Abstract
Life-long hematopoiesis depends on the support of mesenchymal stromal cells within the bone marrow. Therefore, changes in the hematopoietic compartment that occur during development and aging probably correlate with variation in the composition of the stromal cell microenvironment. Mesenchymal stromal cells are a heterogeneous cell population and various subtypes may have different functions. In accordance with others, we show that CD271 and CD146 define distinct colony-forming-unit-fibroblast containing mesenchymal stromal cell subpopulations. In addition, analysis of 86 bone marrow samples revealed that the distribution of CD271brightCD146− and CD271brightCD146+ subsets correlates with donor age. The main subset in adults was CD271brightCD146−, whereas the CD271brightCD146+ population was dominant in pediatric and fetal bone marrow. A third subpopulation of CD271−CD146+ cells contained colony-forming-unit-fibroblasts in fetal samples only. These changes in composition of the mesenchymal stromal cell compartment during development and aging suggest a dynamic system, in which these subpopulations may have different functions.
Keywords: hematopoiesis, bone marrow microenvironment, mesenchymal stem cells
Introduction
Mesenchymal stromal cells (MSC) cultured from adult and fetal tissues constitute a heterogeneous cell population. Although a panel of markers, including CD105 (Endoglin) and CD90 (Thy-1), was introduced to define cultured MSC,1 the cells initiating the culture remain unidentified. Recently, the low-affinity nerve growth factor receptor CD2712–4 and melanoma cell adhesion molecule CD1465,6 were described for prospective isolation of MSC with colony forming unit-fibroblast capacity. The combination of these markers revealed that two different MSC subsets are present in adult BM: CD271+CD146−/lo and CD271+CD146+.7 These subsets had a similar capacity to differentiate and to support hematopoiesis, but in human BM they were found at different sites; CD271+/CD146−lo cells were bone-lining, while CD271+CD146+ had a perivascular localization.7 It is still not clear whether the contribution of these subsets to the MSC population changes during development and aging, two phenomena known to influence BM cellular composition.8–10 Here, we report that MSC subset distribution, characterized by CD271 and CD146, correlates with donor age. CD271+CD146− cells were most common in adults, whereas CD271+CD146+ cells are dominant in children. In addition, fetal CD271−CD146+ cells represent a third MSC subset containing CFU-F.
Design and Methods
Bone marrow was aspirated from 53 adults (mean age 65 years, range 19–86 years). Ten were healthy donors and 43 were patients undergoing cardiac surgery; 27 pediatric patients (mean age 5 years, range 0–16 years) had malignancies (solid n=17, hematologic n=10). Patients’ characteristics are listed in the Online Supplementary Table S1. Because we experienced a large variation in BM aspirate quality, we decided to analyze the relative distribution of MSC subsets. Fetal bones (n=6) were obtained from legally terminated pregnancies (15–20 weeks gestation). Informed consent was obtained for all samples according to the protocol approved by the Medical Research Ethics Committee of the AMC and the Erasmus MC.
Mononuclear cells (MNC) were isolated by Ficoll density gradient centrifugation (GE Health Care Bio-Sciences AB, Uppsala, Sweden) and erythrocyte lysis using NH4Cl. Fetal bones were flushed with IMDM (Lonza, Verviers, Belgium) containing 10% FCS (Bodinco, Alkmaar, The Netherlands), 1% penicillin-streptomycin and erythrocytes were lysed.
MNC were analyzed for expression of: CD34 (clone 8G12), CD73 (AD2), CD90 (5E10), CD45 (HI30), CD140b (PDGFRβ, 28D4), CD146 (P1H12), CXCR4 (12G5), (all from BD, San Jose CA), CD36 (CLB-IVC7, Sanquin, Amsterdam, The Netherlands), CD271 (ME20.4-1.H4), MSCA1 (W8B2) (Miltenyi Biotec, Gladbach, Germany), CXCR7 (358426), NG2 (LHM-2) (R&D systems, Abingdon, UK), CD56 (C5.9), CD235a (JC159) (Dako Cambridgeshire, UK), CD105 (SN6, Ancell, Bayport, MN, USA), isotype matched controls (Sanquin, BD, USA). At least 1,000,000 events were recorded using an LSR II flow cytometer (BD, USA). For six-color sorting (FACS Aria, BD, USA), MNC were stained for CD45, CD34, CD271, CD146, CD105, CD90.
