Abstract
Genetic events underlying pathogenesis of nodal and extranodal marginal zone lymphoma are not completely understood. We report here a novel t(X;14)(p11.4;q32.33) identified in 4 lymphoma cases: 2 with a mucosa-associated lymphoid tissue lymphoma, one with a nodal marginal zone lymphoma and one with gastric diffuse large B-cell lymphoma. In all cases, lymphoma evolved from a previous auto-immune disorder. Fluorescence in situ hybridization and molecular studies showed that t(X;14), which is mediated by immunoglobulin heavy chain locus, targets the GPR34 gene at Xp11.4. Upregulation of GPR34 mRNA and aberrant expression of GPR34 protein has been demonstrated in 3 presented cases by quantitative real-time polymerase chain reaction and immunohistochemistry, respectively. GPR34 belongs to the largest family of cell surface molecules involved in signal transmission that play important roles in many physiological and pathological processes, including tumorigenesis. Although functional consequences of t(X;14) have not been identified, our studies suggest that up-regulated GPR34 activate neither nuclear factor-κB nor ELK-related tyrosine kinase.
Keywords: marginal zone lymphoma, mucosa-associated lymphoid tissue lymphoma, chromosomal translocation, IGH, GPR34
Introduction
Extranodal marginal zone lymphoma (MZL) of mucosa-associated lymphoid tissue (MALT) and nodal MZL are recognized as distinct entities in the World Health Organization classification of lymphoid tumors.1 So far, genetic features of both lymphoma entities have not been well characterized. It has been shown, however, that approximately 25% of MALT lymphoma cases are hallmarked by recurrent chromosomal translocations. The most frequent is t(11;18)(q21:q21) leading to an API2 -MALT1 fusion. Less common translocations include IGH-mediated t(1;14)(p22;q32), t(14;18)(q32;q21) and t(3;14)(p14;q32) targeting the BCL10, MALT1 and FOXP1 genes, respectively.2 Notably, the BCL10 and MALT1 proteins play a physiological role in antigen receptor mediated activation of the nuclear factor-κB (NF-κB) signaling pathway, known to be implicated in tumor growth, survival and chemoresistance.3 Aberrant activation of this pathway not only underlies MALT lymphomas with t(1;14), t(11;18) and t(14;18), but also cases with loss/inactivation of TNFAIP3/A20 (6q23), a known repressor of NF-κB4 and activating mutations of MYD88.5
We report here a novel translocation, t(X;14)(p11.4;q32.33), that was identified in 2 cases of MALT lymphoma, and single cases of nodal MZL and gastric diffuse large B-cell lymphoma (DLBCL).
Design and Methods
Patients
Patients’ samples were selected from the lymphoma database of the Center for Human Genetics, KU Leuven, Leuven. Research on existing material, used anonymously, was approved by the Ethics Committee from the KU Leuven.
Immunohistochemistry
Immunohistochemistry (IHC) using antibody against GPR34 (Atlas Antibodies AB, Stockholm, Sweden) was performed on paraffin-embedded tissue sections. As positive controls, normal testis and bone marrow biopsies from patients with mastocytosis were used. Sections were stained according to the manufacturer’s recommendations, and IHC results were visualized using the En Vision system (Dako Denmark A/S).
Cytogenetic and fluorescence in situ hybridization analysis
Conventional G-banding chromosomal analysis and fluorescence in situ hybridization (FISH) followed routine methods. Probes applied for FISH included LSI IGH (Abbott Molecular, Wiesbaden, Germany) and 16 home-brewed bacterial artificial chromosome (BAC) clones selected from www.ensembl.org (Online Supplementary Figure S1) and labeled with SpectrumOrange- and SpectrumGreen-d-UTP (Abbott Molecular) using random priming.
Agilent CGH-array
The cases were analyzed using Agilent 244k CGH microarrays (Agilent Technologies, Santa Clara, CA, USA). One μg of both reference and tumor DNA was labeled using Agilent Genomic DNA Labeling Kit, mixed with a provided hybridization solution and hybridized to the array, according to the manufacturers’ protocol. After 40 h of hybridization and subsequent washing steps, the arrays were scanned with the GenePix scanner and analyzed using the Feature extraction 9.5.3.1 and the DNA analytics 4.0 software.
