Summary
CD8+ T cells undergo major metabolic changes upon activation, but how metabolism influences the establishment of long-lived memory T (TM) cells after infection remains a key question. We have shown here that CD8+ TM cells, but not effector CD8+ (TE) cells, possessed substantial mitochondrial spare respiratory capacity (SRC). SRC is the extra capacity available in cells to produce energy in response to increased stress or work and as such is associated with cellular survival. We found that interleukin-15 (IL-15), a cytokine critical for CD8+ TM cells, regulated SRC and oxidative metabolism by promoting mitochondrial biogenesis and expression of carnitine palmitoyl transferase (CPT1a), a metabolic enzyme that controls the rate-limiting step to mitochondrial fatty acid oxidation (FAO). These results show how cytokines control the bioenergetic stability of TM cells after infection by regulating mitochondrial metabolism.
Introduction
CD8+ T cells play a crucial role in immunity to infection and cancer. In response to antigen (Ag) and co-stimulation, CD8+ T cells undergo a developmental program characterized by distinct phases encompassing first the expansion, and then contraction, of Ag-specific effector T (TE) cell populations, followed by the persistence of long-lived memory T (TM) cells that mediate immunity to re-infection (Harty and Badovinac, 2008). While this predictable pattern of the response is well characterized, the mechanisms underlying the generation and maintenance of CD8+ TM cells, and in particular how metabolism influences this process, remain unclear.
Upon activation T cells undergo a metabolic switch to glycolysis, which is required to support their growth, proliferation, and effector functions (Krauss et al., 2001; Rathmell et al., 2000; Roos and Loos, 1973). Conventional views suggest that proliferating T cells ferment glucose to make ATP, even though there is sufficient oxygen present to support oxidative phosphorylation (OXPHOS) (Brand and Hermfisse, 1997; Greiner et al., 1994; Wang et al., 1976), a phenomenon known as the Warburg effect (Warburg, 1956). Signals from IL-2 and co-stimulatory CD28 support the activation and expansion of T cells by promoting this metabolic phenotype (Frauwirth et al., 2002; Wieman et al., 2007). In contrast to the glycolytic metabolism of T cells proliferating in response to Ag, it is thought that quiescent T cells (e.g. naive and TM cells), like most cells in normal tissues, use OXPHOS to meet energy demands (Krauss et al., 2001) by interchangeably breaking down glucose, amino acids, and fats to fuel the tricarboxylic acid (TCA) cycle and ATP production (Fox et al., 2005; Jones and Thompson, 2007). Implicit in this divergence in metabolism between activated and quiescent T cells is that the conversion, or switching, between differing metabolic states is required to effectively generate a given T cell fate. This has clearly been shown to be the case for the switch to glycolysis that accompanies naïve T cell activation (Fox et al., 2005; Jones and Thompson, 2007). While it is known that growth factor cytokines support the survival of resting T cells, how cells attain a quiescent state, and the accompanying metabolic transformation to OXPHOS that would presumably occur during the development of stable CD8+ TM cells after infection is incompletely understood.
Previously we demonstrated that pharmacological modulation of fatty acid oxidation (FAO) enhanced CD8+ TM development after vaccination (Pearce et al., 2009). However, understanding the metabolic features of CD8+ TM cells, and the mechanistic insight into why FAO is critical for CD8+ TM, is still lacking. Using extracellular flux analysis we investigated the metabolism of T cells after infection in real time and discovered a striking mitochondrial marker that is unique to CD8+ TM cells. We show here that CD8+ TM cells, unlike CD8+ TE cells or resting naïve CD8+ T cells, maintained substantial spare respiratory capacity (SRC) in their mitochondria. SRC is the extra mitochondrial capacity available in a cell to produce energy under conditions of increased work or stress and is thought to be important for long-term cellular survival and function (Choi et al., 2009; Ferrick et al., 2008; Nicholls, 2009; Nicholls et al., 2010; Yadava and Nicholls, 2007). We show here that SRC in CD8+ TM cells was dependent upon the ability of the cells to oxidize fats in their mitochondria. We demonstrate that IL-15, a cytokine critical for CD8+ TM cells (Kennedy et al., 2000; Ku et al., 2000; Mitchell et al., 2010; Sandau et al., 2010; Schluns et al., 2002; Surh and Sprent, 2008; Tan et al., 2002; Zhang et al., 1998), enhanced SRC by promoting mitochondrial biogenesis and the expression of carnitine palmitoyl transferase 1a (CPT1a), a mitochondrial protein which has been shown to play an important role in the utilization of fatty acids as an alternative energy source (Deberardinis et al., 2006; Ramsay and Zammit, 2004; Zaugg et al., 2011). Our genetic experiments show that CPT1a, and thus FAO, regulated SRC and CD8+ TM development. Our data indicate that maintaining mitochondrial function and SRC in CD8+ T cells is key to stable CD8+ TM formation after infection.
Results
CD8+ TM cells have substantial mitochondrial spare respiratory capacity
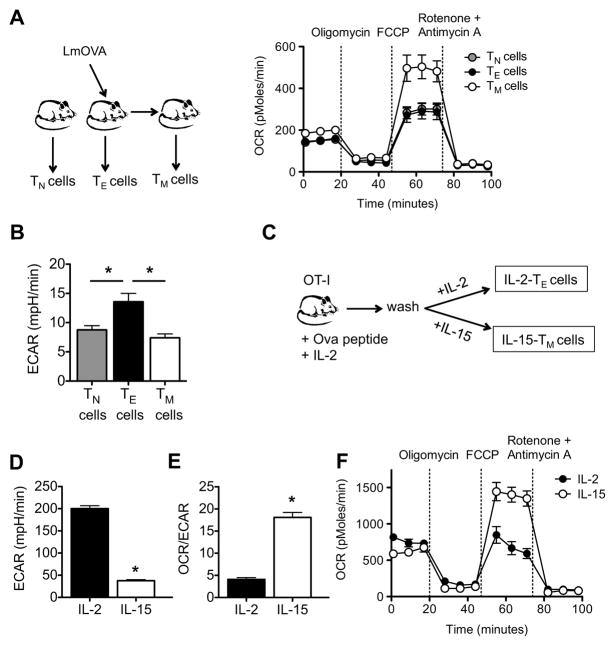
To establish how cellular metabolism is regulated during an immune response we measured the bioenergetic profiles of naïve (TN) CD8+ T cells, and TE and TM CD8+ T cells from wild-type (WT) mice infected with Listeria monocytogenes, in a basal state and after the addition of oligomycin (to block ATP synthesis), FCCP (to uncouple ATP synthesis from the electron transport chain, ETC), and rotenone and antimycin A (to block complex I and III of the ETC, respectively) (Figure S1A) (Gerencser et al., 2009; Nicholls et al., 2010). We found that the O2 consumption rate (OCR), an indicator of OXPHOS (Figure S1B), was slightly higher in TM cells in the basal state when compared to TE and TN cells (Figure 1A), while the basal extracellular acidification rate (ECAR), a consequence of lactic acid production (which is a marker of glycolysis), was greatest in TE cells (Figure 1B). Strikingly however, TM cells demonstrated a substantially larger mitochondrial SRC when compared to TE and TN cells, as indicated by the difference between the maximal OCR (after FCCP injection) and basal OCR (Figure 1A; Figure S1B).
