anti-CD3 in the presence of these cytokines also generated FoxP3+ aTregs, but expansion was severely compromised even when anti-CD28 was included; moreover, the majority of these cells lost FoxP3 expression after transfer into NOD recipients (our unpublished data). Some studies indicated that combining optimal TCR stimulation with anti-CD28 costimulation also elicited the generation of FoxP3+ aTregs (Davidson et al., 2007; Gabrysova et al., 2011). Under these conditions, anti-CD28 stimulation supported the production of endogenous IL-2. In contrast, other studies showed that costimulation through CD28 can inhibit aTreg differentiation, most likely via the PI3K–mTOR pathway (Feuerer et al., 2009; Merkenschlager and von Boehmer, 2010). Therefore, the use of anti-CD28 for aTreg generation introduces additional complexity that may be difficult to define. We find that IL-2, in doses that are optimal for generating Th1 and Th2 effector cells, overcomes the anti-proliferative effects of TGF-β on naïve CD4+ T cells. A similar function of IL-2 in aTreg induction in vitro was reported by Zheng et al. (2007). Thus, these studies demonstrate that aTregs can be elicited in high numbers, which will ultimately be required for clinical translation.
The ultimate goal is the generation of aTregs that will be stable and lack the plasticity to convert to pathogenic effector cells because of the constant threat of reemerging autoimmunity. We have determined that aTregs exhibit stable FoxP3+ expression even after introduction into diabetic mice that have elevated levels of proinflammatory cytokines, including IL-17 and IFNγ, in the circulation (Godebu et al., 2008; our unpublished data). IL-17 and IFNγ are associated with Th17 and Th1 effector/memory cells, respectively, and both cytokines may contribute to the pathogenesis of T1D. In NOD mice that are protected from diabetes by the administration of aTregs, no acquisition of either IFNγ or IL-17 production by aTregs is observed, suggesting that the conversion to pathogenic effectors does not occur (our unpublished data). To test the stability of aTregs in vivo, we introduced them into prediabetic NOD mice or NOD.Scid mice that are devoid of lymphocytes and therefore lack autoimmunity. Under both conditions, aTregs proliferated to a similar extent as naïve T cells. Importantly, aTregs that had undergone division maintained FoxP3 expression and the ability to produce IL-10 as well as TGF-β, and they did not induce or accelerate diabetes (Li et al., 2011). A possible mechanism that could explain the stability is that conditions for aTreg differentiation in vitro result in the downregulation of proinflammatory cytokine receptors, which could otherwise drive the re-differentiation of aTregs into pathogenic effector cells. For instance, TGF-β is required for the differentiation of both Tregs and Th17 cells but the latter cells also require signaling through IL-6. Thus, in the presence of IL-6, conversion of Tregs to Th17 cells is possible. However, it has been demonstrated that under defined in vitro polarizing conditions, TGF-β-induced aTregs downregulate their IL-6R expression. Therefore, the Th17-conversion is blocked in these cells (Zheng et al., 2008). Taken together, these findings indicate that even by stringent criteria, stability of aTregs is maintained, which is crucial for their therapeutic potential.
The development and regulation of memory in aTregs
Of all the facets of Treg regulation that have been studied, the potential for the development of immunological memory has received very little attention. Clearly, the concept that Tregs could function in the long-term control of autoimmunity in T1D just as memory T cells can occur in the prevention of repeated infections is not just appealing, but a critical element for ultimately achieving a cure. Thus, a fundamental aspect of aTregs with respect to their use in T1D is whether their regulation will permit more than transient control of autoimmunity, which is most likely to be a concern with nTregs, whose regulation by IL-2 will ultimately sabotage their long-term function in vivo. Our studies show that one round of in vitro stimulation with anti-CD3, IL-2, and TGF-β is sufficient to elicit stable FoxP3+ aTregs that can persist indefinitely as memory cells in vivo (Godebu et al., 2008), whereas other studies indicate that repeated stimulation drives their terminal differentiation (Gabrysova et al., 2011). As we and others have shown, return of effector cell populations to rest by withdrawal of stimulation is sufficient to drive the development of a memory phenotype (Hu et al., 2001; Harbertson et al., 2002; McKinstry et al., 2007). This is in part due to the transition from anabolic metabolism in the activated effector state to catabolic metabolism with a return to rest. Thus, we hypothesize that aTregs rapidly become memory cells after adoptive transfer because of the loss of strong TCR signals and polarizing cytokines.