Quantitative RT-PCR
RNA isolation and cDNA synthesis were performed using standard protocols. The BJAB cell line was transfected with an eukaryotic expression construct for GPR34 (pcDNA3.1) via electroporation to allow stable GPR34 expression, and single positive clones were obtained via clonal dilution and selection with G418. Quantitative RT-PCR was performed with the LightCycler 480 SYBR Green I master mix (Roche Diagnostics Belgium, Vilvoorde, Belgium) and analyzed using the comparative dCt method. Primer sequences are shown in the Online Supplementary Table S1. In all experiments, the housekeeping gene HPRT1 was used as a reference control.
Western blot analysis
SDS-PAGE and Western blot analysis followed standard protocols. Antibodies used for immunodetection of phospho-IκBα (Ser32/36, clone 5A5) and phospho-p44/42 MAPK (Thr202/Tyr204, clone D13.14.4E) were from Cell Signaling Technology (Danvers, MA, USA). Antibody against ERK2 (p42) (ERK 2 (D-2): sc-1647) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The signal was visualized with the enhanced chemoluminescence (ECL) system (Perkin Elmer, Zaventem, Belgium) on an LAS 3000 mini gene system (Fuji) using the Advanced Image Data Analyzer (AIDA) Software (Raytest BeNeLux B.V., Tilburg, The Netherlands).
Results and Discussion
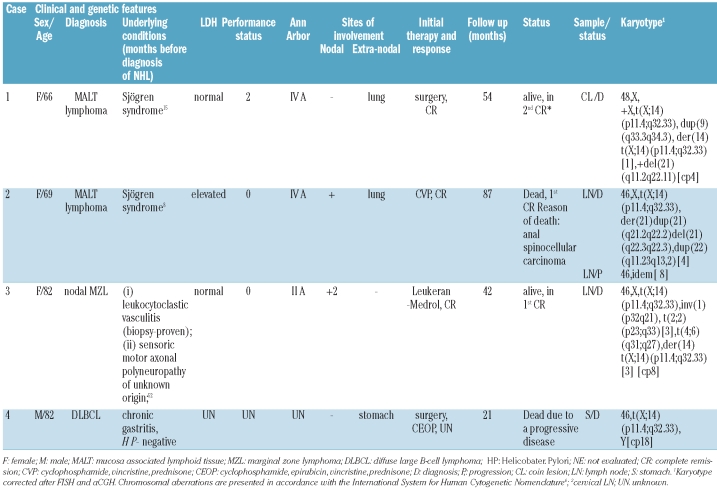
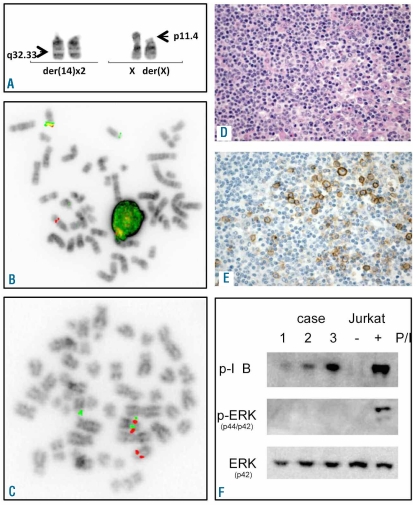
The relevant cytogenetic, clinical and histological characteristics of the reported cases are shown in Table 1. There were 3 females and one male, age ranging from 66 to 82 years (median 75 years). All patients received an initial therapy. Two patients are alive after 42–54 months from diagnosis and 2 have died, either from progressive lymphoma, or from an unrelated cause (other tumor). In each patient, t(X;14) was identified at the time of lymphoma diagnosis. The translocation occurred as the sole abnormality in one case (# 4) and was accompanied by 2 to 4 additional chromosomal abnormalities in other cases. Of note, a subclonal appearance of an additional der(14)t(X;14) was observed in cases 1 and 3 (Table 1 and Figure 1A). The t(X;14)-related rearrangement of IGH was proven by FISH with LSI IGH (Figure 1B). To identify the partner gene targeted by t(X;14), metaphase FISH BACs-walking approach was applied in case 4. In total, 12 BAC clones from the Xp21.3-q11.22 (27–54 Mb) region were used and the breakpoint was narrowed down to the region of CASK at Xp11.4 (Online Supplementary Figure S1A). Given that intron 5 of CASK houses the GPR34 and GPR82 genes (Online Supplementary Figure S1B), 4 additional BAC clones covering CASK were further applied. Eventually, the breakpoint was mapped in the region flanked by RP11-204C16 (covering GPR34 and GPR82) and RP5-1174J21 that hybridized to der(14) and der(X), respectively (Figure 1C). The remaining cases showed the Xp11.4 breakpoint in the same interval.