For CFU-F assays, 500 sorted cells/cm2 were plated in M199 (Gibco, Paisley, UK) containing 10% FCS, 1% penicillin-streptomycin, 20 μg/mL Endothelial Cell Growth factor (Roche Diagnostics, Indianapolis, IN, USA) and 8IU/ml heparin (Leo Pharma, Breda, The Netherlands). For CFU-F with MNC, 20,000–80,000 adult or pediatric cells/cm2 or 2,500–20,000 fetal cells/cm2 were plated. For depletion experiments, 80,000 cells/cm2 were seeded. CFU-F were counted after 14 days.
RNA was isolated using Trizol (Invitrogen, Breda, The Netherlands). cDNA was synthesized using Sensiscript (Qiagen, Venlo, The Netherlands). Primer- and probe-sequences: Nestin (5′>3′) F-GCTGCGGGCTACTGAAAAGT, R-TCTGTAGGCC-CTGTTTCTCCTG, Probe AGCTGGCTGTGGAGGCCCTGG. ABL sequences were published previously.11
For MSC expansion, CFU-F were trypsinized and replated at 2,500 cells/cm2 in M199. The cells were passaged at 70–80% confluency.
Passage three MSC were used for adipogenic and osteogenic differentiation, as previously described,12 and imaged on a Leica DC300 microscope (IM500 software, 20X magnification, Leica, Wetzlar, Germany). Chondrocyte differentiation was performed using NH ChondroDiff medium (Miltenyi), detected by aggrecan-staining (MAB19310, Millipore, Amsterdam, The Netherlands) and imaged on an LSM 510 META confocal microscope (Zeiss, Jena, Germany; ZEN 2007 software, 10X magnification).
Statistical significance was determined by Mann-Whitney’s U test, Wilcoxon’s signed rank test or Spearman’s correlation (SPSS 15.0; SPSS Inc, Chicago, IL, USA). Results were considered significant at P≤0.05.
Results and Discussion
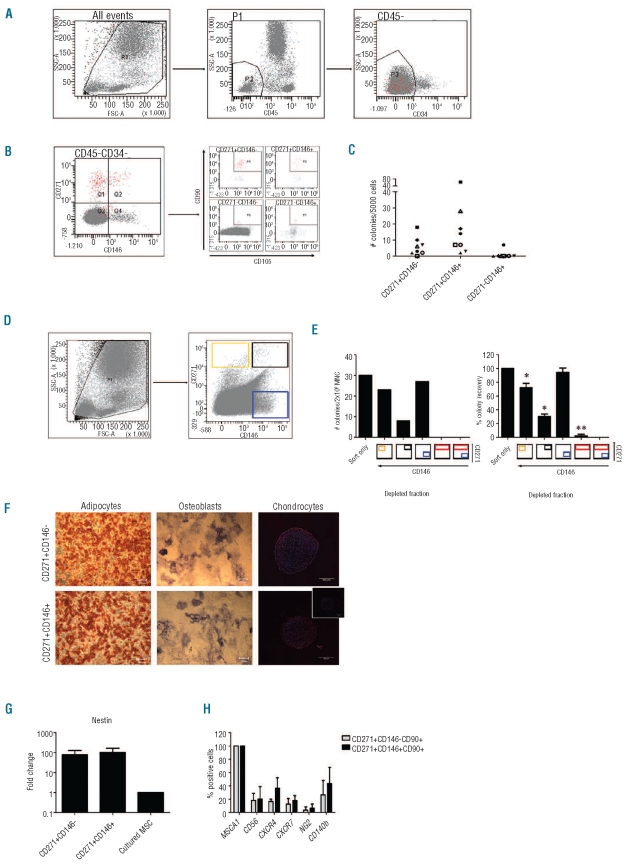
We used the differential distribution of CD271 and CD146 to analyze MSC subsets in human BM at distinct phases during development and aging. We first analyzed the distribution of MSC populations in CD45−/dimCD34−cells in adult BM (n=53, mean age 65 years, range 19–86 years) and identified three subpopulations: CD271brightCD146−, CD271brightCD146+ and CD271−CD146+ (Figure 1A and B). Only the CD271+ populations contained CFU-F, indicative for functional MSC, with a tendency towards more CFU-F in CD271brightCD146+ cells (P<0.072; Figure 1C). CFU-F were absent in CD271-CD146− and CD271−CD146+ fractions, except for one sample (Figure 1C) in accordance with Tormin et al.7 Depletion of CD271−CD146+ did not reduce CFU-F from the remaining BM cells, while CFU-F were absent in CD271bright depleted MNC cells (Figure 1D and E). Thus CFU-F are only present in CD271bright cells and clonogenic MSC do not require support from other BM-derived cells.
