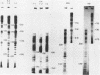
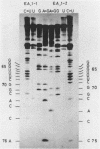
Abstract
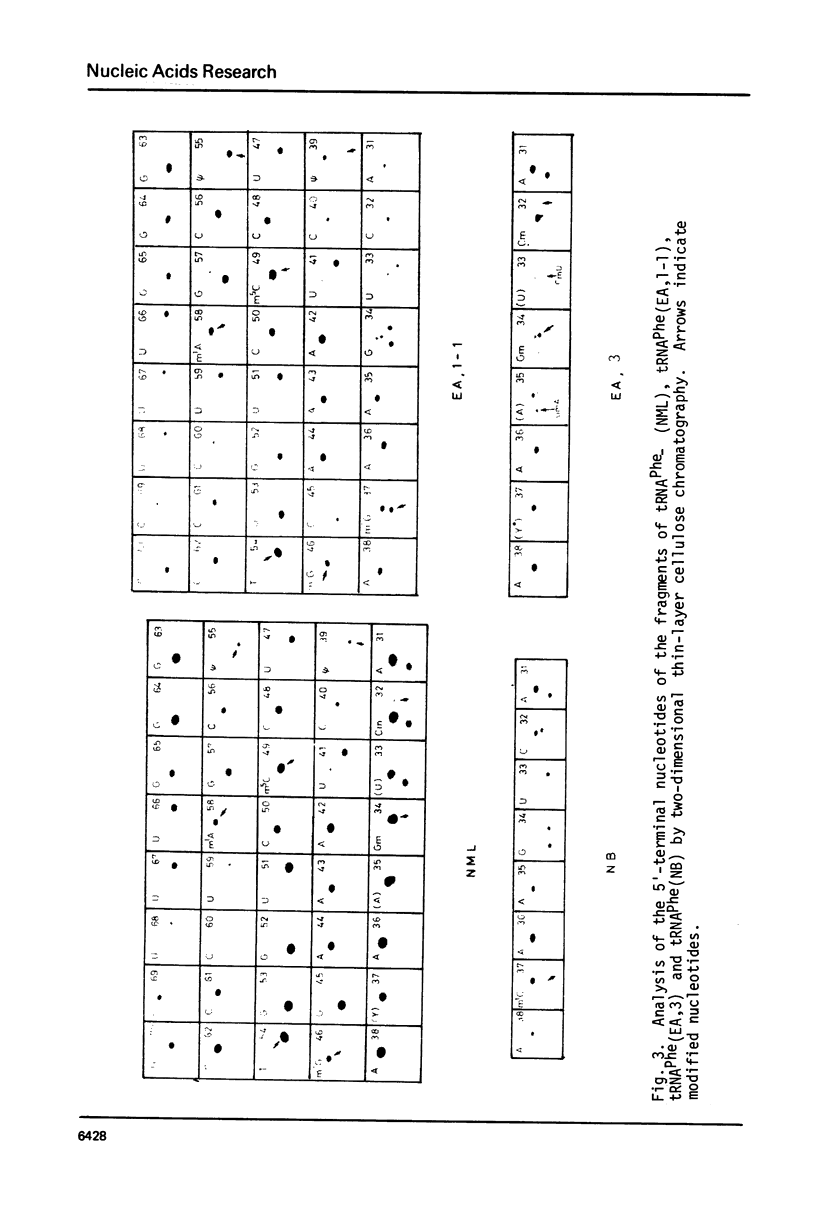
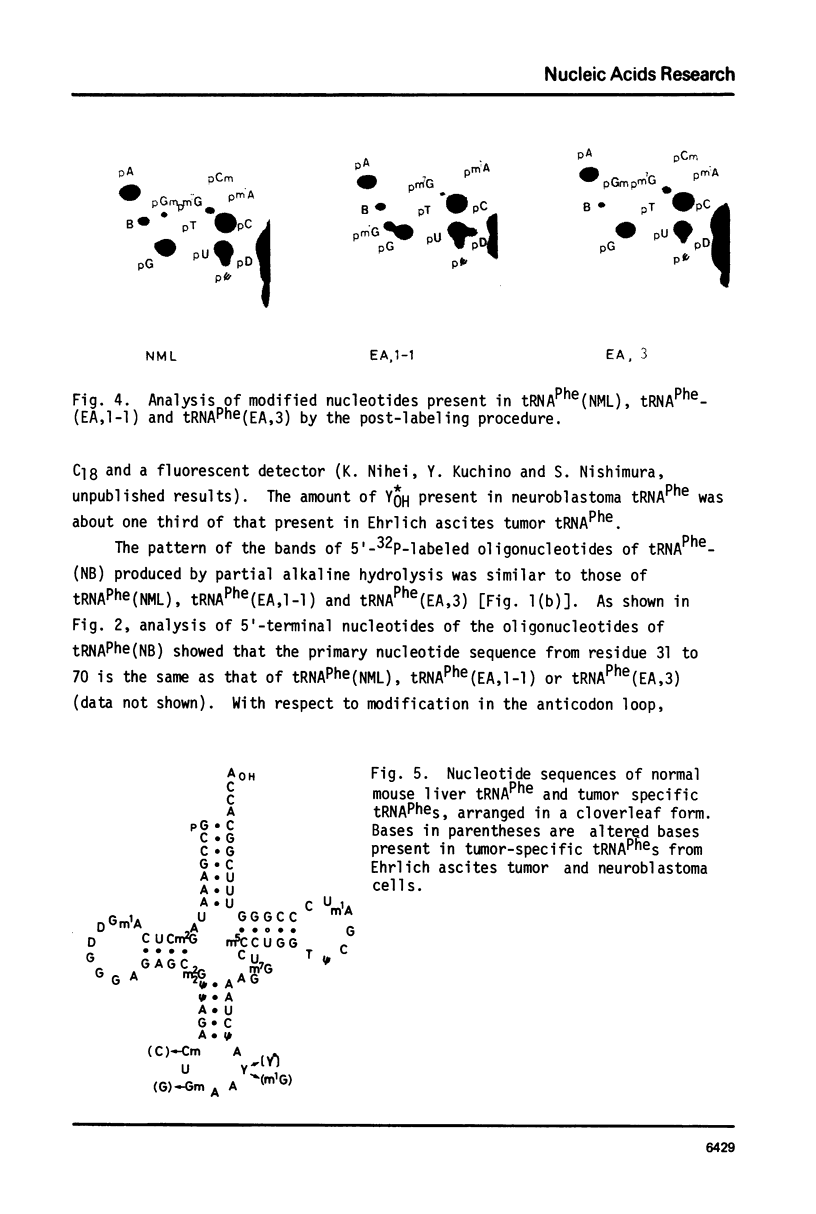
Nucleotide sequences of normal mouse liver tRNAPhe and tumor-specific tRNAPhes isolated from Ehrlich ascites tumor and neuroblastoma cells were examined by post-labeling techniques. The results showed that their sequences are identical, except for changes in post-transcriptional modifications that are located in the anticodon region. Normal mouse liver tRNAPhe contained Cm32, Gm34 and YOH37. On the other hand, tumor-specific tRNAPhes were found in one of two possible configurations: 1) Cm32, Gm34 and Y*OH37 (under-modified YOH) or 2) C32, G34 and m1G37. The ratio of the two forms of tRNAPhes differed in different tumor cells; Ehrlich ascites tumor tRNAPhe had mainly Y*OH-containing tRNAPhe whereas neuroblastoma tRNAPhe has predominantly m1G-containing tRNAPhe. It was concluded that tumor-specific tRNAPhes are products of different extents of modification, rather than of new tRNA transcription.