Table 1.
Relevant clinical and cytogenetic findings.
Figure 1.
Examples of cytogenetic, FISH, IHC and molecular studies. (A) Partial karyotype of case 3 showing t(X;14)(p11.4;q32.33) and an extra copy of der(14). (B and C) Examples of FISH analysis of case 3 using LSI IGH and BAC clones flanking the Xp11.4 region (RP11-204C16-SO/RP11-1174J21-SG), respectively. (D) H&E staining of case 3. (E) IHC with GPR-34 serum in case 3. (F) Immunoblot analysis of cases 1–3 with antibodies that specifically recognize phosphorylated IκBα and p42/p44 MAPK (ERK). Lysates of stimulated Jurkat cells were used as positive control. Blots were re-probed with MAPK p44 to confirm even protein loading. FISH images were acquired with a 63x/1.40 oil-immersion objective in an Axioplan 2 fluorescence microscope equipped with an Axiophot 2 camera (Carl Zeiss Microscopy, Jena, Germany) and a MetaSystems Isis imaging system (MetaSystems). H&E and IHC images were captured with a Leica DMLB microscope (Leica, Wetzlar, Germany) using a Leica PL FLUOTAR lens (40x/0.70) and a Leica DC200 camera. Images were imported directly into PowerPoint (Microsoft, Redmond, WA, USA) using the Leica DC200 camera software (version 2.51).
FISH was followed by qRT-PCR analysis of 5 candidate genes located telomeric to the Xp11.4 breakpoint and translocated to der(14) (GPR82, GPR34, CASK, DDX3X, USP9) performed in 3 available cases (#1–3). As control, RNA from non-malignant spleen was used. This analysis showed that only one of the analyzed genes, GPR34, was up-regulated 11 to 100-fold in the studied cases (Online Supplementary Figure S1C). Further immunostaining with anti-GPR34 serum showed expression of GPR34 protein in 3 out of the 4 analyzed cases (Figure 1D and E). Unfortunately, no archive material from case 4, negative by IHC, was available for qRT-PCR. Whether lack of reactivity with this serum was caused by a technical failure or a low expression level of the GPR34 protein by neoplastic cells, or by activation of another Xp11.4 gene remains unknown.
To check whether any cryptic recurrent genomic imbalances were associated with t(X;14), all 4 cases were subjected to Agilent oligonucleotide 244K array analysis. An average 24 copy number alterations (CNA) (range 11–32) per case were detected, including aberrations previously identified by cytogenetics (Online Supplementary Figure S2). Most of the CNA were smaller than 1 Mb and overlapped with regions of CNVs. Those not associated with CNVs (e.g. del(9)(p24)/1.39 Mb in case 4) were random. The only recurrent aberration was dup(21)(q24qter) (41561927-46914886) detected in cases 1 and 2 (Online Supplementary Figure S3). These data support the concept that t(X;14) was the driving genetic aberration in the presented lymphomas.
The t(X;14) was identified in 2 of 61 (3.3%) cases of MALT lymphoma, in one of 43 (2.3%) cases of nodal MZL and one of 19 (5.2%) cases of extranodal DLBCL with clonal chromosomal abnormalites analyzed in our institution during the last 16 years. To search for additional cases with t(X;14), break apart FISH probes for IGH and CASK were applied in 10 cases of MALT lymphoma with unknown karyotype originating from the lung (n=3), stomach (n=2), spleen (n=2), parotid gland (n=2) and lachrymal gland (n=1). No chromosomal breaks in these loci were found. Moreover, we reviewed the available global expression data of the previously studied 6 t(11;18)-positive and 8 t(11;18)-negative gastric MALT lymphomas (Gene expression Omnibus, accession n. GSE 25550; www.ncbi.nlm.nih.gov/projects/geo/). The level of GPR34 mRNA expression in these cases was comparable with that of control non-malignant spleens (10.045 vs. 9.9725 vs. 10.432, respectively). Additional IHC performed on 6 gastric and 9 pulmonary MALT lymphoma cases showed lack of GRP34 expression in 14 cases. Interestingly, in case of transformed MALT lymphoma, an aberrant expression of GPR34 was found exclusively in a large cell component showing an extra copy of GPR34 by FISH (data not shown).