We demonstrated that monoclonal islet antigen-specific aTregs control and reverse diabetes, are stably maintained at low but detectable levels, and can be greatly re-expanded after challenge with a mimotope peptide (Weber et al., 2006). In an elegant adoptive transfer system using two TCR transgenics specific for different islet antigens, Haskins' group showed that antigen recognition is necessary for aTreg function (Tonkin et al., 2008). Thus, exposure to self-antigen(s) could maintain the functionality of aTregs as occurs with CD4+ memory T cells in general. Importantly, we find that aTregs recovered from primary adoptive recipients transfer protection against diabetes to secondary prediabetic recipients (Godebu et al., 2008). Our studies further demonstrate that aTregs that reverse and thereafter control diabetes can be generated in vitro from polyclonal naïve CD4+ T cells, although larger numbers are required than for islet antigen-specific aTregs to treat T1D. Polyclonal aTregs exhibit greater persistence than monoclonal aTregs over time after adoptive transfer into prediabetic NOD recipients (Godebu et al., 2008). Furthermore, we observe a narrowing of the TCR repertoire to an oligoclonal population that is dominated by the TCR Vβ11 chain, implying in vivo selection by antigen. From our studies, restimulation of polyclonal aTregs by islet antigens in vitro elicits expansion and the appropriate cytokine response (i.e. IL-10 and TGF-β secretion). Thus, as memory cells, polyclonal aTregs will more than likely continue to be selected and may be amplified by antigens as a consequence of homeostatic regulation. This could serve to ‘instruct’ persisting aTregs to become better and more effective memory cells, which would be highly advantageous for long-term control of T1D (Figure 2). The results thus suggest that, as a therapeutic treatment for T1D, there may not be a need to generate antigen-specific aTregs. Despite these indications that aTregs are an important population to consider for clinical translation, a concern that remains to be carefully addressed is the effects of infections on the persistence and function of aTregs, particularly with viruses such as Coxsackievirus that infect the pancreas, release islet antigens, and accelerate diabetes (Horwitz et al., 1998).
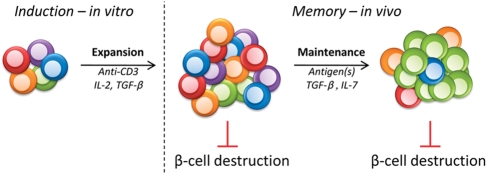
Figure 2.
Generation and maintenance of memory aTregs. One round of in vitro stimulation is sufficient to elicit stable aTregs from polyclonal naïve CD4+ T cells that persist indefinitely as memory cells in vivo. High doses of immobilized anti-CD3 in combination with IL-2 and TGF-β allow for both the optimal induction of FoxP3 and expansion of aTregs in vitro. Following adoptive transfer, these cells express IL-10 and TGF-β and are able to control β-cell destruction and restore normoglycemia. Unlike nTregs, aTregs are long-lived memory cells due to IL-7-dependent homeostatic proliferation. During their maintenance, these cells undergo in vivo selection by antigen(s), which narrows the TCR repertoire to an oligoclonal population that is still very capable of controlling autoreactivity. Thus, aTregs are ideally equipped to safeguard β-cell survival and protect from T1D due to their ease of generation, stability, and longevity.