Full text
PDF




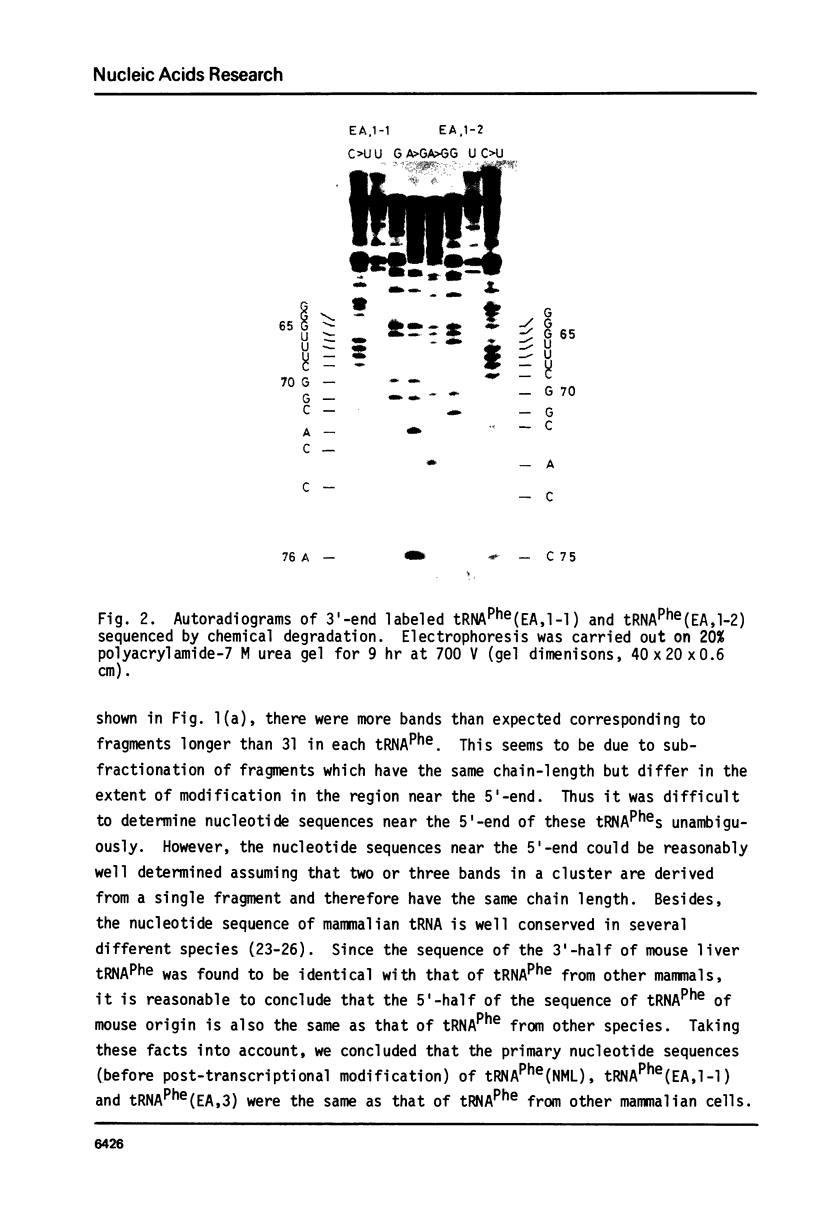




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobstein S. H., Grunberger D., Weinstein I. B., Nakanishi K. Isolation and structure determination of the fluorescent base from bovine liver phenylalanine transfer ribonucleic acid. Biochemistry. 1973 Jan 16;12(2):188–193. doi: 10.1021/bi00726a002. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B., Mushinski J. F. Deficiency of the Y base in a hepatoma phenylalanine tRNA. Nature. 1975 Jan 3;253(5486):66–67. doi: 10.1038/253066a0. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Rice M. J., Mushinski J. F. Comparison of the codon recognition properties and of the utilization of normal and tumor specific Phe-tRNAs in protein synthesis. Cancer Lett. 1981 Apr;12(3):251–258. doi: 10.1016/0304-3835(81)90076-8. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Kasai H., Yamaizumi Z., Kuchino Y., Nishimura S. Isolation of hydroxy-Y base from rat liver tRNAPhe. Nucleic Acids Res. 1979 Mar;6(3):993–999. doi: 10.1093/nar/6.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density to the degree of post-transcriptional modification of tRNALys and tRNAPhe. Biochim Biophys Acta. 1975 Nov 4;407(4):392–398. doi: 10.1016/0005-2787(75)90291-9. [DOI] [PubMed] [Google Scholar]
- Keith G., Dirheimer G. The primary structure of rabbit, calf and bovine liver tRNAPhe. Biochim Biophys Acta. 1978 Jan 26;517(1):133–149. doi: 10.1016/0005-2787(78)90041-2. [DOI] [PubMed] [Google Scholar]
- Keith G., Ebel J. P., Dirheimer G. The primary structure of two mammalian tRNAs Phe: identity of calf liver and rabbit liver tRNAs Phe. FEBS Lett. 1974 Nov 1;48(1):50–52. doi: 10.1016/0014-5793(74)81059-8. [DOI] [PubMed] [Google Scholar]
- Keith G., Picaud F., Weissenbach J., Ebel J. P., Petrissant G., Dirheimer G. The primary structure of rabbit liver tRNA Phe and its comparison with known tRNA Phe sequences. FEBS Lett. 1973 May 1;31(3):345–347. doi: 10.1016/0014-5793(73)80138-3. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Borek E. Tumour-specific phenylalanine tRNA contains two supernumerary methylated bases. Nature. 1978 Jan 12;271(5641):126–129. doi: 10.1038/271126a0. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Kasai H., Yamaizumi Z., Nishimura S., Borek E. Under-modified Y base in a tRHAPhe isoacceptor observed in tumor cells. Biochim Biophys Acta. 1979 Nov 22;565(1):215–218. doi: 10.1016/0005-2787(79)90098-4. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Kato M., Sugisaki H., Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979 Aug 10;6(11):3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Shindo-Okada N., Ando N., Watanabe S., Nishimura S. Nucleotide sequences of two aspartic acid tRNAs from rat liver and rat ascites hepatoma. J Biol Chem. 1981 Sep 10;256(17):9059–9062. [PubMed] [Google Scholar]
- Kuchino Y., Watanabe S., Harada F., Nishimura S. Primary structure of AUA-specific isoleucine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1980 May 13;19(10):2085–2089. doi: 10.1021/bi00551a013. [DOI] [PubMed] [Google Scholar]
