Abstract
Objective
To examine the trajectories of mood, weight and physical activity, and associations between mood, weight, and gender, among 213 obese individuals.
Methods
Prospective, longitudinal design. Assessments at baseline and 6, 12, and 18 months of Profile of Mood States, Paffenbarger Physical Activity Questionnaire, and weight.
Results
Total mood disturbance decreased from baseline to 6 months, with no change thereafter. Weight decreased from baseline to 6 to 12 months, and increased from 12 to 18 months. Physical activity increased from baseline to 6 months, and 12 to 18 months. Increased physical activity predicted greater vigor and less fatigue over time. Females high in distress at 6 months lost less weight than females low in distress and at 18 months gained more weight than those low in distress. There were no such associations among males.
Conclusion
The trajectories of mood, weight and physical activity were synchronous only in the short-term. Distress monitoring, targeted to females who relapse, may be warranted.
Keywords: mood, distress, vigor, weight-loss, gender, physical activity
Introduction
Much attention has been paid to the relationships among mood, eating behavior, and weight ( 1 ). Empirical investigations designed to address this topic have employed disparate methodologies and designs: cross-sectional ( 2, 3 ), longitudinal ( 1, 4 ), and experimental, whereby mood states are manipulated and resultant food consumption is examined ( 5, 6, 7 ), or food consumption is manipulated and resultant mood states are examined ( 8 ). Findings are complex, indicating non-linear and bi-directional relationships. For example, in laboratory-based studies, negative mood states have been associated with both decreased ( 9 ) and increased food consumption ( 10 ). The latter is referred to as the stress-eating paradox, given the typical pattern of appetite suppression and decreased food intake associated with autonomic activation consequent to stress.
In epidemiologic investigations, examination of the relationship between mood and weight has tended to focus specifically on the relationship between mood disorders and obesity. Some studies report a positive relationship between mood disorders and overweight or obesity ( 2, 11, 12, 13, 14, 15 ), whereas others report an inverse relationship ( 16, 17 ), or no relationship ( 18 ). For still others, a positive relationship occurs only among females ( 3, 19, 20 ), and an inverse relationship occurs among males ( 19, 21 ).
It would be remiss to mention such gender differences without considering restrained eating, defined as “the volitional effort to restrict food intake to control body weight” ( 22, p. 16 ). Females are more likely to engage in this behavior than are males ( 23, 24, 25 ). They are also more likely to engage in emotional eating, increased consumption in response to negative emotional experience ( 23, 26 ). The association between negative affect and food intake differs for restrained and unrestrained eaters. Distress increases food consumption among restrained eaters but not unrestrained eaters. This association has been demonstrated in both experimental ( 5, 27 ) and naturalistic settings ( 28 ). The relationship between restrained eating and weight control, moreover, differs by gender, such that restraint is detrimental for females but beneficial for males ( 24 ).
To summarize, results from a large number of investigations, while variant in methodology, suggest important relationships between mood, eating behavior and weight. However, findings are inconsistent and, in some cases, differ by gender. In addition, most studies involving obese populations have focused on mood disorders (e.g., clinical levels of depression or anxiety). Furthermore, few studies have afforded examination of associations between mood and weight within the context of formalized weight-loss programs. An early review indicated deleterious emotional consequences of weight-loss programs ( 29 ), whereas more recent studies indicated either no association( 30 ) or benefit, such as increased vitality ( 31, 32 ), decreased anxiety, decreased depression, and decreased fatigue ( 31 ). The present investigation represents an observational, longitudinal analysis of mood and weight among obese individuals enrolled in a long-term behavioral weight-loss program (i.e., individuals instructed to restrain their eating behavior and increase their energy expenditure through physical activity).
Physical activity, like ingestion, is known to affect mood. Results from a wide range of studies, including randomized controlled trials, suggest that exercise improves mood, even after just single bouts of activity ( 33, 34, 35 ). Conversely, exercise withdrawal is associated with mood decrements and fatigue ( 36 ). In their theoretical model, Baker and Brownell ( 37 ) posit relationships between physical activity, psychological mechanisms, and weight control. Exercise is associated with mood improvement, which in turn is associated with increased motivation and commitment. Such commitment leads to greater dietary compliance and exercise adherence, which in turn results in greater weight-control success. Conversely, limited physical activity and mood decrements result in weight-control failure.
The aims of the present investigation were three-fold. First, we sought to examine the trajectories of mood, weight and physical activity over time, as individuals attempted to lose weight. Synchronous trajectories were expected (e.g., mood improvement concurrent with weight-loss and increased physical activity). Second, we sought to examine the relationship between distress and weight-change as a function of gender. In line with past research ( 3, 19, 20 ), the relationship between distress and weight-change was expected to be stronger for females as compared to males. To note, distress is a “nonspecific term that encompasses sadness, frustration, anxiety, and a number of other negative mood states” ( 38, p. 5 ). Third, we sought to examine the predictive power of physical activity on mood. Increased physical activity was expected to predict better mood over time. Importantly, we chose to focus on mood states as opposed to clinical levels of depression or anxiety per se, as done in prior research.