Since the dysregulation of nTreg homeostasis represents an unalterable characteristic of T1D, in order to achieve long-term protection, memory aTregs require utilization of homeostatic mechanisms that are distinct from those that control nTregs. In our studies, we demonstrated that, immediately after differentiation in vitro, aTregs express high levels of CD25 (IL-2Rα) as nTregs, consistent with many other reports (Zheng et al., 2002; Chen et al., 2003; Tarbell et al., 2004). However, after withdrawal of TCR stimulation and a period of in vitro culture or in vivo incubation in host animals, these cells modulate their expression of γc cytokine receptors with downregulation of CD25 and upregulation of CD127 (IL-7Rα). Moreover, they express low levels of CD62L and high levels of CD44. Thus, in vitro-differentiated aTregs acquire a typical effector memory phenotype (Harbertson et al., 2002). This phenotype is maintained in vivo in NOD recipient mice indefinitely (up to 2 years), suggesting that aTregs persist in the hosts as effector memory cells (Godebu et al., 2008; Li et al., 2011). The CD25–CD127+ phenotype is reciprocal to that of nTregs, which are CD25+CD127–. This suggests that aTregs may use IL-7 rather than IL-2 for homeostatic regulation. Indeed, in an adoptive transfer model, we confirmed this prediction (Li et al., 2011). The IL-7 dependency of aTregs mirrors that of other CD4+ memory T cell subsets (Kondrack et al., 2003), suggesting that memory aTregs use mechanisms of homeostatic regulation that are similar to conventional memory T cells.
In addition to IL-7, we find that TGF-β may play an important role in maintaining memory aTregs in the pancreas. Our studies and those of others indicate that aTregs can control the autoimmune response within the microenvironment of the islets themselves (Tonkin and Haskins, 2009; Li et al., 2011). It is thought that islet-specific T cells persist in the context of the islets primarily because of the local availability of antigens (Lennon et al., 2009), which could be important for their local maintenance and survival. However, our studies show that TGF-β can also play a role in the ability of aTregs to control the autoimmune response either directly or indirectly by contributing to their expansion and/or survival. Although it is unclear whether TGF-β can be used in an autocrine or paracrine manner by aTregs, anti-TGF-β treatment abolished their capacity for protection against pathogenic cells from diabetic mice after adoptive transfer (Li et al., 2011). Although IL-10 has been reported to regulate FoxP3 expression in aTregs in the gut mucosal tissues (Murai et al., 2009), we did not find a role for this cytokine in aTreg homeostasis in T1D (Li et al., 2011).
One effect of TGF-β on T cells is to induce expression of β7 integrin (Lim et al., 1998), which can pair with either α4 or αE (CD103) integrin. It is therefore noteworthy that aTregs express both integrin α chains in addition to β7. The mucosal addressin cell adhesion molecule, MAdCAM-1, which is the ligand for α4β7, is highly upregulated on vascular endothelial cells in inflamed islets in NOD mice (Hanninen et al., 1993; Yang et al., 1994, 1997). Importantly, we find that α4β7 is required to mediate the localization of aTregs in the islets (Weber et al., 2006; Li et al., 2011). Furthermore, in the absence of β7 expression, aTregs are no longer protective (Li et al., 2011), demonstrating the need for their localization in the target organ for function. It has been previously shown that α4β7 is highly expressed on islet-infiltrating lymphocytes in NOD mice, suggesting that the mechanisms underlying aTreg migration are shared with those of pathogenic effector cells. This is consistent with the recent concept that similarities between Tregs and effector T cells are important for effective regulation. For example, the expression of the Th2 associated transcription factor IRF4 by Tregs is necessary for their control of Th2 responses (Zheng et al., 2009). The sharing of adhesion/migration mechanisms by aTregs and effector cells would facilitate their localization in the same microenvironments. Furthermore, integrin αEβ7 could play an important role in retaining aTregs in pancreatic islets, which express the ligand E-cadherin (Kilshaw and Higgins, 2002).
Taken together, current data indicate that aTregs can persist as memory cells and reverse and/or protect against diabetes through IL-2-independent homeostatic regulation, which can be exploited in developing cell-based treatments to restore self-tolerance in T1D.
