Abstract
Here we investigate the regulation of hepcidin, a hormone that inhibits dietary iron absorption and macrophage iron recycling, by the serum iron-binding protein transferrin. Mice deficient in transferrin (Tfhpx/hpx) and hemojuvelin (Hjv−/−), a gene mutated in juvenile hemochromatosis, a disease of hepcidin deficiency and iron overload, were generated. While Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/− phenotypes did not differ markedly, transferrin treatment and RBC transfusions robustly increased hepcidin levels in Tfhpx/hpx Hjv+/+ but not Tfhpx/hpx Hjv−/−mice. These results suggest that, while hemojuvelin is not essential for the establishment or maintenance of hepcidin deficiency in transferrin-deficient mice, hemojuvelin is essential for transferrin-dependent and tansferrin-independent hepcidin expression in conditions of iron overload.
Keywords: hemojuvelin, transferrin dependent, transferrin independent, hepcidin, mice
Introduction
Synthesized predominantly by the liver, the serum peptide hepcidin inhibits duodenal iron absorption and macrophage iron efflux.1 Hepcidin expression is regulated by several factors including iron, anemia, hypoxia and inflammation. Using the hypotransferrinemic mouse, a model of transferrin deficiency characterized by anemia, hepcidin deficiency and iron overload, we recently demonstrated that the serum iron-binding protein transferrin is a key determinant of hepcidin expression.2 Given that iron-dependent hepcidin expression is mediated in part by genes mutated in hereditary hemochromatosis, a disease of hepcidin deficiency and iron overload, we hypothesized that transferrin-dependent hepcidin expression in hpx mice requires function of hemochromatosis genes. To test this hypothesis, we generated mice deficient in both transferrin (Tfhpx/hpx) and hemojuvelin (Hjv−/−), a gene mutated in juvenile hemochromatosis,3,4 and characterized the response of these mice to transferrin treatment and red blood cell (RBC) transfusions.
Design and Methods
Tfhpx/hpx and Hjv−/− mice were maintained respectively on the BALB/cJ and C57BL/6J backgrounds, as previously described.2,5 Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/−mice were generated by breeding Tf+/hpx mice to Hjv+/−mice then intercrossing the Tf+/hpx Hjv+/− progeny. Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/− pups were injected intraperitoneally with 3 mg purified human transferrin (Roche) once a week until weaning at Day 21 to ensure their survival. All experiments were performed on 8-week old mice. For transferrin treatment of adult mice, mice were injected intraperitoneally with 10 mg transferrin (Roche) every other day. For RBC transfusions, EDTA-anticoagulated blood was collected, centrifuged and washed with PBS four times to remove plasma, then injected into recipients intraperitoneally. Parameters of erythropoiesis and iron metabolism were measured as previously described.2 All statistical analyses were performed using Microsoft Excel. Student’s two-tailed t-test P values (unpaired; unequal variance) at <0.05 were considered significantly different.
Results and Discussion
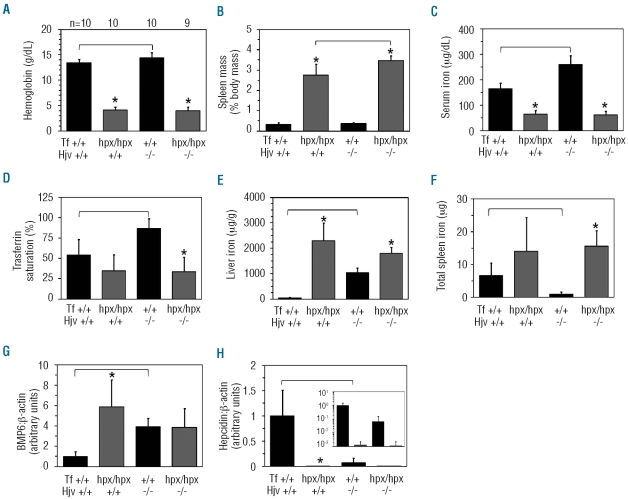
To generate Tfhpx/hpx Hjv−/− mice, we first bred Tf+/hpx mice to Hjv+/− mice then intercrossed the Tf+/hpx Hjv+/− progeny (Figure 1). The phenotype of Tfhpx/hpx Hjv+/+ mice relative to Tf+/+ Hjv+/+ mice was similar to that of BALB/cJ Tfhpx/hpx mice:2 decreased hemoglobin, serum iron and liver hepcidin RNA levels, increased spleen masses, liver iron and Bmp6 RNA levels (Figure 1A-C,E,H,G), where bone morphogenetic protein 6 (Bmp6) is an endogenous stimulator of hepcidin expression, abundantly expressed in conditions of iron overload.1 The phenotype of Tf+/+ Hjv−/− mice relative to Tf+/+ Hjv+/+ mice was similar to that of C57BL/6J Hjv−/− mice: decreased liver hepcidin and spleen iron levels, and increased serum iron levels, transferrin saturations, liver iron and Bmp6 RNA levels (Figure 1C–H), although hemoglobin levels were also increased by 1 g/dL. As far as Tfhpx/hpx Hjv+/+ mice are concerned, Tfhpx/hpx Hjv−/− mice displayed only a mild 0.5% increase in spleen mass, expressed as a percentage of total body mass (Figure 1B). Relative to Tf+/+ Hjv−/− mice, Tfhpx/hpx Hjv−/− mice had decreased hemoglobin, serum iron levels, transferrin saturations and increased spleen masses, liver iron and total spleen iron levels; hepcidin levels were decreased but this was not significant (P=0.06) (Figure 1A–F,H).