Methods
Design and Participants
Participants were recruited from the Minneapolis community in two waves to the Lose It Forever (LIFE) study - a randomized controlled trial designed to test the efficacy of different combinations of behavioral (diet and exercise) prescriptions for weight-loss and then weight-maintenance. In the trial, participants were randomly assigned to receive either standard behavioral therapy or a maintenance-tailored treatment emphasizing variety and adaptation to change. Repeated assessments occurred at baseline, 6 months, 12 months, and 18 months. We focus here on the observational, prospective, longitudinal aspect of the study, collapsing across treatment condition. Primary results of the trial, with weight-loss and weight-maintenance as outcomes, are reported elsewhere ( 39 ) and beyond the scope of the present manuscript.
Recruitment methods included mass media advertising and, to attract men and minorities, specialized advertising targeted to those populations. Eligible participants were at least 18 years old, with a BMI of 30–39. Exclusion criteria were: current use of weight-loss medications or participation in another organized weight loss program; history or presence of cancer, cardiovascular disease, diabetes, chronic fatigue, arthritis, fibromyalgia, or stroke; inability to walk at least 10 minutes without stopping; current pharmacologic or behavioral treatment for a major psychological disorder; and current use of a synthroid drug. In addition, females were excluded if they were pregnant, < 6 months postpartum, breastfeeding, or planning to become pregnant in the ensuing 30 months.
Procedures
Study procedures were IRB-approved, and informed consent was obtained from all participants. Clinic-measured assessments included weight, height and waist/hip ratio. Weight and height were measured in light clothing, without shoes, using a wall-mounted stadiometer in centimeters with a horizontal measuring block, and a Tanita BWB 800 digital scale, read in kilograms (Tanita corp., Arlington Heights, IL). Participants also completed a battery of pencil-and-paper measures, including demographic items, the Profile of Mood States (POMS), and the Paffenbarger Activity Questionnaire.
The POMS is a measure of affect or mood ( 40 ). Respondents rate the extent to which they experienced feelings such as sad “during the past week”. Ratings are made on a 5-point (0 = not at all; 4 = extremely) scale. Six subscale scores are calculated, assessing distinct mood states: tension-anxiety (9 items such as tense, panicky, restless), depression-dejection (15 items such as unhappy, sorry, hopeless, lonely), anger-hostility (12 items such as spiteful, annoyed, furious), fatigue-inertia (7 items such as listless, sluggish, weary, exhausted), confusion-bewilderment (7 items such as unable to concentrate, muddled, forgetful) and vigor-activity (8 items such as lively, active, full of pep, cheerful, carefree). A Total Mood Disturbance (TMD) score is also calculated, used as an indicator of overall distress. The POMS manual suggests summary scoring for each subscale. However, given the variant number of items per subscale, and therefore different theoretical ranges per subscale, mean scores are reported here, for ease of interpretation and comparison across subscales (theoretical range = 0–4, with higher values indicative of greater negativity in all cases but vigor). Mean TMD scores are also reported, calculated by averaging items for all subscales after reverse-scoring the vigor items (theoretical range = 0–4, with higher numbers indicative of greater distress). The developers ( 40 ) report strong internal consistencies (Kuder-Richardson Formula 20 values ranging from 0.84 to 0.95) and good validity as evidenced by appropriate correlations with similar constructs of depression as measured by the Beck Depression Inventory ( 41 ), and appropriate changes in mood following a mood-induction paradigm. In the present sample, internal consistency (Cronbach alpha) values at the baseline time point were as follows: 0.87 for tension-anxiety, 0.92 for depression-dejection, 0.91 for anger-hostility, 0.89 for vigor-activity, 0.91 for fatigue-inertia, 0.76 for confusion-bewilderment, and 0.96 for TMD.
The Paffenbarger Activity Questionnaire is a well-known measure of physical activity ( 42 ). In this questionnaire, respondents are asked to report the number of stairs climbed, blocks walked, and the type and duration of all leisure-time physical activities during the past week. Responses are scored to provide an estimate of kilocalories expended per week in overall leisure time activity, and in activities of light (5 kcal/min), mixed (7.5 kcal/min), and high (10 kcal/min) intensity. We focus here on the former, average weekly caloric expenditure. Test-retest reliability of the scale was demonstrated in several studies spanning disparate samples ( 43, 44, 45 ). Validity was demonstrated by appropriate relationships with BMI ( 46 ), body fat ( 43 ), maximum oxygen consumption ( 43, 44 ), and self-reported physical activity and sweating ( 46 ).
Statistical Analyses
Descriptive and inferential analyses were performed using SPSS 13.0 and SAS 8.1. A series of linear mixed model regression analyses were conducted to examine the effects of time on several dependent variables: POMS TMD, each of the POMS subscales, weight, and weekly average energy expenditure in kilocalories. Mixed models adjust for the correlation of repeated measures over time and allow for missing data. In all models, time was treated as a fixed effect, with baseline serving as the referent. Study wave, gender and treatment condition were also included in the models as fixed effects, as was a time x treatment condition interaction for the POMS models.