Translating aTregs into cell-based therapy for T1D
There are many differences between human and mouse T cells that need to be addressed in order to develop aTregs for treatment of T1D or other inflammatory diseases. For instance, in mouse CD4+ T cells FoxP3 expression is exclusively Treg-specific, whereas in human CD4+ T cells, FOXP3 can be transiently expressed during activation and does not necessarily confer suppressor function (Wang et al., 2007). In addition, although numerous studies have demonstrated stable regulatory functions of IL-2- and TGF-β-induced mouse aTregs, it has been reported that although this combination of cytokines induced stable FOXP3-expressing human CD4+ T cells, they lacked regulatory function (Tran et al., 2007). This result raised concerns that FOXP3 expression may not be sufficient to confer aTreg function in human cells, and/or that the IL-2 plus TGF-β condition may not be sufficient to elicit aTreg differentiation (Shevach et al., 2008). However, other studies showed that IL-2 and TGF-β supported the differentiation of stable, functional Tregs from human CD4+CD25– T cells (Horwitz et al., 2008). These contrasting results are likely due to different experimental conditions, starting cell populations, or even reagents. Indeed, many different culture conditions have been reported to elicit regulatory functions from human CD4+CD25– T cells. For instance, studies have shown that, even without the addition of TGF-β, TCR stimulation in the presence of IL-2 was able to generate functional human Tregs (Walker et al., 2003, 2005). Under these conditions, TGF-β from serum and/or from activated T cells may have been sufficient to support the differentiation, and extended periods of culture could have contributed to the stable differentiation (Ziegler, 2007).
In addition to TGF-β, other factors or pharmacological agents have been reported to be able to differentiate, stabilize, and expand human Tregs. For instance, the vitamin A metabolite ATRA has been shown to promote Treg differentiation in mouse models (Mucida et al., 2007). Recently, ATRA was demonstrated to promote and stabilize functional human aTregs that were able to protect against xenogeneic graft-versus-host disease (GvHD) (Lu et al., 2010; Zhou et al., 2010). Another example is rapamycin. By inhibiting the activation of the mTOR, rapamycin can suppress effector cell activation/proliferation while allowing Treg differentiation and/or expansion (Powell and Delgoffe, 2010). In combination with IL-2 or TGF-β, rapamycin was able to induce and expand regulatory T cells from naïve human CD4+ T cells (Long and Buckner, 2008; Hippen et al., 2011a; Qian et al., 2011). Furthermore, these aTregs protected recipient animals from xenogeneic GvHD (Hippen et al., 2011a; Qian et al., 2011). Rapamycin was also shown to expand Tregs from T1D patients (Battaglia et al., 2006). A third example is the extracellular matrix component hyaluronic acid (HA), which is a ligand for CD44. By binding to CD44, high molecular weight (MW), but not low MW, HA can promote the suppressive function and stability of human aTregs (Bollyky et al., 2007, 2009). It is possible that more agents may be identified that can facilitate the in vitro differentiation, stabilization, and expansion of human aTregs that can be used to restore self-tolerance in vivo. Therefore, future studies are needed to optimize and standardize conditions and protocol(s) for therapeutic purposes.
Concluding remarks
As with other autoimmune and allergic diseases, there is a need for strategies to treat T1D that restore tolerance. Although the detailed mechanisms remain under investigation, difficulties with nTregs have emerged that currently preempt their use in a therapeutic setting. Our recent studies, in addition to those of others, suggest that in vitro-differentiated aTregs may prove to be a better, more readily translatable alternative to nTregs for T1D treatment because of their development of protective memory and unique homeostatic regulation. Of substantial relevance is that aTregs can be generated by stimulating naïve (CD45RA+)CD4+ T cells isolated from human blood samples, even from T1D patients, and with islet autoantigens (Long et al., 2009; Lu et al., 2010; Dromey et al., 2011). These findings support the concept that aTregs have significant potential for use as a treatment for T1D as well as other autoimmune conditions (Hippen et al., 2011b). However, it is critical to determine if the administration of aTregs could induce generalized immune suppression that may make recipients more susceptible to infections or cancer. It is also important to determine if by controlling autoimmunity, aTreg treatment can lead to restoration of measurable β-cell function, ultimately reducing or even eliminating insulin dependence.
Funding
L.M.B. was supported by grants from the National Institutes of Health (NIH, AI081238) and Juvenile Diabetes Research Foundation (JDRF, 31-2008-353).
Conflict of interest: none declared.
Acknowledgements
The authors would like to thank M.F. Douglass and J.T. Nguyen for assistance with the manuscript preparation. We apologize for not being able to cite many excellent works due to page limitations.