Figure 1. CD8+ TM cells have substantial mitochondrial spare respiratory capacity.
Spleens and lymph nodes were harvested from naive and LmOVA infected mice, and TN, TE, and TM cells were isolated. (A) O2 consumption rates (OCR) were measured in real time under basal conditions and in response to indicated mitochondrial inhibitors; P < 0.0001 after FCCP injection. Data are representative of 4 independent experiments. (B) Extracellular acidification rates (ECAR) were measured under basal conditions; *P < 0.01 for TE versus TN cells, and < 0.01 for TE versus TM cells. Data are representative of 2 independent experiments. (C-F) OT-I cells were activated with OVA peptide for 3 days, and subsequently cultured in either IL-2 or IL-15 to generate IL-2 TE and IL-15 TM cells, respectively. Basal extracellular acidification rate (ECAR) (D), basal OCR/ECAR ratio (E), and OCR under basal conditions and in response to indicated mitochondrial inhibitors (F) in IL-2 TE and IL-15 TM cells are shown; *P < 0.0001 (D), < 0.0001 (E), <0.0001 (F after FCCP). Data are representative of at least 3 independent experiments. Data are shown as mean ± SEM. See also Figure S1.
CD8+ TM cells are characterized by their ability to persist for long periods of time and to respond vigorously to antigen re-encounter (Prlic et al., 2007). Since SRC is thought to be important for cellular survival and function (Choi et al., 2009; Ferrick et al., 2008; Nicholls, 2009; Nicholls et al., 2010; Yadava and Nicholls, 2007) we explored the role of SRC in CD8+ T cells. We investigated whether the cytokines IL-2 and IL-15, which are critical for the development and maintenance of CD8+ TE and TM cells, respectively (Kennedy et al., 2000; Mitchell et al., 2010; Sandau et al., 2010; Schluns et al., 2002; Surh and Sprent, 2008; Tan et al., 2002; Zhang et al., 1998), influence SRC. We isolated CD8+ T cells from major histocompatibility (MHC) class I-restricted OT-I transgenic mice and measured their bioenergetic profiles after stimulation with OVA peptide and IL-2 for 3 days, followed by 4 days of culture in either IL-2 or IL-15 (Figure 1C) (Carrio et al., 2004). These in vitro culture conditions approximate the program of TE and TM development after the response to an infection in vivo. Upon infection, antigen and IL-2 promote CD8+ TE activation and proliferation, followed by the contraction of these cell populations as infection is cleared and antigen and growth factors such as IL-2 decline. During this time presumably, exposure to cytokines such as IL-15 promotes the development and maintenance of CD8+ TM cells (Sandau et al., 2010; Schluns et al., 2002; Zhang et al., 1998). Culture with IL-2 and IL-15 induced TE-like (IL-2 TE) and TM-like (IL-15 TM) cells respectively, which displayed activation marker phenotypes similar to TE and TM cells generated after infection (Figure S1C) (Carrio et al., 2004). Consistent with the idea that proliferating T cells use glycolysis for energy (Frauwirth et al., 2002; Jones and Thompson, 2007) we found that IL-2 TE cells displayed high basal ECAR (Figure 1D) when compared to IL-15 TM cells. In contrast, while IL-15 TM cells were less metabolically active in a basal state, as shown by lower basal ECAR (Figure 1D) and OCR (Figure S1D) when compared to IL-2 TE cells, they possessed a higher OCR/ECAR ratio (Figure 1E). This would indicate that IL-15 TM cells preferentially use OXPHOS rather than glycolysis. We found that IL-15 promoted SRC in activated CD8+ T cells, whereas CD8+ T cells cultured in the presence of IL-2 lacked this energy reserve (Figure 1F). In addition, to rule out effects from substrate limitations during the assay, we performed the assay in the absence or presence of 10% serum and observed similar results (data not shown). Consistent with the idea that SRC is an intrinsic feature of CD8+ TM cells, we found that culture with IL-7, which also promotes the development of CD8+ TM-like cells in vitro (Carrio et al., 2004), and is important for the CD8+ TM cell homeostasis in vivo (Tan et al., 2002), enhanced SRC (data not shown).