Additional analyses afforded examination of physical activity (energy expenditure) as a predictor of mood over time (POMS TMD and each of the subscales). Two variables were created to indicate both between- and within-person variability in expenditure. Between-person variability can be thought of as simple mean differences in energy expenditure between participants. Within-person variability can be thought of as the time-specific deviation from an individual’s own mean energy expenditure. These two variables were included in a mixed linear regression model as time-varying covariates, along with wave, gender, and treatment condition.
For all of the models detailed above, the covariance matrix was unstructured. This is a conservative approach because it does not assume that correlation within a subject (in this case, a subject’s mood, weight or energy expenditure) is fixed and constant over time. Three females who became pregnant during the course of the study were excluded from analyses.
Finally, linear regression analysis was used to examine the relationship between mood, weight-change, and gender. Three regressions were run for each of three weight- change dependent variables: baseline to 6-month weight-change, 6-month to 12-month weight-change, and 12-month to 18-month weight-change. This was done separately for males and females. The change variable was calculated by subtracting weight at a given time point from weight at the subsequent time point, with positive values indicating gain and negative values indicating loss. For each analysis, POMS TMD scores were entered as a predictor of weight-change, treated as a dichotomy based on a median split (0 = low distress, 1 = high distress). For each regression, POMS TMD at the later of the two time points was used, e.g., 6 months for the analysis involving baseline to 6-month weight change.
Results
Table 1 displays baseline demographic and clinical characteristics of a) the total sample (n = 213) and b) the “completer” subsample of those still participating at 18 months (n = 159). Looking at the enrolled/ total sample, participants were, on average, 49 years old, 53% female, and 32% non-Caucasian. By self-report, 77% were college-educated. Mean baseline weight and BMI values were 103 kg and 35 kg/m2, respectively. Over one-half of participants had previously participated in an organized weight-loss program. Descriptively, the “completer” subsample did not differ appreciably from the enrolled sample.
Table 1.
Baseline Demographic and Clinical Characteristics of the Total Sample and those Still Participating at 18 Months
| Total sample | Completers | |
|---|---|---|
| N | 213 | 159 |
| Age, M (SD) | 48.81 (10.53) | 50.21 (10.44) |
| Gender, n (%) | ||
| Male | 100 (46.9) | 79 (49.7) |
| Female | 113 (53.1) | 80 (50.3) |
| Ethnicity, n (%) | ||
| Hispanic | 6 (2.8) | 5 (3.1) |
| Not Hispanic | 207 (97.2) | 154 (96.9) |
| Race, n (%) | ||
| American Indian or Alaska Native | 1 (.5) | 0 (0.0) |
| Asian | 5 (2.3) | 4 (2.5) |
| African American | 50 (23.5) | 23 (14.5) |
| Native Hawaiian or Pacific Islander | 1 (0.5) | 1 (0.6) |
| Caucasian | 143 (67.1) | 122 (76.7) |
| More than one race | 7 (3.3) | 4 (2.5) |
| Other | 4 (1.9) | 3 (1.9) |
| Unknown | 2 (0.9) | 2 (1.3) |
| Education, n (%) | ||
| ≤ High school degree | 7 (3.3) | 3 (1.9) |
| Some college or vocational school | 41 (19.2) | 31 (19.5) |
| College or technical degree | 77 (36.2) | 58 (36.5) |
| Some graduate school | 19 (8.9) | 16 (10.1) |
| Baccalaureate degree | 68 (31.9) | 50 (31.4) |