References
- Akerblom H.K., Virtanen S.M., Ilonen J., et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–837. doi: 10.1007/s00125-005-1733-3. doi:10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Lee D.U., Rao A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. doi:10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- Atkinson M.A. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can't we prevent or reverse this disease? Diabetes. 2005;54:1253–1263. doi: 10.2337/diabetes.54.5.1253. doi:10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. doi:10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Barrat F.J., Cua D.J., Boonstra A., et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. doi:10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J.C., Clayton D.G., Concannon P., et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009;41:703–707. doi: 10.1038/ng.381. doi:10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M., Stabilini A., Migliavacca B., et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., et al. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J. Exp. Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. doi:10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone J.A., Abbas A.K. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. doi:10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Bollyky P.L., Lord J.D., Masewicz S.A., et al. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J. Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- Bollyky P.L., Falk B.A., Long S.A., et al. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J. Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. doi:10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson D., Togher L., Rodrigo E., et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 2006;116:1371–1381. doi: 10.1172/JCI27191. doi:10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder D., Westendorf A.M., Hansen W., et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. doi:10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- Brusko T.M., Wasserfall C.H., Clare-Salzler M.J., et al. Functional defects and the influence of age on the frequency of CD4+CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. doi:10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- Brusko T., Wasserfall C., McGrail K., et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. doi:10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. doi:10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Yu A., Malek T.R. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol. Rev. 2011;241:63–76. doi: 10.1111/j.1600-065X.2011.01004.x. doi:10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson S.W., Shultz L.D., Leiter E.H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. doi:10.2337/diabetes.42.1.44. [DOI] [PubMed] [Google Scholar]
- Cobbold S.P., Nolan K.F., Graca L., et al. Regulatory T cells and dendritic cells in transplantation tolerance: molecular markers and mechanisms. Immunol. Rev. 2003;196:109–124. doi: 10.1046/j.1600-065x.2003.00078.x. doi:10.1046/j.1600-065X.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. doi:10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.D., Carayanniotis G., Sercarz E. Antigen processing by autoreactive B cells promotes determinant spreading. Cell. Mol. Immunol. 2005;2:169–175. [PubMed] [Google Scholar]
- Davidson T.S., DiPaolo R.J., Andersson J., et al. Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- Dendrou C.A., Wicker L.S. The IL-2/CD25 pathway determines susceptibility to T1D in humans and NOD mice. J. Clin. Immunol. 2008;28:685–696. doi: 10.1007/s10875-008-9237-9. doi:10.1007/s10875-008-9237-9. [DOI] [PubMed] [Google Scholar]
- Dromey J.A., Lee B.H., Yu H., et al. Generation and expansion of regulatory human CD4+ T-cell clones specific for pancreatic islet autoantigens. J. Autoimmun. 2011;36:47–55. doi: 10.1016/j.jaut.2010.10.005. doi:10.1016/j.jaut.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Fantini M.C., Becker C., Monteleone G., et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Hill J.A., Mathis D., et al. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. doi:10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- Feuerer M., Hill J.A., Kretschmer K., et al. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc. Natl Acad. Sci. USA. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. doi:10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floess S., Freyer J., Siewert C., et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. doi:10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrysova L., Christensen J.R., Wu X., et al. Integrated T-cell receptor and costimulatory signals determine TGF-beta-dependent differentiation and maintenance of Foxp3+ regulatory T cells. Eur. J. Immunol. 2011;41:1242–1248. doi: 10.1002/eji.201041073. doi:10.1002/eji.201041073. [DOI] [PubMed] [Google Scholar]
- Gepts W., Lecompte P.M. The pancreatic islets in diabetes. Am. J. Med. 1981;70:105–115. doi: 10.1016/0002-9343(81)90417-4. doi:10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Godebu E., Summers-Torres D., Lin M.M., et al. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J. Immunol. 2008;181:1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanninen A., Taylor C., Streeter P.R., et al. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J. Clin. Invest. 1993;92:2509–2515. doi: 10.1172/JCI116859. doi:10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbertson J., Biederman E., Bennett K.E., et al. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J. Immunol. 2002;168:1095–1102. doi: 10.4049/jimmunol.168.3.1095. [DOI] [PubMed] [Google Scholar]