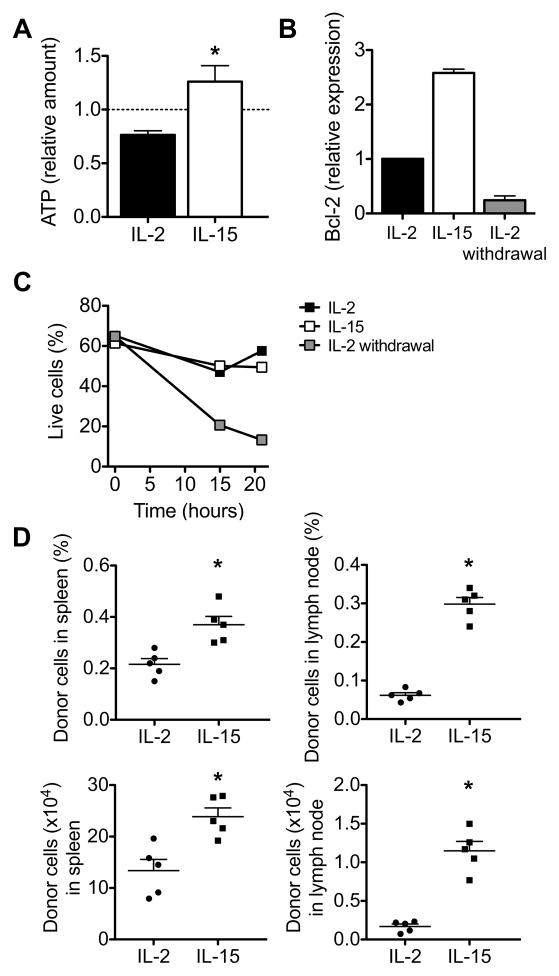
To determine how SRC correlates with the ability to produce energy in response to increased work, we re-stimulated IL-2 TE and IL-15 TM cells with anti-CD3 and anti-CD28, and found that IL-15 TM cells have an increased amount of ATP after re-stimulation when compared to IL-2 TE cells (Figure 2A). Moreover, upon re-stimulation IL-2 TE cells had decreased mitochondrial membrane potential (Ψmito) and enhanced superoxide production (Figures S2A and S2B), two parameters that indicate poor mitochondrial health (Grayson et al., 2003; Lambert and Brand, 2009; Mookerjee et al., 2010). Meanwhile IL-15 TM cells showed increased Ψmito and lower superoxide concentration. Consistent with this, IL-15 TM cells survived better (Figure S2C) after re-stimulation when compared to IL-2 TE cells. Since upon re-stimulation TM cells should convert to a TE phenotype, we tested whether re-stimulating IL-15 TM cells resulted in a metabolic switch from OXPHOS to glycolysis. Indeed, upon stimulation with PMA and ionomycin, IL-15 TM cells rapidly increased their ECAR (Figure S2D), and decreased their OCR/ECAR ratio (Figure S2E), while a longer stimulation with anti-CD3 and anti-CD28 also resulted in a lower OCR/ECAR ratio (Figure S2F) in those cells. Since all cells in these cultures were first activated with OVA peptide in the presence of IL-2, we wanted to establish that signals from IL-15, rather than simply the withdrawal of IL-2, regulated CD8+ T cell survival. Indeed we found that Bcl-2 expression, which correlates with CD8+ T cell survival (Grayson et al., 2000), was highest in IL-15 TM cells (Figure 2B), and that CD8+ T cells after IL-2 withdrawal were not long-lived without signals from IL-15 (Figure 2C). To explore whether IL-15 TM cells survived better in vivo, we adoptively transferred IL-2 TE cells or IL-15 TM cells into congenic mice and after 2 days we found significantly more IL-15 TM than IL-2 TE donor cells in spleen and lymph nodes (Figure 2D), which is consistent with data showing that IL-15 is critical for CD8+ T cell persistence (Kennedy et al., 2000; Sandau et al., 2010; Schluns et al., 2002). Together the data suggest that enhanced SRC enables TM cells to survive as functional long-lived cells and that this process is modulated by IL-15.
Figure 2. IL-15 signals promote CD8+ T cell survival.
OT-I cells were activated with OVA peptide for 3 days, and subsequently cultured in either IL-2 or IL-15. (A) IL-2 TE and IL-15 TM cells were re-stimulated with anti-CD3 and anti-CD28 for 5 hours and the amounts of re-stimulation induced ATP are shown relative to non-re-stimulated cells (dashed line); *P = 0.03. Data are shown as mean ± SEM and are representative of 2 independent experiments. (B-C) IL-2 cultured cells were withdrawn from IL-2 for 6–8 hours, and (B) relative Bcl-2 mRNA expression and (C) percentage live cells (based on 7-AAD analysis) are shown. Data are shown as the mean ± SEM (figure generated from 2 independent experiments) (B) or as a representative experiment of 3 independent experiments (C). (D) IL-2 TE and IL-15 TM cells were adoptively transferred into congenic recipients (n = 5 per group), and organs were harvested 2 days later, dot plots show percentages, top panels, or total numbers, bottom panels, of donor cells. Data are shown as mean ± SEM. Data are representative of 2 independent experiments. *P = 0.004 (top left), < 0.0001 (top right), 0.005 (bottom left), and < 0.0001 (bottom right). See also Figure S2.
IL-15 induces mitochondrial biogenesis in CD8+ T cells
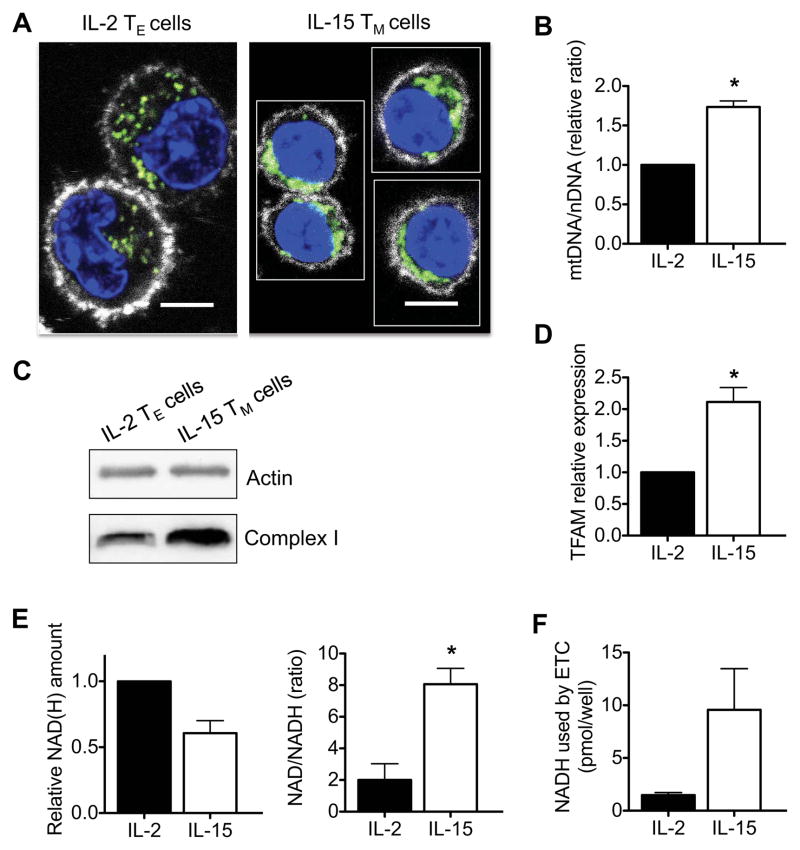
Since SRC is a measure of how efficiently the ETC can move protons from the mitochondrial matrix into the intermembrane space in the presence of the uncoupler FCCP as compared to the basal state (Figure S1A) (Mookerjee et al., 2010; Nicholls et al., 2010), we reasoned that the enhanced SRC evident in IL-15 TM cells might be explained by an increase in mitochondrial mass. IL-2 TE cells are larger than IL-15 TM cells (Carrio et al., 2004; Rathmell et al., 2000), and we found that their larger cytoplasm had a dispersed mitochondrial distribution (Figure 3A). In contrast, IL-15 TM cells had a smaller cytoplasmic space with tightly packed mitochondria, indicating a difference in the ratio between mitochondrial mass and total cell mass between TE and TM cells (Figure 3A and Figure S3). We next tested whether IL-15 induced mitochondrial biogenesis in CD8+ T cells by quantifying the ratio of mitochondrial DNA to nuclear DNA (mtDNA/nDNA) and found that this ratio was higher in IL-15 TM cells than in IL-2 TE cells (Figure 3B). We also found that IL-15 TM cells expressed more ETC protein complex I (Figure 3C), which has previously been reported to be important for TM cell function (Yi et al., 2006), Moreover, IL-15 TM cells had greater expression of mitochondrial transcription factor A (TFAM) mRNA (Figure 3D). Together these data demonstrate the enhanced mitochondrial content of IL-15 TM cells. NADH is generated by the TCA cycle and donates electrons to complex I as part of OXPHOS (Saraste, 1999) (Figure S1A). To test whether enhanced NADH availability further contributes to the greater SRC in IL-15 TM cells we measured the amount of NAD(H) (total NAD+ and NADH) and found that IL-15 TM cells actually had less NADH, as demonstrated by a lower amount of total NAD(H) and a higher NAD/NADH ratio, than IL-2 TE cells (Figure 3E). Importantly, when NADH consumption by the ETC was blocked with rotenone and antimycin A, IL-15 TM cells accumulated more NADH than IL-2 TE cells (Figure 3F). This would suggest that IL-15 TM cells preferentially use OXPHOS to generate energy, correlating with our results showing that IL-15 TM cells have higher OCR/ECAR ratios (Figure 1E). Together these data show that IL-15 induces mitochondrial biogenesis in CD8+ T cells and that greater mitochondrial mass, rather than greater NADH availability, contributes to enhanced SRC in these IL-15 TM cells.