| Unknown | 1 (0.5) | 1 (0.6) |
| Marital status, n (%) | ||
| Married or cohabiting | 140 (65.7) | 111 (69.8) |
| Separated or divorced | 34 (16.0) | 20 (12.6) |
| Widowed | 4 (1.9) | 3 (1.9) |
| Never married | 34 (16.0) | 24 (15.1) |
| Unknown | 1 (0.5) | 1 (0.6) |
| Current full- or part-time employment, n (%) | 190 (89.2) | 139 (87.4) |
| Baseline weight in kg, M (SD) | 103.19 (14.19) | 103.00 (14.49) |
| Body mass index, M (SD) | 34.9 (2.8) | 34.76 (2.78) |
| Systolic blood pressure, M (SD) | 119.41 (15.56) | 120.11 (16.21) |
| Diastolic blood pressure, M (SD) | 71.53 (8.90) | 71.40 (8.87) |
| Waist/ hip ratio, M (SD) | 0.92 (.09) | 0.92 (.09) |
| Smoked ≥ 100 cigarettes in lifetime, n (%) | 82 (38.5) | 63 (39.9) |
| Current smoker, n/ denominator (%) | 12/ 82 (14.6) | 8/ 63 (12.7) |
| Past participation in weight-loss program, n (%) | 119 (55.9) | 92 (57.9) |
Trajectories of Mood, Weight, and Physical Activity
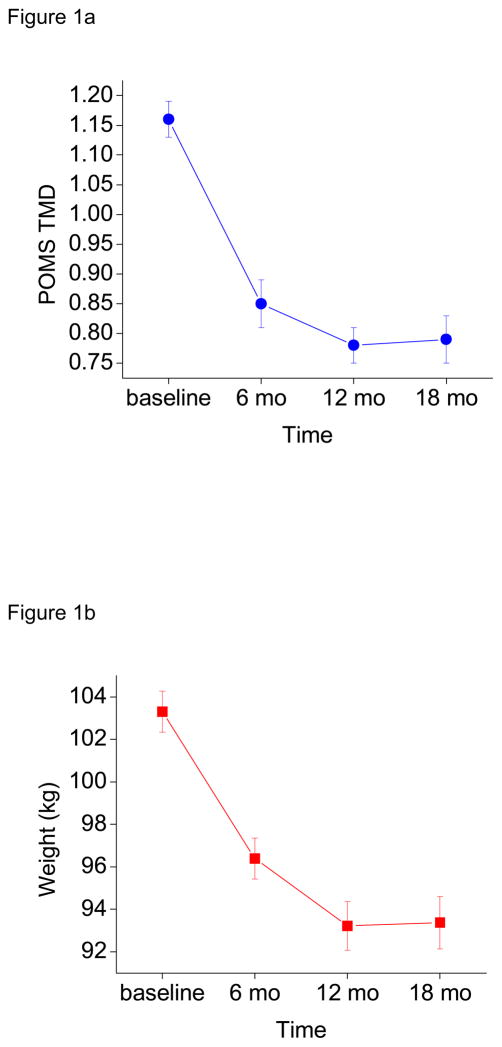
Means and standard errors for TMD, weight, and energy expenditure (as a function of time) are displayed in Figures 1a–c, respectively. As seen in the top panel of Figure 1, POMS TMD significantly decreased from baseline to 6 months (beta = −0.31, p < 0.0001), with no further significant change from 6 to 12 months (beta = −0.05, p = 0.121) or 12 to 18 months (beta = 0.01, p = 0.824). As seen in the middle panel of Figure 1, weight significantly decreased from baseline to 6 months (beta = −6.69 kg, p < 0.0001), and 6 to 12 months (beta = −2.93 kg, p < 0.0001), but significantly increased from 12 to 18 months (beta = 0.64 kg, p = 0.005). Finally, as seen in the bottom panel of Figure 1, weekly average energy expenditure significantly increased from baseline to 6 months (beta = 842.77 kcal, p < 0.0001) and 12 to 18 months (beta = 197.45, p = 0.045). Energy expenditure did not significantly change from 6 to 12 months (beta = −165.53, p = 0.153).
Figure 1.
a–c Profile of Mood States Total Mood Disturbance (POMS TMD, top panel), Weight (middle panel), and Weekly Average Caloric Expenditure (bottom panel) as a Function of Time, M (SE). Values reflect all available data per time point (pairwise deletion).
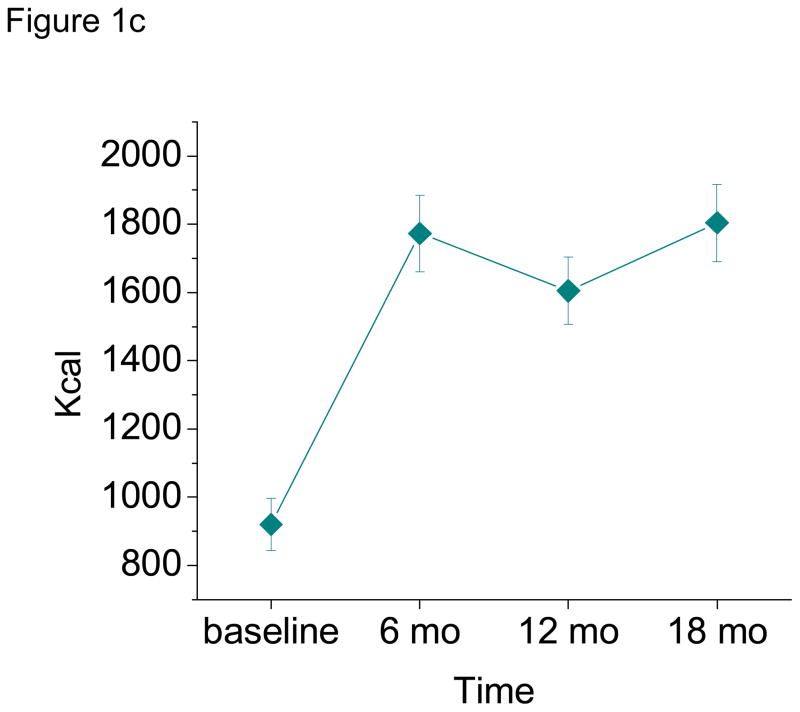
Means and standard errors for each of the POMS subscales are displayed in Figure 2. The patterns of change over time were similar for tension-anxiety, anger-hostility, fatigue-inertia and confusion-bewilderment. Negative affect significantly decreased from baseline to 6 months (beta = −0.10, p = 0.023 for tension-anxiety; beta = −0.12, p = 0.007 for anger-hostility; beta = −0.24, p = 0.0009 for fatigue-inertia; and beta = −0.14, p = 0.001 for confusion-bewilderment), with no significant change thereafter, either from 6 to 12 months or 12 to 18 months (all p values > 0.05). Vigor-activity significantly increased from baseline to 6 months (beta = 0.36, p < 0.0001), with stabilization thereafter (p values > 0.05). The trajectory for depression-dejection was distinct, showing no significant change for any time interval (all p values > 0.05). None of the aforementioned models yielded main effects of gender, wave or treatment condition (p values > 0.05), with one exception. Participants receiving the treatment emphasizing variety and adaptation to change reported greater tension-anxiety over time as compared to those receiving standard behavioral therapy (beta = 0.19, p = 0.025). The time x treatment condition interaction was not significant for any of the POMS models, p > 0.05.