- Herold K.C., Hagopian W., Auger J.A., et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. doi:10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- Herold K.C., Gitelman S.E., Masharani U., et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. doi:10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra H.S., Schloot N.C., van Veelen P.A., et al. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc. Natl Acad. Sci. USA. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. doi:10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen K.L., Merkel S.C., Schirm D.K., et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am. J. Transplant. 2011a;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen K.L., Riley J.L., June C.H., et al. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin. Immunol. 2011b;23:462–468. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M.S., Bradley L.M., et al. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 1998;4:781–785. doi: 10.1038/nm0798-781. doi:10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- Horwitz D.A., Zheng S.G., Wang J., et al. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur. J. Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. doi:10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- Hu H., Huston G., Duso D., et al. CD4+ T cell effectors can become memory cells with high efficiency and without further division. Nat. Immunol. 2001;2:705–710. doi: 10.1038/90643. doi:10.1038/90643. [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. doi:10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem S.A., Ariel I., Thornton P.S., et al. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. doi:10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- Keenan H.A., Sun J.K., Levine J., et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. doi:10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet N.I., Steppan L.B., Vorachek W., et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3+ T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilshaw P.J., Higgins J.M. Alpha E: no more rejection? J. Exp. Med. 2002;196:873–875. doi: 10.1084/jem.20021404. doi:10.1084/jem.20021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrack R.M., Harbertson J., Tan J.T., et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. doi:10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K., Heng T.S., von Boehmer H. De novo production of antigen-specific suppressor cells in vivo. Nat. Protoc. 2006;1:653–661. doi: 10.1038/nprot.2006.105. doi:10.1038/nprot.2006.105. [DOI] [PubMed] [Google Scholar]
- Kukreja A., Cost G., Marker J., et al. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J.M., Tremble J., Dayan C., et al. Increased resistance to CD4+CD25hi regulatory T cell-mediated suppression in patients with type 1 diabetes. Clin. Exp. Immunol. 2008;154:353–359. doi: 10.1111/j.1365-2249.2008.03810.x. doi:10.1111/j.1365-2249.2008.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon G.P., Bettini M., Burton A.R., et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–653. doi: 10.1016/j.immuni.2009.07.008. doi:10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.R., Deiro M.F., Godebu E., et al. IL-7 uniquely maintains FoxP3+ adaptive Treg cells that reverse diabetes in NOD mice via integrin-beta7-dependent localization. J. Autoimmun. 2011;37:217–227. doi: 10.1016/j.jaut.2011.06.002. doi:10.1016/j.jaut.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.P., Leung E., Krissansen G.W. The beta7 integrin gene (Itgb-7) promoter is responsive to TGF-beta1: defining control regions. Immunogenetics. 1998;48:184–195. doi: 10.1007/s002510050422. doi:10.1007/s002510050422. [DOI] [PubMed] [Google Scholar]
- Lindley S., Dayan C.M., Bishop A., et al. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. doi:10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- Lo J., Peng R.H., Barker T., et al. Peptide-pulsed immature dendritic cells reduce response to beta cell target antigens and protect NOD recipients from type I diabetes. Ann. NY Acad. Sci. 2006;1079:153–156. doi: 10.1196/annals.1375.023. doi:10.1196/annals.1375.023. [DOI] [PubMed] [Google Scholar]
- Long S.A., Buckner J.H. Combination of rapamycin and IL-2 increases de novo induction of human CD4+CD25+FOXP3+ T cells. J. Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. doi:10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.A., Walker M.R., Rieck M., et al. Functional islet-specific Treg can be generated from CD4+CD25– T cells of healthy and type 1 diabetic subjects. Eur. J. Immunol. 2009;39:612–620. doi: 10.1002/eji.200838819. doi:10.1002/eji.200838819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.A., Cerosaletti K., Bollyky P.L., et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. doi:10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhou X., Wang J., et al. Characterization of protective human CD4+CD25+FOXP3+ regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. doi:10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Tarbell K.V., Yang H., et al. Dendritic cells with TGF-beta1 differentiate naive CD4+CD25– T cells into islet-protective Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2007;104:2821–2826. doi: 10.1073/pnas.0611646104. doi:10.1073/pnas.0611646104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Herold K.C., Miller S.D. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity. 2010;32:488–499. doi: 10.1016/j.immuni.2010.04.002. doi:10.1016/j.immuni.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R.A., von Andrian U.H. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. doi:10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClymont S.A., Putnam A.L., Lee M.R., et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. doi:10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry K.K., Golech S., Lee W.H., et al. Rapid default transition of CD4 T cell effectors to functional memory cells. J. Exp. Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. doi:10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J. Exp. Med. 2010;207:1347–1350. doi: 10.1084/jem.20101156. doi:10.1084/jem.20101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki A., Hanafusa T., Yamada K., et al. Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin. Exp. Immunol. 1985;60:622–630. [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. doi:10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Murai M., Turovskaya O., Kim G., et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. doi:10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Gabrysova L., Spits H. Quantitative events determine the differentiation and function of helper T cells. Nat. Immunol. 2011;12:288–294. doi: 10.1038/ni.2003. doi:10.1038/ni.2003. [DOI] [PubMed] [Google Scholar]
- Oliveira V.G., Caridade M., Paiva R.S., et al. Sub-optimal CD4+ T-cell activation triggers autonomous TGF-beta-dependent conversion to Foxp3+ regulatory T cells. Eur. J. Immunol. 2011;41:1249–1255. doi: 10.1002/eji.201040896. doi:10.1002/eji.201040896. [DOI] [PubMed] [Google Scholar]
- Peng Y., Laouar Y., Li M.O., et al. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl Acad. Sci. USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. doi:10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.D., Delgoffe G.M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. doi:10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam A.L., Vendrame F., Dotta F., et al. CD4+CD25high regulatory T cells in human autoimmune diabetes. J. Autoimmun. 2005;24:55–62. doi: 10.1016/j.jaut.2004.11.004. doi:10.1016/j.jaut.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Qian X., Wang K., Wang X., et al. Generation of human regulatory T cells de novo with suppressive function prevent xenogeneic graft versus host disease. Int. Immunopharmacol. 2011;11:630–637. doi: 10.1016/j.intimp.2010.11.036. doi:10.1016/j.intimp.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia L., Cahill C.J., Finegood D.T., et al. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. doi:10.1210/en.138.4.1736. [DOI] [PubMed] [Google Scholar]
- Schneider A., Rieck M., Sanda S., et al. The effector T cells of diabetic subjects are resistant to regulation via CD4+FOXP3+ regulatory T cells. J. Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R.K., Geiger T.L. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J. Immunol. 2007;179 11 p following 1390. [PubMed] [Google Scholar]
- Setoguchi R., Hori S., Takahashi T., et al. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. doi:10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M., Tran D.Q., Davidson T.S., et al. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol. 2008;38:915–917. doi: 10.1002/eji.200738111. doi:10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda L.K., Young D.L., Ramanujan S., et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. doi:10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Stadinski B., Kappler J., Eisenbarth G.S. Molecular targeting of islet autoantigens. Immunity. 2010;32:446–456. doi: 10.1016/j.immuni.2010.04.008. doi:10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Stene L.C., Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am. J. Clin. Nutr. 2003;78:1128–1134. doi: 10.1093/ajcn/78.6.1128. [DOI] [PubMed] [Google Scholar]
- Sun C.M., Hall J.A., Blank R.B., et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. doi:10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Penaranda C., et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. doi:10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V., Yamazaki S., Olson K., et al. CD25+CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. doi:10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J.A. A protective role of the environment in the development of type 1 diabetes? Diabet. Med. 1991;8:906–910. doi: 10.1111/j.1464-5491.1991.tb01528.x. doi:10.1111/j.1464-5491.1991.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Todd J.A., Walker N.M., Cooper J.D., et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 2007;39:857–864. doi: 10.1038/ng2068. doi:10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y., Furuuchi K., Kojima Y., et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2008;9:194–202. doi: 10.1038/ni1549. doi:10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- Tonkin D.R., Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur. J. Immunol. 2009;39:1313–1322. doi: 10.1002/eji.200838916. doi:10.1002/eji.200838916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin D.R., He J., Barbour G., et al. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J. Immunol. 2008;181:4516–4522. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
- Tran D.Q., Ramsey H., Shevach E.M. Induction of FOXP3 expression in naive human CD4+FOXP3– T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. doi:10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S., Poirot L., Hattori M., et al. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. doi:10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.S., Kane L.P., Morel P.A. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J. Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. doi:10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehik K., Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab. Res. Rev. 2011;27:3–13. doi: 10.1002/dmrr.1141. [DOI] [PubMed] [Google Scholar]