As constitutive NF-κB activity is frequently associated with B-cell lymphoma development,3 we examined whether the NF-κB pathway was active in 3 lymphomas with t(X;14) from which material was available. In all of them, Western blot analysis showed phosphorylation of the NF-κB inhibitor protein Iκ-Bα (Figure 1F) which indicates its degradation and is a hallmark of activation of NF-κB pathway. Jurkat T cells stimulated with PMA/Ionomycin served as a positive control. Quantitative RT-PCR further confirmed upregulation of a number of NF-κB target genes in t(X;14) lymphoma samples, with transcript levels comparable to a t(11;18)-positive MALT lymphoma (Online Supplementary Figure S4A). Whether GPR34 activates NF-κB is unknown. Knock-out studies in mice revealed a physiological role for GPR34 in the immune response7 which is tightly regulated by NF-κB. To examine the role for GPR34 in NF-κB activation, we over-expressed GPR34 in the B-cell lymphoma cell line BJAB, in which the NF-κB pathway is relatively quiescent. Although GPR34 transcript levels increased 40 to 800-fold, the set of NF-κB target genes was not up-regulated, in contrast to its induced upregulation by overexpression of API2-MALT1 or mutant CARD11 (L232LI), known activators of NF-κB signaling in B cells (Online Supplementary Figure S4B). These data suggest that activation of NF-κB signaling in MALT lymphomas with t(X;14) is not mediated by GPR34 overexpression and most likely involves other mechanisms.
In summary, our study provides evidence that the new IGH-mediated t(X;14)(p11.4;q32) is a primary aberration involved in the pathogenesis of B-NHL, including extranodal and nodal MZL, and gastric DLBCL. This translocation and the well known t(1;14), t(3;14), t(11;18), and t(14;18) are mutually exclusive in MALT lymphoma. Of note, all 4 reported patients had an underlying disorder, including Sjögren’s syndrome (case 1 and 2), a leukocytoclastic vasculitis and polyneuropathy (case 3), and HP-negative chronic gastritis with intestinal metaplasia (case 4). The observation of Sjögren’s syndrome in both MALT lymphoma cases remains in line with the general concept of MALT lymphoma development suggesting that this neoplasm arises as a result of chronic B-cell stimulation.8
Molecular analysis showed that t(X;14) targets GPR34. Upregulation of GPR34 mRNA and protein was demonstrated by qRT-PCR and IHC, respectively, performed in 3 cases. The finding of a subclonal gain of der(14)t(X;14) in 2 cases suggests that an extra dosage of the GPR34 product may provide a growth advantage for tumor cells. GPR34, like the neighboring GPR82, codes for a G-protein coupled receptor that belongs to the largest family of cell surface molecules involved in signal transmission.9 These integral membrane proteins contain 7 putative transmembrane domains and mediate signals to the interior of the cell. They activate heterotrimeric G proteins that in turn activate various effector proteins, eventually leading to the activation of multiple intracellular signaling pathways and a physiological response. An aberrant expression and activity of GPRs has been implicated in a wide spectrum of diseases, including tumors/malignancies.10 Importantly, due to their unique structure, localization and ligand binding activity, GPRs have been extensively used for drug development and are the most common targets of commercial drugs.9
GPR34 was originally cloned from a human fetal brain cDNA library based on the platelet-activating factor (PAF) receptor sequence.11,12 The receptor mRNA is ubiquitously expressed in all human tissues, particularly in placenta, brain and spleen.13 Recent studies suggest that GPR34, found to be abundantly expressed in mast cells, is the functional mast cell receptor for lysophosphatidyl-L-serine (lysoPS).14 The latter molecule seems to stimulate degranulation of mast cells. High functional expression of GPR34 in mast cells suggests that the receptor is involved in a basic function of these cells and is possibly activated in damaged and inflamed tissues. Genetic studies in mice, however, did not confirm that the known effects of lyso-PS, such as mast cell degranulation and cell migration, are mediated by GPR34. However, GPR34 function appeared to be required for an adequate immune response, suggesting that its upregulation could contribute to lymphomagenesis.7
So far, there has been only a preliminary report of involvement of GPR34 in tumorigenesis by Novak et al. (ASH abstract15). The authors presented one case of MALT lymphoma with the same t(X;14)/IGH-GPR34 rearrangement and suggested a role for ERK signaling in tumor development. Hela cells with stable overexpression of GPR34 but not a signaling deficient mutant indeed displayed increased phosphorylation of ERK1/2. In addition, proliferation was significantly higher in GPR34 over-expressing cells and was hampered by MAPK inhibitor. In our model of BJAB cells over-expressing GPR34, however, phosphorylation of ERK was not observed (Online Supplementary Figure S5) which may suggest a cell type-dependent effect. The observation that ERK is also not constitutively phosphorylated in the present MALT lymphomas with t(X;14) (Figure 1F) further questions its role in the pathogenesis of these tumors.