Figure 3. IL-15 induces mitochondrial biogenesis in CD8 +T cells.
IL-2 TE and IL-15 TM cells were analyzed (A-F). (A) Confocal images show cells stained with Mitotracker (green), Hoechst (blue), and anti-CD8 (white), scale bars are 5 microns. (B) mtDNA/nDNA ratio (figure generated from 2 independent experiments) *P = 0.01, (C) Western analysis for complex I expression, and (D) relative mitochondrial transcription factor A (TFAM) mRNA expression (figure generated from 5 independent experiments) in IL-2 TE and IL-15 TM cells; *P = 0.008. (E) Relative total amount of NAD(H), NAD/NADH ratio, and (F) NADH consumed by the ETC (measured as NADH built up after ETC blockade) in IL-2 TE and IL-15 TM cells (figures generated from 3 independent experiments); *P = 0.03. Data are shown as mean ± SEM. Data in A and C are representative of at least 3 independent experiments. See also Figure S3.
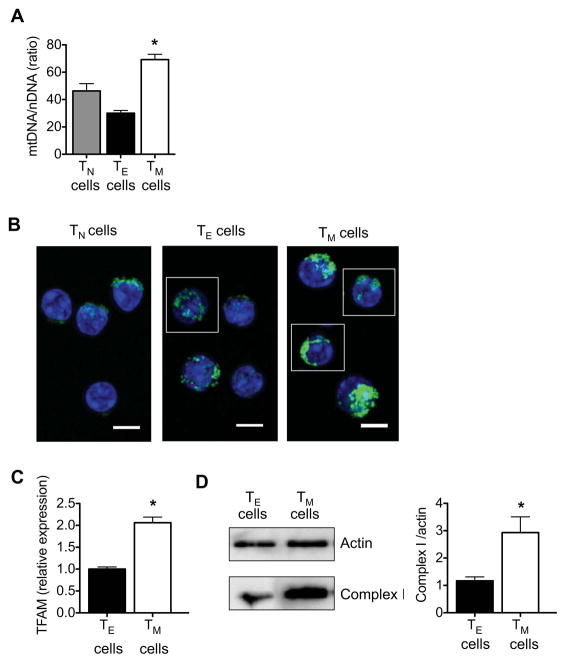
To confirm that bona fide CD8+ TM cells isolated after L. monocytogenes infection had enhanced mitochondrial content, we quantified the ratio of mtDNA/nDNA and found that this ratio was higher in CD8+ TM cells than in CD8+ TE cells (Figure 4A). Notably, CD8+ TN cells had fewer mitochondria than TM cells, indicating that enhanced mitochondrial mass is not a characteristic of resting T cells in general, but is unique to TM cells. This correlates with our finding that only TM cells, and not TN and TE cells, have considerable SRC. In addition, bona fide CD8+ TM cells had a denser mitochondrial distribution, while mitochondria in CD8+ TE cells were generally more disperse (Figure 4B). We also found that CD8+ TM cells had significantly higher TFAM mRNA (Figure 4C) and complex I protein expression when compared to CD8+ TE cells (Figure 4D). Together our data indicate that CD8+ TM cells have greater mitochondrial mass and this underlies their enhanced SRC.
Figure 4. CD8+ TM cells have greater mitochondrial mass than CD8+ TE cells.
Spleens and lymph nodes were harvested from naive and LmOVA infected mice, and TN, TE, and TM cells were isolated. (A) mtDNA/nDNA ratio (data are shown as mean ± SEM, figure generated from 2 independent experiments) *P < 0.01 for TE vs TM cells, and < 0.05 for TN vs TM cells. (B) Confocal images show TN, TE and TM cells stained with Mitotracker (green) and Hoechst (blue), scale bars are 5 microns. (C) Relative mitochondrial transcription factor A (TFAM) mRNA expression (data are shown as mean ± SEM, figure generated from 2 independent experiments) *P = 0.01, and (D) Western analysis for complex I expression (bars depict quantification of densitometry from 3 blots) *P = 0.008. Data are representative of 2 independent experiments.