- Minna J., Nelson P., Peacock J., Glazer D., Nirenberg M. Genes for neuronal properties expressed in neuroblastoma x L cell hybrids. Proc Natl Acad Sci U S A. 1971 Jan;68(1):234–239. doi: 10.1073/pnas.68.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski J. F., Marini M. Multiple chromatographic peaks of phenylalanyl-tRNA associated with spontaneous hydrolysis of Y base during isolation. Biochim Biophys Acta. 1977 Jun 17;476(4):345–351. doi: 10.1016/0005-2787(77)90299-4. [DOI] [PubMed] [Google Scholar]
- Mushinski J. F., Marini M. Tumor-associated phenylalanyl transfer RNA found in a wide spectrum of rat and mouse tumors but absent in normal adult, fetal, and regenerating tissues. Cancer Res. 1979 Apr;39(4):1253–1258. [PubMed] [Google Scholar]
- Nishimura S., Weinstein I. B. Fractionation of rat liver transfer ribonucleic acid. Isolation of tyrosine, valine, serine, and phenylalanine transfer ribonucleic acids and their coding properties. Biochemistry. 1969 Mar;8(3):832–842. doi: 10.1021/bi00831a011. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi R. G., Engelhardt D. L., Grunberger D. Formation of phenylalanine transfer RNA lacking the wye base in Vero cells during methionine starvation. J Biol Chem. 1978 Sep 25;253(18):6341–6343. [PubMed] [Google Scholar]
- Pergolizzi R. G., Engelhardt D. L., Grunberger D. Incorporation of lysine into Y base of phenylalanine tRNA in Vero cells. Nucleic Acids Res. 1979;6(6):2209–2216. doi: 10.1093/nar/6.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi R. G., Grunberger D. The effect of exogenous nutrients on the biosynthesis of Y base in tRNAPHe from Ehrlich ascites carcinoma. Cancer Lett. 1980 Feb;8(4):329–333. doi: 10.1016/0304-3835(80)90149-4. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Anandaraj M. P., Chia L. S., Randerath E., Gupta R. C., Randerath K. Sequence studies on tRNAPhe from placenta: comparison with known sequences of tRNAPhe from other normal mammalian tissues. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1097–1105. doi: 10.1016/0006-291x(75)90470-2. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Rizi H. L., Weisz C., DiLauro M. N., Pike D., Chen C. Y., Chen E. Y. Comparison of rat liver and Walker 256 carcinosarcoma tRNAs. Nucleic Acids Res. 1979 Feb;6(2):673–688. doi: 10.1093/nar/6.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Giveon D., Kimhi Y., Littauer U. Z. Abundance of tRNAPhe lacking the peroxy Y-base in mouse neuroblastoma. Biochemistry. 1976 Nov 30;15(24):5258–5262. doi: 10.1021/bi00669a010. [DOI] [PubMed] [Google Scholar]
- Shindo-Okada N., Kuchino Y., Harada F., Okada N., Nishimura S. Biological and structural differences between tRNAVal species isolated from rat ascites hepatoma cells and normal rat liver. J Biochem. 1981 Aug;90(2):535–544. doi: 10.1093/oxfordjournals.jbchem.a133502. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]