Figure 1.
Characterization of MSC subsets in adult BM. (A and B) Representative figures showing the gating strategies to eliminate hematopoietic cells from BM mononuclear cells (MNC) (A), and selection of CD271 and/or CD146 positive cells from BM CD45−CD34− cells, including co-expression of CD90 and CD105 in each quadrant of the first panel (B). The CD271−CD146− cells predominantly contained nucleated erythrocytes, defined by co-staining for CD36 and CD235a (data not shown). (C) CFU-F content in 5000 CD271brightCD146−, CD271brightCD146+ and CD271−CD146+ sorted BM MNC (n=8). (D) Gating strategy for the depletion of CD271 and/or CD146 positive fractions from BM MNC by FACS without eliminating hematopoietic cells. (E) CFU-F content in 2x106 depleted cells or cells that only passed through the sorter. Left panel shows one representative experiment, while the percentage of CFU-F is averaged in the right panel (mean ± SD, n=3). The colored boxes below the bars indicate which fraction is depleted. *P<0.05, **P<0.01. (F) Cultured CD271brightCD146− and CD271brightCD146+ MSC were differentiated towards adipocytes (left panel, scale bar 200 μm), osteoblasts (right panel, scale bar 200 μm) and chondrocytes (right panel: scale bar 500 μm; inset: isotype control; red: aggrecan; blue: dapi).
Images were taken on a Leica DC300 microscope, magnification 20X; chondrocyte images were made using a Zeiss LSM 510 META confocal microscope, magnification 10X. (G) Nestin mRNA expression detected by RQ-PCR in uncultured CD271brightCD146−, CD271brightCD146+ relative to cultured unfractionated MSC (mean ± SD, n=2). (H) Co-expression (% of all cells) of indicated markers on uncultured CD271brightCD146− or CD271brightCD146+ MSC detected by flow cytometry (mean ± SD, n=3).
Expanded CD271bright subsets showed trilineage differentiation potential in vitro (Figure 1F) and maintained expression of MSC markers CD73, CD90 and CD105 (data not shown) fulfilling the ISCT criteria.1 Next, the expression of recently described MSC markers, including Nestin,13 CD140b (PDGFRβ),3 CD562 and MSCA-12 was analyzed. High levels of Nestin mRNA were detected in CD271brightCD146− and CD271brightCD146+ cells. Far less Nestin mRNA was detected in culture-expanded MSC (Figure 1G). Both subsets contained CD56- and CD140b-positive subpopulations. Uniform expression was observed for MSCA-1 and chemokine receptors CXCR4 and CXCR7; however, only 15–40% of the cells expressed CXCR4 and CXCR7 above background (Figure 1H).
Cellular composition of BM changes during development9 and aging.8,10,14 The frequency of CFU-F declines with age,8 but the effect on the composition of the MSC compartment is unknown. Considering all adult BM samples, the largest subpopulation that co-expressed CD90 and CD105 was CD271brightCD146− (Online Supplementary Figure S1A; n=53, 60.4±3.0%; 0.011±0.001% of nucleated cells), whereas CD271brightCD146+ and CD271−CD146+ populations accounted for 30.6±2.9% (0.006±0.001%) and 9.4±2.1% (0.003±0.001%), respectively (Online Supplementary Figure S1A).
When separating samples from younger adults (19–55 years, i.e. the eligible age for stem cell donors; www.europ-donor.nl) and elderly adult donors (>55 years), it was observed that CD271brightCD146− and CD271brightCD146+ occurred at a similar frequency in younger adults whereas the CD271brightCD146+ fraction was significantly reduced in elderly adults (Online Supplementary Figure S1B and C). This suggests that CD146 expression on CD271bright cells decreases with aging, as illustrated by representative donor samples of distinct ages (Online Supplementary Figure S1D-G).