Spare respiratory capacity in CD8+ T cells is dependent on mitochondrial fatty acid oxidation
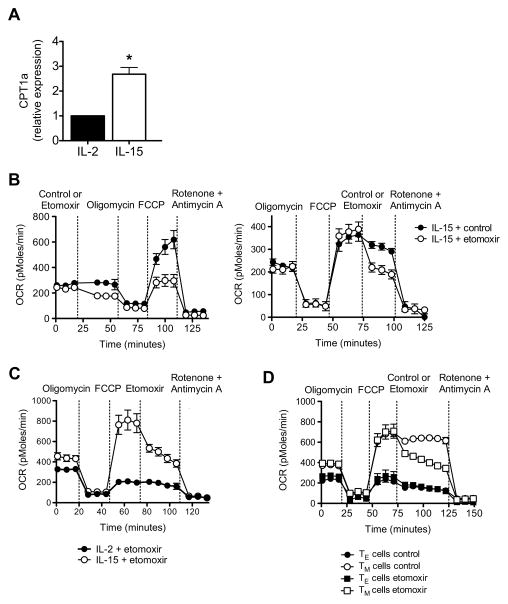
Our previous studies showed that pharmacologically modulating FAO after infection could promote the generation of CD8+ TM cells (Pearce et al., 2009). Since IL-15 is known to promote TM development (Sandau et al., 2010; Schluns et al., 2002; Zhang et al., 1998), and we have shown here that IL-15 supports SRC in CD8+ T cells, we sought to determine whether IL-15-enhanced SRC is associated with mitochondrial FAO. The rate-limiting step to FAO is the transfer of fatty acids from the cytosol into mitochondria by carnitine palmitoyltransferase 1 (CPT1) (Deberardinis et al., 2006; Ramsay and Zammit, 2004). We measured CPT1a mRNA in IL-2 TE and IL-15 TM cells and found that IL-15 TM cells expressed more CPT1a mRNA (Figure 5A). This indicates that IL-15 promotes the use of fatty acids for energy, and correlates with our finding that IL-15 induces mitochondrial biogenesis.
Figure 5. Spare respiratory capacity in CD8+ T cells is dependent on mitochondrial FAO.
(A) Relative CPT1a mRNA expression in IL-2 TE and IL-15 TM cells (figure generated from data from 5 independent experiments); *P = 0.003. Oxygen consumption rates (OCR) in IL-15 TM cells (B), in IL-2 TE and IL-15 TM cells (C), and bona fide TE and TM cells isolated from LmOVA infected B6 mice (D), under basal conditions and in response to indicated drugs; P < 0.0001 (after FCCP in B left panel), < 0.01 (after etomoxir in B right panel), and < 0.0001 (for TM cells after etomoxir versus control, D). See also Figure S4.
To determine whether the enhanced SRC in IL-15 TM cells depends on FAO we exposed IL-15 TM cells to the CPT1 inhibitor etomoxir, which blocks mitochondrial FAO (Deberardinis et al., 2006; Lopaschuk et al., 1988). Treatment with etomoxir, either before or after FCCP injection, impaired SRC in IL-15 TM cells (Figure 5B). Also evident is that basal OCR was inhibited by etomoxir (Figure 5B, left panel), further demonstrating that IL-15 TM cells use FAO for energy in a resting state. In contrast, OCR in IL-2 TE cells was not affected by etomoxir (Figure 5C). Consistent with these findings, SRC in bona fide CD8+ TM cells isolated after L. monocytogenes infection was also impaired by etomoxir, whereas the OCR after FCCP in CD8+ TE cells was not affected (Figure 5D). To test whether the inhibition of FAO alters the survival of IL-15 TM cells in vitro, we cultured cells with etomoxir and found that CPT1a inhibition impaired their survival (Figure S4).
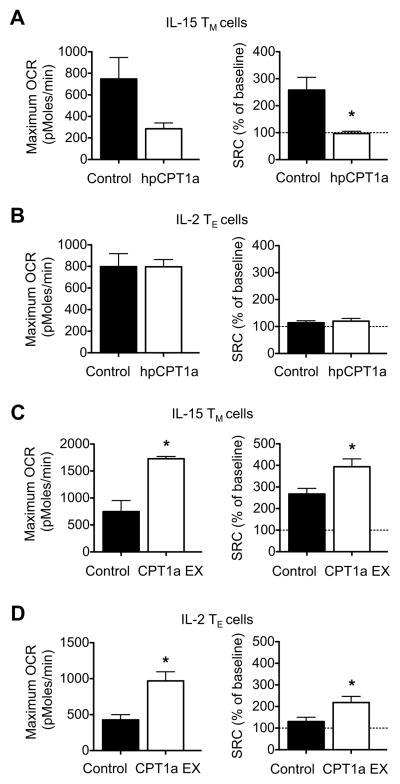
To further substantiate that SRC is regulated by FAO we next used a genetic approach. We modulated the expression of CPT1a by transducing CD8+ T cells with retrovirus expressing shRNA against CPT1a (hpCPT1a), and found that inhibiting CPT1a expression in IL-15 TM cells reduced maximum OCR after injection of FCCP (Figure 6A), while hpCPT1a expression did not affect maximum OCR in IL-2 TE cells (Figure 6B). To assess the effect of a genetic gain of function for FAO, we retrovirally expressed CPT1a (CPT1a EX) in CD8+ T cells. Retroviral expression of CPT1a significantly enhanced maximal OCR after FCCP in both IL-15 TM cells (Figure 6C) and IL-2 TE cells (Figure 6D). The same results were found when the data were expressed as SRC (Figure 6A-D, where maximum OCR after FCCP is calculated as a percentage of baseline OCR). The efficacy of the hpCPT1a and CPT1a EX to modulate the expression of CPT1a was confirmed (Figure S5A). We further verified that CPT1a EX directly promoted FAO by showing that etomoxir inhibits the enhanced maximum OCR in CPT1a EX IL-15 TM cells to a greater extent than in control IL-15 TM cells (Figure S5B). Consistent with increased SRC, retroviral CPT1a expression enhanced the survival of IL-15 TM cells in vitro (Figure S5C). Together these data establish that increased FAO positively regulates SRC in CD8+ T cells and thereby favors cellular survival.
Figure 6. Spare respiratory capacity in CD8+ T cells is dependent on CPT1a expression.
Maximum OCR (as indicated by OCR after injection of oligomycin and subsequent FCCP) and spare respiratory capacity (indicated by maximum OCR calculated as percentage of baseline OCR) in IL-15 TM cells(A, C) and IL-2 TE cells (B, D) cells transduced with either control (virus expressing shRNA against luciferase) or virus expressing shRNA against CPT1a (hpCPT1a) (A, B), and either control (empty vector) or CPT1a expressing (EX) retrovirus (C, D); *P = 0.03 (A), 0.003 (max OCR) and 0.02 (SRC) (C), 0.006 (max OCR) and 0.03 (SRC) (D). Data are representative of at least 2 independent experiments, and shown as mean ± SEM. See also Figure S5.