- Vendrame F., Pileggi A., Laughlin E., et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010;59:947–957. doi: 10.2337/db09-0498. doi:10.2337/db09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. doi:10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron-Lynch F., Herold K.C. Immunomodulatory therapy to preserve pancreatic beta-cell function in type 1 diabetes. Nat. Rev. Drug Discov. 2011;10:439–452. doi: 10.1038/nrd3402. doi:10.1038/nrd3402. [DOI] [PubMed] [Google Scholar]
- Walker M.R., Kasprowicz D.J., Gersuk V.H., et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J. Clin. Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.R., Carson B.D., Nepom G.T., et al. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25– cells. Proc. Natl Acad. Sci. USA. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. doi:10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ioan-Facsinay A., van der Voort E.I., et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. doi:10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Weber S.E., Harbertson J., Godebu E., et al. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J. Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- Weinberg A.D., Whitham R., Swain S.L., et al. Transforming growth factor-beta enhances the in vivo effector function and memory phenotype of antigen-specific T helper cells in experimental autoimmune encephalomyelitis. J. Immunol. 1992;148:2109–2117. [PubMed] [Google Scholar]
- Weiner H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. doi:10.1034/j.1600-065X.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Wen L., Peng J., Li Z., Wong F.S. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol. 2004;172:3173–3180. doi: 10.4049/jimmunol.172.5.3173. [DOI] [PubMed] [Google Scholar]
- Wen L., Ley R.E., Volchkov P.Y., et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. doi:10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett D.K., Bundy B., Becker D.J., et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319–327. doi: 10.1016/S0140-6736(11)60895-7. doi:10.1016/S0140-6736(11)60895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L.S., Clark J., Fraser H.I., et al. Type 1 diabetes genes and pathways shared by humans and NOD mice. J. Autoimmun. 2005;25(Suppl):29–33. doi: 10.1016/j.jaut.2005.09.009. doi:10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Willcox A., Richardson S.J., Bone A.J., et al. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. doi:10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K., Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. doi:10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Wong J., Mathis D., Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J. Exp. Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. doi:10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W.F., Kohu K., Chiba T., et al. Interplay of transcription factors in T-cell differentiation and function: the role of Runx. Immunology. 2011;132:157–164. doi: 10.1111/j.1365-2567.2010.03381.x. doi:10.1111/j.1365-2567.2010.03381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Jin H., Korn T., et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.D., Michie S.A., Tisch R., et al. A predominant role of integrin alpha 4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 1994;91:12604–12608. doi: 10.1073/pnas.91.26.12604. doi:10.1073/pnas.91.26.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.D., Sytwu H.K., McDevitt H.O., et al. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes. 1997;46:1542–1547. doi: 10.2337/diacare.46.10.1542. doi:10.2337/diabetes.46.10.1542. [DOI] [PubMed] [Google Scholar]
- You S., Leforban B., Garcia C., et al. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc. Natl Acad. Sci. USA. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. doi:10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccone P., Burton O.T., Gibbs S., et al. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J. Biomed. Biotechnol. 2010;2010:795210. doi: 10.1155/2010/795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.G., Gray J.D., Ohtsuka K., et al. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25– precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J.H., Gray J.D., et al. Natural and induced CD4+CD25+ cells educate CD4+CD25– cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Wang P., et al. IL-2 is essential for TGF-beta to convert naive CD4+CD25– cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Horwitz D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Chaudhry A., Kas A., et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. doi:10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Bailey-Bucktrout S.L., Jeker L.T., et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. doi:10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Kong N., Wang J., et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. doi:10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S.F. FOXP3: not just for regulatory T cells anymore. Eur. J. Immunol. 2007;37:21–23. doi: 10.1002/eji.200636929. doi:10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- Ziegler A.G., Pflueger M., Winkler C., et al. Accelerated progression from islet autoimmunity to diabetes is ausing the escalating incidence of type 1 diabetes in young children. J. Autoimmun. 2011;37:3–7. doi: 10.1016/j.jaut.2011.02.004. doi:10.1016/j.jaut.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]