Figure 2.
Profile of Mood States (POMS) Subscale Scores as a Function of Time, M (SE). Values reflect all available data per time point (pairwise deletion).
Physical Activity as a Predictor of Mood
Additional analyses afforded examination of energy expenditure as a predictor of mood over time. Increased energy expenditure was associated with greater vigor (beta = 0.01, p = 0.0006) and less fatigue (beta = −0.01, p = 0.033) over time. In other words, for every one unit increase in energy expenditure (one kilocalorie expended per week), there was an increase in vigor and a decrease in fatigue. Energy expenditure did not predict depression-dejection, tension-anxiety, anger-hostility, confusion-bewilderment, or TMD over time (p values > 0.05).
Mood, Weight-change and Gender
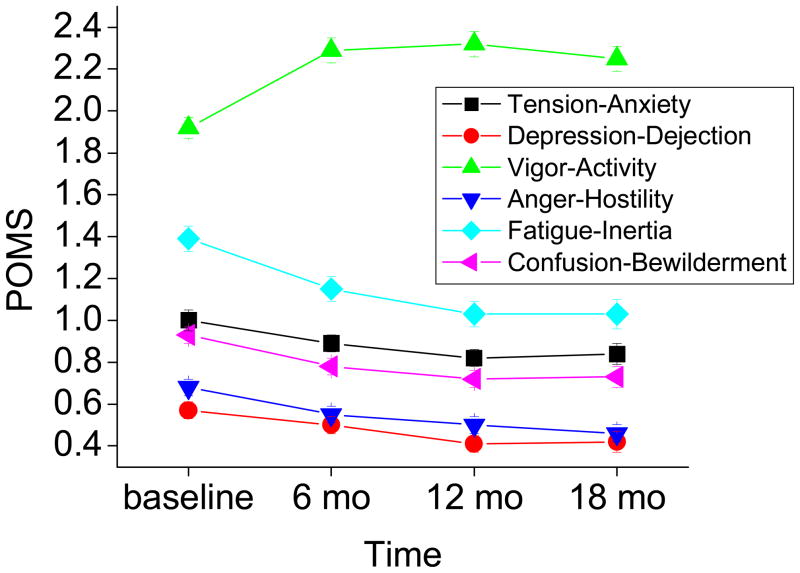
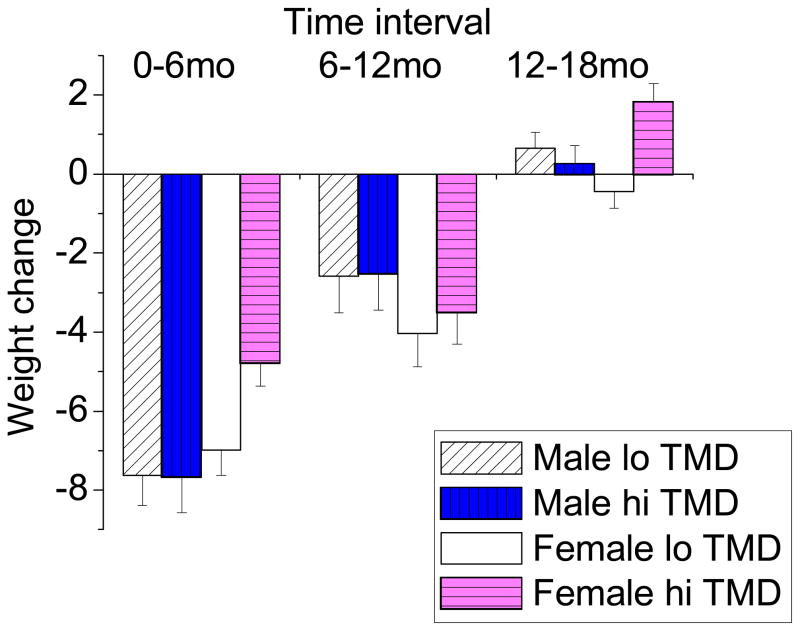
Results for the linear regressions conducted on baseline to 6-month, 6-month to 12-month, and 12-month to 18-month weight-change (separately for males and females) were as follows. POMS TMD did not predict weight-change at any time interval for males (beta = −0.04, p = 0.971 for baseline to 6-month weight-change; beta = 0.06, p = 0.961 for 6-month to 12-month weight-change; and beta = −0.39, p = 0.524 for 12-month to 18-month weight-change). A different result emerged for females. Greater distress at 6 months predicted greater baseline to 6-month weight gain (beta = 2.20, p = 0.012). Similarly, greater distress at 18 months predicted greater 12-month to 18-month weight gain (beta = 2.27, p = 0.001). Distress at 12 months did not predict 6-month to 12-month weight-change (beta = 0.53, p = 0.652). See Figure 3 for a graphical presentation of these data. As illustrated in the figure, weight-change did not differ as a function of concurrent distress among males, but did so among females. Females high in distress at six months lost less initial (baseline to 6 months) weight as compared to females low in distress at six months (negative values in the figure indicate weight loss). Similarly, females high in distress at 18 months gained more weight between 12 and 18 months as compared to females low in distress at 18 months (positive values in the figure indicate weight gain).