Mitochondrial FAO enhances T cell survival and promotes CD8+ TM cell development
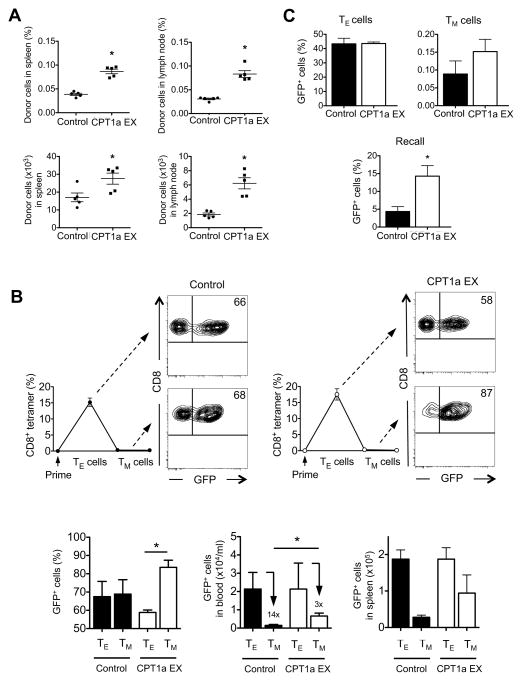
To explore whether CPT1a, and thus FAO, supports Ag-specific CD8+ T cell survival in vivo, we adoptively transferred activated control-transduced or CPT1a EX-transduced OT-I T cells into congenic mice and found significantly more CPT1a EX-transduced donor cells in spleen and lymph nodes than control-transduced donor cells after 2 days (Figure 7A), indicating that CPT1a increases survival of Ag-specific CD8+ T cells in vivo. Finally, to establish that FAO regulates development of CD8+ TM cells we adoptively transferred activated control-transduced and CPT1a-EX-transduced OT-I T cells into congenic recipients and tracked OVA-specific CD8+ T cell responses after immunization with L. monocytogenes. The frequency of OVA-specific cells that are control-transduced remains the same from the TE phase to the TM phase (Figure 7B, compare 66% in TE to 68% in TM, contour plots and left bar graph), indicating that there was no advantage to expressing the control vector in promoting cell survival. However, the frequency of CPT1a EX-transduced cells significantly increased from the peak of the TE response through contraction (Figure 7B, compare 58% in TE to 87% in TM, contour plots and left bar graph). The advantage to expressing the CPT1a EX vector was demonstrated by less contraction (Figure 7B, middle bar graph), and resulted in higher absolute numbers of CPT1a EX-transduced TM cells as compared to control-transduced TM cells, both in blood (Figure 7B, middle bar graph) and in spleen (Figure 7B, right bar graph). Furthermore, total TM cells increased in mice that received adoptive transfers of CPT1a EX transduced cells, suggesting that the CPT1a pathway is a limiting factor in TM cell generation (Figure S6A and S6B). Together, these data show that CPT1a, and thus FAO, promotes the development of CD8+ TM cells after infection.
Figure 7. Mitochondrial FAO enhances T cell survival and promotes CD8+ TM cell development.
OVA peptide activated OT-I cells transduced with control (empty vector) or CPT1a expressing (EX) retrovirus were intravenously injected into congenic recipients (n = 5 per group), and (A) organs were harvested 2 days later, dot plots show percentages (top panels), or total numbers (bottom panels) of donor cells (mean ± SEM); *P < 0.0001 (top left), < 0.0001 (top right), 0.03 (bottom left), and 0.0006 (bottom right), or (B) mice were infected with LmOVA, and peripheral blood CD8+ T cells were analyzed by KbOVA tetramer (line graphs) and GFP for retroviral expression (contour plots) at the peak of the response (TE cells, day 7) and after contraction (TM cells, day 14). Contour plots and bar graphs show the frequency of transduced (GFP+) cells within the CD8+ and KbOVA tetramer positive gate in blood (left bar graph; *P = 0.0003), absolute numbers of transduced (GFP+) cells/ml blood (middle bar graph, data are normalized to control TE cells; P = 0.02, numbers above bars indicate fold contraction), and absolute numbers of transduced (GFP+) cell in spleen (right bar graph, data are normalized to control TE cells). Data are shown as mean ± SEM and are representative of at least 2 independent experiments with at least 5 mice per group. (C) OT-I cells transduced with control (empty vector) or CPT1a expressing (EX) retrovirus were intravenously injected into IL-15 deficient recipients (n = 5 per group) and mice were infected with LmOVA. Bar graphs show the frequency of transduced (GFP+) cells within the KbOVA tetramer gate at the peak of the response (TE cells, day 7), after contraction (TM cells, day 14) and after challenge (recall, day 5 post challenge); *P = 0.01. Data are shown as mean ± SEM and are representative of 2 independent experiments. See also Figure S6.
To determine the extent to which the effects of IL-15 signals on CD8+ TM cells in vivo are CPT1a- dependent we adoptively transferred activated control-transduced and CPT1a-EX-transduced OT-I T cells into IL-15 deficient recipients and tracked OVA-specific CD8+ T cell responses after immunization with L. monocytogenes. While the frequencies of control and CPT1a EX transduced cells were similar at the peak of the response (TE), more CPT1a EX transduced cells survived contraction and subsequently were able to respond to a challenge infection (Figure 7C). We found, however, that the survival of both control and CPT1a EX transduced cells was reduced in IL-15 deficient recipients when compared to WT recipient mice (Figure S6C). These results suggest that IL-15 signals promote CPT1a expression and FAO to support CD8+ TM cells, but that this is not the only function of IL-15.
Discussion
We propose a model where mitochondrial respiratory capacity regulates T cell survival after infection. As TE cells undergo clonal expansion during an immune response they primarily use glycolysis to support rapid cell growth. We speculate that during this process the majority of CD8+ TE cells fail to maintain and/or induce mitochondrial biogenesis and this results in a reduction in the ratio between mitochondrial mass and overall cell mass, and a consequential loss of reserve energy-generating capacity, i.e. SRC. Reduced mitochondrial mass increases dependence on glycolysis and renders TE cells bioenergetically unstable as they lose the ability to create energy from diverse substrates via OXPHOS, and thus are unable to maintain viability when infection associated signals decline and elements such as IL-2 that support glycolysis dissipate. Some Ag-specific T cells from the primary infection survive as long-lived TM cells because they maintain mitochondrial mass via exposure to IL-15, or perhaps other common γ cytokines such as IL-7. Since FAO occurs in mitochondria, more mitochondrial mass allows greater use of fatty acids for energy via OXPHOS, thereby facilitating cell survival in the absence of pro-glycolysis signals. Moreover, the enhanced SRC provided by increased mitochondrial mass, might allow TM cells to respond quickly (“rapid recall”) if pathogen is reencountered.