In conclusion, we identified GPR34 as a new player of B-NHL pathogenesis. The gene is recurrently targeted by the IGH-mediated t(X;14)(p11.4;q32.33) associated with MZ/MALT lymphoma evolving from a previous auto-immune disorder. The functional consequences of t(X;14) remain elusive, but our data indicate that up-regulated GPR34 activates neither NF-κB nor ERK. Further studies are required to determine GPR34 natural ligand(s) and signal pathways. Given that G protein-couple receptors (GPCR) represent the very important therapeutic targets and new GPCR-based drugs are under development,9 lymphoma patients with t(X;14) might benefit in future from cancer-tailored treatments.
Acknowledgments
The authors thank Prof. Guy Jerusalem (CHU Sart Tilman, Liège) for clinical data, Ursula Pluys and Riet Somers for technical assistance, and Rita Logist for editorial help.
Footnotes
Funding: this work was supported by a concerted action grant from the K.U.Leuven, an IWT-Flanders SBO grant (n. 060848), the Research Foundation-Flanders (FWO, grant G.0552.08) and the ‘Belgian Foundation against Cancer’. XS and PV are senior clinical investigators of the FWO.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Sagaert X, Tousseyn T. Marginal zone B-cell lymphomas. Discov Med. 2010;10(50):79–86. [PubMed] [Google Scholar]
- 3.Staudt LM. Oncogenic Activation of NF-kappa B. Cold Spring Harbor Perspectives in Biology. 2010;2(6):a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanudet E, Huang Y, Ichimura K, Dong G, Hamoudi RA, Radford J, et al. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia. 2010;24(2):483–7. doi: 10.1038/leu.2009.234. [DOI] [PubMed] [Google Scholar]
- 5.Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–9. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009: International System for Human Cytogenetic Nomenclature. Karger; 2009. [Google Scholar]
- 7.Liebscher I, Muller U, Teupser D, Engemaier E, Engel KMY, Ritscher L, et al. Altered Immune Response in Mice Deficient for the G Protein-coupled Receptor GPR34. J Biol Chem. 2011;286(3):2101–10. doi: 10.1074/jbc.M110.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4(8):644–53. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 9.Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10(1):47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27(5):1329–39. [PubMed] [Google Scholar]
- 11.Marchese A, Sawzdargo M, Nguyen T, Cheng R, Heng HHQ, Nowak T, et al. Discovery of three novel orphan G-protein-coupled receptors. Genomics. 1999;56(1):12–21. doi: 10.1006/geno.1998.5655. [DOI] [PubMed] [Google Scholar]
- 12.Schoneberg T, Schulz A, Grosse R, Schade R, Henklein P, Schultz G, et al. A novel sub-group of class I G-protein-coupled receptors. Biochim Biophys Acta. 1999;1446(1–2):57–70. doi: 10.1016/s0167-4781(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 13.Engemaier E, Rompler H, Schoneberg T, Schulz A. Genomic and supragenomic structure of the nucleotide-like G-protein-coupled receptor GPR34. Genomics. 2006;87(2):254–64. doi: 10.1016/j.ygeno.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341(4):1078–87. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 15.Novak AJ, Akasaka T, Manske M, Price-Troska T, Gupta M, Witzig TE, et al. Elevated Expression of GPR34 in Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma and Its Association with Increased Cell Growth, Erk Activation, and AP-1 and CRE-Mediated Transcription. Blood. 2009;114(22):1510. [Google Scholar]