Figure 3.
Weight-change as a Function of Gender and Profile of Mood States Total Mood Disturbance (TMD), M (SE). Values negative in sign indicate weight-loss; values positive in sign indicate gain.
Discussion
The first aim of this study was simply to examine the trajectories of mood, weight and physical activity over time. On average, weight decreased from baseline to 6 months, and from 6 to 12 months, but increased from 12 to 18 months. Physical activity increased from baseline to 6 months, and again from 12 to 18 months, with no change in the interceding period. Total mood disturbance, tension-anxiety, anger-hostility, fatigue-inertia, and confusion-bewilderment decreased from baseline to 6 months, with stabilization thereafter. Similarly, vigor-activity increased from baseline to 6 months, with stabilization thereafter. Depression-dejection did not change from one time point to the next. This may be explained by a floor effect. Looking at Figure 2, we see that values for depression-dejection are quite low, with limited room for improvement.
Weight-loss was associated with improved mood only during the early period of rapid change. Weight decreased from baseline to 6 months, as did TMD. However, a further reduction in negative affect was not seen as weight continued to drop, nor was an increase seen as weight was regained. Perhaps the weight-loss itself became less reinforcing over time, or the secondary benefits of weight-loss such as positive attention and praise diminished over time. This is in line with theoretical work on motivation ( 47 ). As goals are initially met (e.g., as weight is first lost), motivation strengthens. However, motivation weakens with repeated exposure to the goal.
The relationship between distress and exercise was inverse (as expected), but only in the short-term. Exercise increased from baseline to 6 months, just as TMD decreased. This is in line with past research on the emotional benefits of physical activity ( 33 ). Interestingly, we did not see a concomitant improvement in mood as energy expenditure increased from 12 to 18 months. Moreover, while energy expenditure was associated with greater vigor and less fatigue over time, it was not associated with overall distress, sadness, anxiety or anger. This finding is commensurate with experimental studies designed to examine the effects of exercise on mood utilizing the POMS ( 48 ). It could be that the effects of physical activity on mood are more specific to energy and activation as opposed to discrete emotional states such as sadness or anger. Given the non-experimental nature of our study, we do not know whether positive mood states fostered better dedication to an exercise program or if physical activity led to increased vitality. According to Baker and Brownell ( 37 ), exercise enhances mood, which in turn increases commitment and adherence.
Consistent with past research ( 3, 20 ), the relationship between mood and weight was stronger for females than for males. Females high in emotional distress at 6 months had lost less weight since the start of the study than females low in emotional distress at 6 months. Similarly, females high in emotional distress at 18 months had lost less weight since 12 months as compared to females low in emotional distress at 18 months. In fact, on average, females high in distress at 18 months had gained weight, whereas females low in distress had lost weight. As others have noted ( 19, 21 ), the emotional impact of being overweight may be stronger for females, in part because the social stigma of being overweight is greater ( 49 ). The social consequences, therefore, are more significant. It is possible that such pressures are exacerbated within the context of a formal weight-loss program, a situation in which participants are expected to “perform”. It makes sense, then, that females who had been less successful in terms of weight-loss would report higher levels of distress. Accordingly, distress monitoring, targeted to females who relapse, may be warranted. It may also be helpful to consider emotion-focused coping strategies. In an intriguing study of obesity-specific coping, greater helplessness and intrusion, and less fighting spirit were found among individuals who, at two-year follow-up after treatment for obesity, had gained versus lost weight ( 50 ).
It is important to consider who was not represented in the study, either by exclusion or drop-out over time. Persons with a chronic disease (e.g., diabetes) were excluded from participation. This may have resulted in a sample with lower baseline levels of distress. While the enrolled/ total sample did not differ appreciably from the subsample of participants who completed the study, there were fewer African Americans. This is fitting with research indicating greater obesity and less weight-loss success among African Americans ( 51, 52, 53 ).
There are multiple facets of emotion and the present investigation sought to examine just one, that of self-reported emotional experience. It would be interesting, in future research, to examine an observable indicator of emotion among weight- loss intervention participants, such as the emotional valence of words uttered during group-format treatment sessions. In addition, self-reported assessments in our study were few and far between. Diary studies, or the use of ecological momentary assessment methods, may prove exceedingly informative. An extended follow-up is also warranted.
Finally, future research should consider individual differences and the potential moderating role of such differences. Examples include baseline or concurrent differences in body image, real/ideal body weight synchrony or lack thereof, and stress reactivity. With respect to the latter, research indicates associations between high stress reactivity, increased cortisol, increased intake of calorically dense food, and weight, specifically, central adiposity ( 54, 55, 56 ). With respect to cognitions regarding weight, weight perceptions, not BMI or weight per se, have been associated with elevations in psychological distress (both overweight and underweight perceptions) ( 57 ). As we did not assess perceptions of weight in the present study, we cannot address the relationship of such to affective functioning. Weight-related cognitions, whether accurate or distorted, are likely to be affectively-laden, particularly among individuals sufficiently motivated to lose weight so as to enroll in an extensive weight-loss program.