Whether T cells receive growth factor signals can be controlled at many levels, including cytokine and cytokine receptor expression, trans-presentation of the cytokine to the T cells by antigen presenting cells or other accessory cells (McGill et al., 2010; Stonier et al., 2008), or T cell localization to niches where a cytokine is expressed. It is likely that whether T cells are able to receive signals from growth factors in vivo is a result of a combination of these mechanisms. Our preliminary observations indicate that lymph node homing receptors are upregulated immediately upon IL-2 withdrawal in vitro, suggesting that once infection associated signals such as IL-2 dissipate, cells become competent to traffic to lymph nodes where they gain access to IL-15 or IL-7. Additionally, it has been shown that Ag-specific CD8+ cells that have not fully differentiated into terminal TE cells during primary infection express IL-7R and persist as long-lived TM cells (Kaech et al., 2003). This suggests the possibility that these T cells have maintained mitochondrial function throughout contraction because of continual signals from IL-7.
The IL-2 and IL-15 receptors share identical subunits and their signaling pathways overlap (Hofmann et al., 2002; Waldmann et al., 1998), implying that the strength of signal through the cytokine receptor, rather than the mere induction of these pathways, may lead to the differential effects of IL-2 compared to IL-15 on TE cells versus TM cell differentiation (Carrio et al., 2004). However, our unpublished observations suggest that overall Stat5 phosphorylation is similar between our cultured IL-2 TE and IL-15 TM cells, indicating that at least the degree of Stat5 activation per se is not sufficient to dictate the differential effects of these cytokines. More work needs to be done to determine whether there is a unique signaling element, perhaps shared by IL-15 and IL-7 that supports TM cell development (Carrio et al., 2004).
Our data showing that retroviral expression of CPT1a facilitates CD8+ TM development in IL-15 deficient mice, but not to the same extent as in WT recipients, suggest that while IL-15 does promote FAO to support TM cells that this is not the only function of IL-15 during an immune response. We speculate that mitochondrial biogenesis is key to stable TM cell formation and that this is regulated by IL-15. While CPT1a EX transduced cells increase FAO due to enhanced CPT1a expression, they lack the mitochondrial mass, and thus energy producing machinery to maintain full bioenergetic stability when in an IL-15 deficient environment.
While we believe that our findings show how metabolism regulates T cell responses after infection, several questions remain. For example, how do TE cells come to have less SRC than TM cells? Although it has been described that mitochondrial biogenesis is up-regulated early after CD8+ T cell activation (D'Souza et al., 2007), we speculate that Ag-specific TE cells that continue to proliferate during infection might eventually outpace their own mitochondrial biogenesis, explaining why TE cells have less mitochondrial mass. It is also possible that mitochondria segregate unequally as T cells asymmetrically divide so that terminally differentiated TE cells acquire fewer mitochondria (Chang et al., 2007). Another unresolved issue is that of why TN cells have less SRC than TM cells. We have shown here that TN have less mitochondrial mass than TM cells, which correlates with their decreased SRC. It is also possible that qualitative differences exist between the mitochondria in TN and TM cells, such as differences in expression of ETC complexes on a per mitochondrion basis, which could in part be explained by the effects of IL-15 on TM cells after infection. This suggests that cell-intrinsic bioenergetic differences underlie the disparity in functionality between TN and TM cells, i.e. the rapid recall of TM cells is possible because of enhanced SRC. All of these areas are actively under study.
Agents that protect cells against loss in mitochondrial SRC, or that induce mitochondrial biogenesis, are thought to have potential for treating numerous pathologies (Beeson et al., 2010). Our findings here suggest that drugs that target mitochondrial SRC could hold promise as immunotherapeutics and might warrant further study for their ability to alter T cell responses. These results may serve to focus scientific efforts toward investigating how mitochondria dictate T cell function and lifespan, and thus impact how successful therapies and vaccines are designed.
Experimental Procedures
Mice and immunizations
C57BL/6, C57BL/6 CD45.1, C57BL/6 CD90.1, and major histocompatibility-complex class-I-restricted OVA specific T-cell-receptor (OT-I) transgenic mice were purchased from The Jackson Laboratory, and re-derived stocks were maintained at the Trudeau Institute under specific pathogen-free conditions under protocols approved by the Institutional Animal Care and Use Committee. IL-15 deficient mice (IL-15−/−) were purchased from Taconic. We used the attenuated strain of recombinant Listeria monocytogenes deleted for actA (LmOVA) for immunizations. Mice were infected intravenously or intraperitoneally with a sub-lethal dose of 1×107 to 5×107 colony forming units (CFU), and if indicated challenge infected with 5×107 CFU (Pearce et al., 2009). TN, TE and TM cell isolations from spleens and lymph nodes were done based on CD8+, CD44 and CD62L expression (CD44lo CD62Lhi, CD44hi CD62Llo and CD44hi CD62Lhi, respectively) or donor marker, at 6–7 days (TE) or 14–40 days (TM) after infection, using the BD Influx sorter or MACS purification (Miltenyi Biotech).
Flow cytometry
All fluorochrome-conjugated monoclonal antibodies were purchased from BD Pharmingen or eBioscience. All staining was performed as previously described (Pearce et al., 2009). OVA-specific CD8+ T cells were quantified by direct staining with H2-Kb OVA257–264 (KbOVA) MHC–peptide tetramers, either by killing animals and collecting cells from organs or by collecting blood from live animals (serial bleeds), as indicated. Mitochondrial membrane potential was measured using 3,3’-dihexyloxacarbocyanine iodide (DiOC6) (Invitrogen) and superoxide production using dihydroethidium (HE) (Sigma).
In vitro cultures and retroviral transductions
OT-I splenocytes were activated with OVA-peptide and IL-2 (100 U/ml) for 3 days, and subsequently cultured in the presence of either IL-2 or IL-15 (10 ng/ml) for 4 days. For restimulation assays, activated T cells were re-activated with either anti-CD3 (5.0 μg/ml) + anti-CD28 (0.5 μg /ml). For retroviral transduction experiments, OT-I splenocytes were activated, transduced with control (empty vector or virus expressing shRNA against luciferase), CPT1a expressing virus (CPT1a EX), or virus expressing shRNA against CPT1a (hpCPT1a) 1 day later, and subsequently cultured as described. GFP is a marker of retroviral expression. For in vitro survival assays, OT-I splenocytes were activated for 3 days as described, then plated (1×105 cells) in a 96-well plate in the presence of the indicated cytokine and (if indicated) 200 μM etomoxir, and daily analyzed for 7-AAD staining.