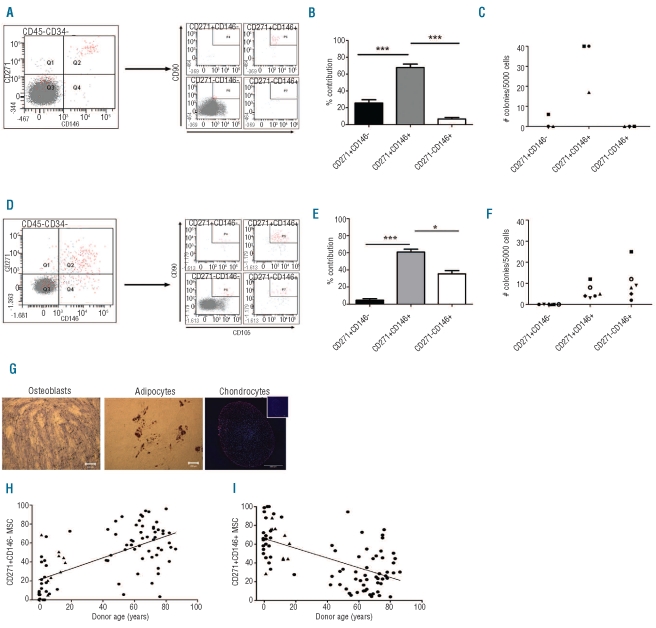
To further investigate the age-related distribution of MSC subsets, we studied pediatric and fetal BM. In contrast to adults, CD271brightCD146+CD90+CD105+ cells were dominant in children (Figure 2A and B; n=27, 67.7±4.2%; 0.010±0.002% MNC count). This fraction also contained most CFU-F (Figure 2C; n=3). The CD271brightCD146−CD90+CD105+ (0.003±0.001% from MNC) and CD271−CD146+ CD90+CD105+ (0.0006±0.0002%) populations represented 25.6 and 6.6% of the MSC, respectively.
Figure 2.
MSC subsets are differentially distributed in pediatric and fetal BM. (A) Gating strategy to analyze CD271 and/or CD146 positive cells from pediatric BM CD45−CD34− cells. Each quadrant of the left panel was subsequently analyzed for co-expression of CD90 and CD105. Importantly, CD90 and CD105 co-expressing cells are now only observed in CD271brightCD146+ cells. (B) Distribution of CD271brightCD146−, CD271brightCD146+ and CD271−CD146+ cells in the CD45−CD34−CD105+CD90+ population of pediatric BM (mean ± SD, n=27). ***P<0.0001 (C) 5000 CD271brightCD146−, CD271brightCD146+ and CD271−CD146+ were sorted from BM MNC, and CFU-F content per fraction was evaluated (n=3).
(D) Gating strategy to analyze CD271 and/or CD146 positive cells in fetal BM CD45−CD34− cells. Each quadrant of the left panel was subsequently analyzed for co-expression of CD90 and CD105. CD90 and CD105 co-expressing cells are observed in CD271brightCD146+ and CD271−CD146+ cells. (E) Distribution of CD271brightCD146−, CD271brightCD146+ and CD271−CD146+ cells in fetal CD45−CD34−CD105+CD90+ BM (mean ± SD, n=6). *P<0.05 ***P<0.0001. (F) CFU-F content in 5000 CD271brightCD146−, CD271brightCD146+ or CD271−CD146+ sorted BM MNC (n=6). Fetal CD271−CD146+ cells now contain CFU-F, in contrast to adult and pediatric BM. (G) Cultured MSC obtained from CD271−CD146+ fetal BM cells were differentiated towards osteoblasts (right panel, scale bar 200 μm), adipocytes (left panel, scale bar 200 μm), and chondrocytes (right panel, scale bar 500 μm; inset: isotype control; red: aggrecan; blue: dapi). Images were taken on a Leica DC300 microscope, magnification 20X; chondrocyte images were made using a Zeiss LSM 510 META confocal microscope, magnification 10X. (H-I) The percentage of CD271brightCD146− (H) or CD271brightCD146+ (I) relative to the sum of all CD271+ and/or CD146+ cells that co-expressed CD105 and CD90 per donor was plotted versus donor age. Triangles represent patients with hematopoietic malignancy. The variation in distribution between donors was explained by donor age for 36% (P<0.0001) and 26% (P<0.0001) for CD271brightCD146− cells and CD271brightCD146+ cells, respectively.
A relatively large subpopulation of fetal CD271−CD146+ cells co-expressed CD105 and CD90. They accounted for 35.5±8.8% (Figure 2D and E; n=6, 0.107±0.004% from MNC) of all MSC. Interestingly, fetal CD271−CD146+ cells contained CFU-F (Figure 2F). CD271brightCD146+ cells represented 60.8±8.2% (0.174±0.025%) and also formed CFU-F (Figure 2D and F). CD271brightCD146− cells were nearly absent (0.012±0.004%) and they did not contain CFU-F (Figure 2D-F). The newly defined fetal CD271−CD146+ subset had trilineage differentiation potential (Figure 2G). The fetal subsets had a comparable proliferation rate (data not shown). These data show that fetal BM contains a third MSC subset, identified as CD271−CD146+CD105+CD90+ that unlike their adult counterparts do generate CFU-F. In addition, these results indicate that CFU-F content and the distribution of MSC subsets differs between adult, pediatric and fetal BM.