Limitations aside, findings from this study extend the literatures on weight-loss and weight-maintenance by examining the trajectories of mood, weight, and physical activity among participants enrolled in a long-term behavioral weight-loss intervention. Trajectories were synchronous only in the short-term, offering implications for interventions designed to enhance mood within the context of formal weight-loss programs, not necessarily at the outset (as weight is lost and mood improves) but perhaps mid-way or later on in the process, as individuals begin to lapse. Females in particular should be monitored for distress, especially if their weight-loss efforts have been unsuccessful. Lastly, exercise is recommended, not just for weight-loss but also for psychosocial functioning, specifically, increased vigor and decreased fatigue.
Acknowledgments
Supported by grant R01 DK064596 from the National Institute of Diabetes and Digestive and Kidney Diseases, awarded to Dr. Jeffery.
Special thanks to all of the participants and Emily Finch.
References
- 1.Blaine B. Does depression cause obesity? A meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008;13:1190–7. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- 2.Bruffaerts R, Demyttenaere K, Vilagut G, Martinez M, Bonnewyn A, De Graaf R, et al. The relation between body mass index, mental health, and functional disability: A European population perspective. Can J Psychiatry. 2008;53:679–88. doi: 10.1177/070674370805301007. [DOI] [PubMed] [Google Scholar]
- 3.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 4.Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, et al. Obesity and depression: Results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes. 2006;30:520–7. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- 5.Baucom DH, Aiken PA. Effect of depressed mood in eating among obese and nonobese dieting and nondieting persons. J Pers Soc Psychol. 1981;41:577–85. doi: 10.1037//0022-3514.41.3.577. [DOI] [PubMed] [Google Scholar]
- 6.Chua JL, Touyz S, Hill AJ. Negative mood-induced overeating in obese binge eaters: An experimental study. Int J Obes Relat Metab Disord. 2004;28:606–10. doi: 10.1038/sj.ijo.0802595. [DOI] [PubMed] [Google Scholar]
- 7.Heatherton TF, Herman CP, Polivy J. Effects of physical threat and ego threat on eating behavior. J Pers Soc Psychol. 1991;60:138–43. doi: 10.1037//0022-3514.60.1.138. [DOI] [PubMed] [Google Scholar]
- 8.Macht M, Dettmer D. Everyday mood and emotions after eating a chocolate bar or an apple. Appetite. 2006;46:332–6. doi: 10.1016/j.appet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Stone A, Brownell K. The stress-eating paradox: Multiple daily measurements in adult males and females. Psychol Health. 1994;9:425–36. [Google Scholar]
- 10.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 11.Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;109:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- 12.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–97. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 13.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: Evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27:514–21. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000;152:163–70. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- 15.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisp AH, McGuiness B. Jolly fat: Relation between obesity and psychoneurosis in general population. Br Med J. 1976;1:7–9. doi: 10.1136/bmj.1.6000.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart AL, Brook RH. Effects of being overweight. Am J Public Health. 1983;73:171–8. doi: 10.2105/ajph.73.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallstrom T, Noppa H. Obesity in women in relation to mental illness, social factors and personality traits. J Psychosom Res. 1981;25:75–82. doi: 10.1016/0022-3999(81)90093-3. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: Results from a general population study. Am J Public Health. 2000;90:251–7. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992;16:999–1003. [PubMed] [Google Scholar]
- 21.Palinkas LA, Wingard DL, Barrett-Connor E. Depressive symptoms in overweight and obese older adults: A test of the “jolly fat” hypothesis. J Psychosom Res. 1996;40:59–66. doi: 10.1016/0022-3999(95)00542-0. [DOI] [PubMed] [Google Scholar]
- 22.Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46:16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Burton P, Smit HJ, Lightowler HJ. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–7. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Drapeau V, Provencher V, Lemieux S, Despres JP, Bouchard C, Tremblay A. Do 6-y changes in eating behaviors predict changes in body weight? Results from the Quebec Family Study. Int J Obes Relat Metab Disord. 2003;27:808–14. doi: 10.1038/sj.ijo.0802303. [DOI] [PubMed] [Google Scholar]