Adoptive transfers
For adoptive transfer experiments, naïve OT-I cells were obtained from the blood or spleen, KbOVA tetramers were used to determine numbers of OT-I cells, and then 1–3×104 OT-I cells, were transferred into CD45.1 or CD90.1 congenic recipient mice. For in vivo survival analysis of cultured IL-2 TE and IL-15 TM cells, 1×106 cells were transferred into congenic recipient mice. For adoptive transfer of retrovirally transduced OT-I cells for in vivo survival analysis, GFP+ cells were sorted and 1×106 cells were transferred into CD45.1 congenic recipient mice. For adoptive transfer of retrovirally transduced OT-I cells for TM development, 1×105 GFP+ cells were transferred into CD45.1 congenic or IL-15 deficient recipient mice.
Metabolism assays
Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured in XF media (non-buffered RPMI 1640 containing 25 mM glucose, 2 mM L-glutamine and 1 mM sodium pyruvate), under basal conditions and in response to 200 μM etomoxir, 1 μM oligomycin, 1.5 μM fluoro-carbonyl cyanide phenylhydrazone (FCCP) and 100 nM rotenone + 1 μM antimycin A (Sigma), or PMA (50 ng/ml) + ionomycin (500 ng/ml) using the XF-24 Extracellular Flux Analyzer (Seahorse Bioscience). NAD(H) measurements were performed using the NAD+ and NADH Quantification Kit (BioVision), and ATP measurements using the ATP determination kit (Invitrogen).
Imaging
IL-2 TE and IL-15 TM cells were stained for CD8 (BD bioscience), Mitotracker green and Hoechst (Invitrogen). TN, TE and TM cells were sorted by CD44, CD8, and CD62L after L. monocytogenes infection, and stained with Mitotracker green and Hoechst.
RT-PCR and western blot analysis
RNA isolations were done using the RNeasy kit (Qiagen) and single strand cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Genomic DNA was extracted using the phenol-chloroform method to determine mtDNA/nDNA ratios (Guo et al., 2009). Primers were purchased from Applied Biosystems and real-time PCR was performed by the Taqman method using an Applied Biosystems 7500 sequence detection system. The expression of mRNA for genes of interest was normalized to the expression of a housekeeping gene (GAPDH). Cell lysate preparation, SDS–PAGE, electrophoretic transfer, immunoblotting and development using enhanced chemiluminescence were accomplished as previously described (Pearce et al., 2009). The Complex I antibody (subunit NDUFB8) for western analysis was purchased from Mitosciences.
Statistical analysis
Comparisons for two groups were calculated using unpaired two-tailed Student’s t-tests, comparisons for more than two groups were calculated using 1-way ANOVA followed by Bonferroni’s multiple comparison tests. Comparisons over time were calculated using 2-way ANOVA followed by Bonferroni’s multiple comparison tests.
Supplementary Material
Highlights.
CD8+ TM cells possess substantial mitochondrial spare respiratory capacity (SRC)
IL-15 regulates SRC by promoting mitochondrial biogenesis and CPT1a expression
SRC in CD8+ TM cells is dependent on mitochondrial fatty acid oxidation (FAO)
Mitochondrial FAO enhances T cell survival and promotes CD8+ TM cell development
Acknowledgments
We thank Mike Tighe, Debbie Duso, Ron Lacourse, Brandon Sells, and Georgia Perona-Wright for technical assistance, and David Woodland and Jake Kohlmeier for critical reading of the manuscript. This work was supported in part by the Trudeau Institute, and grants from the NIH (E.L.P, E.J.P), NIAID Institutional Training Grant (E.A.), Netherlands Organisation for Scientific Research (G.J.W.W.) and the Emerald Foundation (E.L.P). There are no conflicts of interest. G.J.W.W., B.E., J.D.C., E.A., E.J.P., and E.L.P. designed the research and analyzed the data. G.J.W.W., B.E., C.H.C, J.D.C., T.C.F. and E.L.P. performed experiments. G.J.W.W., B.E., E.J.P., and E.L.P. contributed to the preparation of the manuscript. G.J.W.W. wrote the manuscript with E.L.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beeson CC, Beeson GC, Schnellmann RG. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem. 2010;404:75–81. doi: 10.1016/j.ab.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza AD, Parikh N, Kaech SM, Shadel GS. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion. 2007;7:374–385. doi: 10.1016/j.mito.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281:37372–37380. doi: 10.1074/jbc.M608372200. [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson JM, Laniewski NG, Lanier JG, Ahmed R. Mitochondrial potential and reactive oxygen intermediates in antigen-specific CD8+ T cells during viral infection. J Immunol. 2003;170:4745–4751. doi: 10.4049/jimmunol.170.9.4745. [DOI] [PubMed] [Google Scholar]
- Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- Greiner EF, Guppy M, Brand K. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. J Biol Chem. 1994;269:31484–31490. [PubMed] [Google Scholar]
- Guo W, Jiang L, Bhasin S, Khan SM, Swerdlow RH. DNA extraction procedures meaningfully influence qPCR–based mtDNA copy number determination. Mitochondrion. 2009;9:261–265. doi: 10.1016/j.mito.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Hofmann SR, Ettinger R, Zhou YJ, Gadina M, Lipsky P, Siegel R, Candotti F, O'Shea JJ. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr Opin Allergy Clin Immunol. 2002;2:495–506. doi: 10.1097/00130832-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Brand MD, Buttgereit F. Signaling takes a breath--new quantitative perspectives on bioenergetics and signal transduction. Immunity. 2001;15:497–502. doi: 10.1016/s1074-7613(01)00205-9. [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Circ Res. 1988;63:1036–1043. doi: 10.1161/01.res.63.6.1036. [DOI] [PubMed] [Google Scholar]
- McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech Ageing Dev. 2010;131:463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37:1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. 2010:46. doi: 10.3791/2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med. 2004;25:475–493. doi: 10.1016/j.mam.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Roos D, Loos JA. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes. II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp Cell Res. 1973;77:127–135. doi: 10.1016/0014-4827(73)90561-2. [DOI] [PubMed] [Google Scholar]
- Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T, Tagaya Y, Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998;16:205–226. doi: 10.3109/08830189809042995. [DOI] [PubMed] [Google Scholar]
- Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JS, Holbrook BC, Michalek RD, Laniewski NG, Grayson JM. Electron transport complex I is required for CD8+ T cell function. J Immunol. 2006;177:852–862. doi: 10.4049/jimmunol.177.2.852. [DOI] [PubMed] [Google Scholar]
- Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, Huang P, Sawyer SK, Fuerth B, Faubert B, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.