The proportion of CD271brightCD146− cells in BM was significantly correlated to donor age (n=86, R2=0.36, P<0.0001, Figure 2H) while the distribution of CD271brightCD146+ cells was inversely correlated (n=86, R2=0.26, P<0.0001, Figure 2I). Therefore, the previously reported age-related decrease in BM CFU-F8,15,16 may now be explained by the loss of CD271brightCD146+ cells that tend to generate more CFU-F. The aspiration site may influence CFU-F content and stromal cell phenotype.17 In our study, BM from cardiac patients was sternum-derived; fetal femurs were flushed, whereas BM from other patients was iliac crest-derived. Excluding cardiac patients and fetal samples, thereby correcting for aspiration site-related effects, did not affect the age-related correlation (n=37, age 0–86, CD271brightCD146− cells, R2=0.13; P<0.031; CD271brightCD146+ cells, R2=0.12; P<0.036).
In conclusion, we have shown for the first time that the distribution of defined MSC subsets significantly correlates with donor age, and we identified a novel MSC subset specific for fetal BM. Tormin et al. reported that CD271+CD146−/lo and CD271+CD146+, respectively, localize to endosteal or perivascular niches in vivo,7 while we report an age-related distribution. This suggests that the relative size of specialized BM niches is dynamic, and that distinct phases in life require different MSC subtypes. The increase in CD271brightCD146− MSC in aged BM may, therefore, correspond to the increase in long-term hematopoietic stem cells (HSC) in aged murine BM.18,19 These quiescent HSC predominantly localize to the endosteal niche, where the human CD271+CD146−/lo also reside.7 Accordingly, our preliminary co-culture data suggest that adult CD271brightCD146−cells provide better long-term hematopoietic support than CD271brightCD146+ cells. Although the ontogeny and the relationship between the subsets remain unclear, MSC seem to comprise a dynamic system during human life, in which the subpopulations could have different functions during bone marrow development, homeostasis and regeneration.
Acknowledgments
We would like to thank Dr BJ Biemond, A van der Laan, Dr SE Dohmen, K. de Heer and the Dept. of Cardiothoracic Surgery, Academic Medical Center Amsterdam for collection of bone marrow aspirates and Ms N Papazian and Dr T Cupedo for collection of fetal bone marrow. Furthermore, the authors would like to thank Ms T Schaap and Dr D Hamman for technical assistance, and Dr M von Lindern for critically reading the manuscript and helpful discussions.
Footnotes
Funding: this work was supported by DPTE grant n. 06728 and Sanquin PPO-C.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94(2):173–84. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann NY Acad Sci. 2007;1106:262–71. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 4.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 5.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B, Funari A, Michienzi S, Di CS, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Tormin A, Li O, Brune JC, Walsh S, Schutz B, Ehinger M, et al. CD146 expression on primary non-hematopoietic bone marrow stem cells correlates to in situ localization. Blood. 2011;117(19):5067–77. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21(3):429–35. [PubMed] [Google Scholar]
- 9.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133(19):3733–44. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 10.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102(26):9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474–86. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 12.Maijenburg MW, Noort WA, Kleijer M, Kompier CJ, Weijer K, van Buul JD, et al. Cell cycle and tissue of origin contribute to the migratory behaviour of human fetal and adult mesenchymal stromal cells. Br J Haematol. 2010;148(3):428–40. doi: 10.1111/j.1365-2141.2009.07960.x. [DOI] [PubMed] [Google Scholar]
- 13.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6(3):265–78. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznetsov SA, Mankani MH, Bianco P, Robey PG. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009;2(1):83–94. doi: 10.1016/j.scr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuznetsov SA, Cherman N, Riminucci M, Collins MT, Robey PG, Bianco P. Age-dependent demise of GNAS-mutated skeletal stem cells and “normalization” of fibrous dysplasia of bone. J Bone Miner Res. 2008;23(11):1731–40. doi: 10.1359/jbmr.080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Orgel M, Treml S, et al. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 2010;19(5):669–77. doi: 10.1089/scd.2009.0290. [DOI] [PubMed] [Google Scholar]
- 18.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–6. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 19.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]