Figure 1.
Phenotype of Tfhpx/hpx Hjv−/− mice. Mice were characterized at two months of age for (A) hemoglobin levels, (B) spleen masses, (C) serum iron levels, (D) transferrin saturations, (E) liver iron levels, (F) total spleen iron levels and (G) liver Bmp6 and (H) hepcidin RNA levels relative to β-actin levels as measured by quantitative polymerase chain reaction. (A) ‘n’ refers to number of mice analyzed per group; each group contains male and female mice. Inset in (H) represents data replotted with a logarithmic y axis. Asterisks indicate statistical significance (P<0.05) relative to Tf+/+ mice of the same Hjv genotype; brackets indicate statistical significance (P<0.05) between two genotypes. Bars on graphs represent one standard deviation.
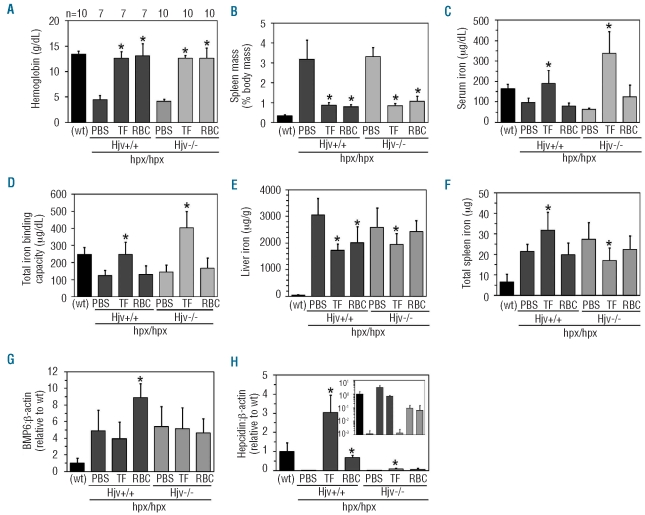
To determine the role of hemojuvelin in transferrin-dependent hepcidin expression, we next treated Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/− mice with phosphate-buffer saline (PBS), transferrin or RBCs every other day for one week. As far as PBS treatment is concerned, both transferrin treatment and RBC transfusions increased hemoglobin levels and decreased spleen masses in both genotypes of mice (Figure 2A and B), while only transferrin treatment increased serum iron levels and total iron binding capacities in both genotypes (Figure 2C and D). Transferrin saturations decreased from 80 to 60% in RBC-transfused Tfhpx/hpx Hjv+/+ mice and increased from 55 to 75% in Tfhpx/hpx Hjv−/− mice treated with transferrin or RBC transfusions (data not shown). There was no increase in liver iron levels with either treatment of either genotype (Figure 2E) while total spleen iron levels increased and decreased, respectively, in transferrin-treated Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/−mice (Figure 2F). Liver Bmp6 RNA levels increased in RBC-transfused Tfhpx/hpx Hjv+/+ but not Tfhpx/hpx Hjv−/− mice (Figure 2G). Hepcidin levels increased prominently in both transferrin-treated and RBC-transfused Tfhpx/hpx Hjv+/+ mice but only modestly in transferrin-treated Tfhpx/hpx Hjv−/− mice, and this increase was without significance (P=0.07) in RBC-transfused Tfhpx/hpx Hjv−/− mice (Figure 2H).
Figure 2.
Treatment of Tfhpx/hpx Hjv−/− mice. Tfhpx/hpx Hjv+/+ and Tfhpx/hpx Hjv−/− mice were treated at two months of age with 0.5 mL PBS, 10 mg transferrin or 0.5 mL washed wild-type RBCs every other day for one week then analyzed for (A) hemoglobin levels, (B) spleen masses, (C) serum iron levels, (D) total iron binding capacities, (E) liver iron levels, (F) total spleen iron levels and (G) liver Bmp6 and (H) hepcidin RNA levels relative to β-actin levels as measured by quantitative polymerase chain reaction. (A) ‘n’ refers to number of mice analyzed per group; each group contains male and female mice. Inset in (H) represents data replotted with a logarithmic y axis. Asterisks indicate statistical significance (P<0.05) relative to PBS-treated mice of the same genotype. ‘(wt)’ indicates Tf+/+ Hjv+/+ mouse values from Figure 1 included as historical reference. Bars on graphs represent one standard deviation.