- 25.Neumark-Sztainer D, Sherwood NE, French SA, Jeffery RW. Weight control behaviors among adult men and women: Cause for concern? Obes Res. 1999;7:179–88. doi: 10.1002/j.1550-8528.1999.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 26.Larsen JK, van Strien T, Eisinga R, Engels RC. Gender differences in the association between alexithymia and emotional eating in obese individuals. J Psychosom Res. 2006;60:237–43. doi: 10.1016/j.jpsychores.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Wallis DJ, Heatherington MM. Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004;43:39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosom Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 29.Stunkard AJ, Rush J. Dieting and depression reexamined. A critical review of reports of untoward responses during weight reduction for obesity. Ann Intern Med. 1974;81:526–33. doi: 10.7326/0003-4819-81-4-526. [DOI] [PubMed] [Google Scholar]
- 30.Vander Wal JS, McBurney MI, Cho S, Dhurandhar NV. Ready-to-eat cereal products as meal replacements for weight loss. Int J Food Sci Nutr. 2007;58:331–40. doi: 10.1080/09637480701240802. [DOI] [PubMed] [Google Scholar]
- 31.Annesi JJ, Unruh JL. Relations of exercise, self-appraisal, mood changes and weight loss in obese women: Testing propositions based on Baker and Brownell's (2000) model. Am J Med Sci. 2008;335:198–204. doi: 10.1097/MAJ.0b013e318152010c. [DOI] [PubMed] [Google Scholar]
- 32.Jensen GL, Roy MA, Buchanan AE, Berg MB. Weight loss intervention for obese older women: Improvements in performance and function. Obes Res. 2004;12:1814–20. doi: 10.1038/oby.2004.225. [DOI] [PubMed] [Google Scholar]
- 33.Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–93. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull. 2006;132:866–76. doi: 10.1037/0033-2909.132.6.866. [DOI] [PubMed] [Google Scholar]
- 35.Yeung RR. The acute effects of exercise on mood state. J Psychosom Res. 1996;40:123–41. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]
- 36.Kop WJ, Weinstein AA, Deuster PA, Whittaker KS, Tracy RP. Inflammatory markers and negative mood symptoms following exercise withdrawal. Brain Behav Immun. 2008;22:1190–6. doi: 10.1016/j.bbi.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Baker C, Brownell K. Physical activity and maintenance of weight loss: Physiological and psychological mechanisms. In: Bouchard C, editor. Physical Activity and Obesity. Champaign, IL: Human Kinetics; 2000. pp. 311–28. [Google Scholar]
- 38.Carney RM, Freedland KE. Psychological distress as a risk factor for stroke-related mortality. Stroke. 2002;33(1):5–6. [PubMed] [Google Scholar]
- 39.Levy RL, Langer SL, Welsh EM, Flood AP, Jaeb MA, Laqua PS, et al. Maintenance-tailored treatment improves long-term weight loss. Gastroenterology. 2008;134(Suppl 1):A-230-1. [Google Scholar]
- 40.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 41.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 42.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 43.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46:1403–11. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 45.LaPorte RE, Black-Sandler R, Cauley JA, Link M, Bayles C, Marks B. The assessment of physical activity in older women: Analysis of the interrelationship and reliability of activity monitoring, activity surveys, and caloric intake. J Gerontol. 1983;38:394–7. doi: 10.1093/geronj/38.4.394. [DOI] [PubMed] [Google Scholar]
- 46.Washburn RA, Smith KW, Goldfield SR, McKinlay JB. Reliability and physiologic correlates of the Harvard Alumni Activity Survey in a general population. J Clin Epidemiol. 1991;44:1319–26. doi: 10.1016/0895-4356(91)90093-o. [DOI] [PubMed] [Google Scholar]
- 47.McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychol Bull. 1999;125:437–57. [Google Scholar]
- 48.Bartholomew JB, Morrison D, Ciccolo JT. Effects of acute exercise on mood and well-being in patients with major depressive disorder. Med Sci Sports Exerc. 2005;37:2032–7. doi: 10.1249/01.mss.0000178101.78322.dd. [DOI] [PubMed] [Google Scholar]
- 49.Puhl RM, Andreyeva T, Brownell KD. Perceptions of weight discrimination: prevalence and comparison to race and gender discrimination in America. Int J Obes. 2008;32:992–1000. doi: 10.1038/ijo.2008.22. [DOI] [PubMed] [Google Scholar]
- 50.Ryden A, Karlsson J, Sullivan M, Torgerson JS, Taft C. Coping and distress: What happens after intervention? A 2-year follow-up from the Swedish Obese Subjects (SOS) study. Psychosom Med. 2003;65:435–42. doi: 10.1097/01.psy.0000041621.25388.1a. [DOI] [PubMed] [Google Scholar]
- 51.Akan GE, Grilo CM. Sociocultural influences on eating attitudes and behaviors, body image, and psychological functioning: A comparison of African-American, Asian-American, and Caucasian college women. Int J Eat Disord. 1995;18:181–7. doi: 10.1002/1098-108x(199509)18:2<181::aid-eat2260180211>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 52.Anderson WA, Greene GW, Forse RA, Apovian CM, Istfan NW. Weight loss and health outcomes in African Americans and whites after gastric bypass surgery. Obesity. 2007;15:1455–63. doi: 10.1038/oby.2007.174. [DOI] [PubMed] [Google Scholar]
- 53.Buffington CK, Marema RT. Ethnic differences in obesity and surgical weight loss between African-American and Caucasian females. Obes Surg. 2006;16:159–65. doi: 10.1381/096089206775565258. [DOI] [PubMed] [Google Scholar]
- 54.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Newman E, O'Connor DB, Conner M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology. 2007;32:125–32. doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–32. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Atlantis E, Ball K. Association between weight perception and psychological distress. Int J Obes. 2008;32:715–21. doi: 10.1038/sj.ijo.0803762. [DOI] [PubMed] [Google Scholar]