Hepcidin regulation in hpx mice can be described in terms of the stores- and erythroid-regulators. Proposed decades ago to explain the regulation of iron homeostasis by various phenomena,6 and described here in the context of the central role of hepcidin in iron homeostasis, the stores-regulator stimulates hepcidin expression in conditions of iron overload, while the erythroid-regulator inhibits hepcidin expression in conditions of aberrant erythropoiesis. Our studies implicate transferrin and hemojuvelin as essential components of the stores-regulator but not the erythroid-regulator. A role for transferrin and hemojuvelin in stores-regulator activity is suggested by the increase in hepcidin levels in myeloablated hpx mice treated with transferrin2 and the minimal increase in hepcidin levels in transferrin-treated Tfhpx/hpx Hjv−/− mice. The fact that these two factors do not play a role in erythroid-regulator activity is suggested by the profound hepcidin deficiency in untreated hpx mice and the persistently low hepcidin levels in both Tfhpx/hpx Hjv−/− and Tfhpx/hpx Hjv+/+ mice despite severe iron overload.
While our studies suggest that transferrin and hemojuvelin are not essential components of the erythroid-regulator, the lack of an increase in hepcidin levels in RBC-transfused Tfhpx/hpx Hjv−/− mice, and the modest increase in hepcidin levels in Tf-transfused Tfhpx/hpx Hjv−/− mice, suggests that the erythroid-regulator active in untreated hpx mice may inhibit hepcidin expression by modulating activity along the hemojuvelin-hepcidin signaling pathway. Possible targets of erythroid-regulator activity include BMP receptors or co-receptors such as hemojuvelin, endogenous stimulators of hepcidin expression like BMP6 or downstream mediators of BMP signaling. In vivo modulators of hepcidin expression have already been identified; for example, the hepatic membrane protein transmembrane protease serine 6 (Tmprss6) decreases hepcidin expression by cleaving hemojuvelin from the cell membrane.7 Given that our preliminary data suggest that Tfhpx/hpx Tmprss6−/− mice are not viable (data not shown), exploration of the role of Tmprss6 in hepcidin regulation in hpx mice will require the construction of conditional Tmprss6 alleles or the use of other methods. While a role for BMP antagonists Gdf15 and Twsg1 in hepcidin regulation has yet to be demonstrated in vivo, both antagonize hepcidin expression in vitro and are over-expressed in Tfhpx/hpx mice.2,8 Examination of the role of these factors in hepcidin regulation in Tfhpx/hpx mice will require an approach similar to that taken with hemojuvelin in this report.
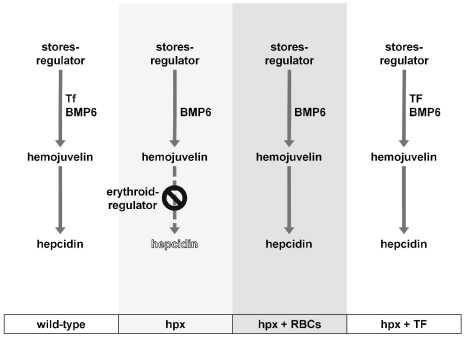
We propose the following model for transferrin-centric hepcidin regulation (Figure 3). Erythropoiesis in untreated hpx mice leads to activation of the erythroid-regulator which inhibits stores-regulator mediated hepcidin expression. With RBC transfusions, the inhibitory effects of the erythroid-regulator are lifted and hepcidin expression, wholly hemojuvelin-dependent, is stimulated by factors other than transferrin such as Bmp6. A similar process occurs in hpx mice treated with transferrin, except that in these mice hepcidin expression is stimulated additionally by administered transferrin. Overall, transferrin and hemojuvelin play essential yet distinct functions: transferrin directly stimulates liver hepcidin expression and delivers iron for erythropoiesis so that the inhibitory effects of anemia and hypoxia on hepcidin expression are minimized, while hemojuvelin mediates the stimulation of hepcidin expression by transferrin and other factors.
Figure 3.
Model of transferrin-dependent regulation of hepcidin expression in hypotransferrinemic mice. Hepcidin expression is regulated by the activity of the stores-and erythroid-regulators. Stores-regulator activity, mediated by transferrin (Tf) and BMP6, stimulates hepcidin expression in a hemojuvelin-dependent manner. Erythroid-regulator activity, possibly mediated by Tmprss6, Gdf15 and Twsg1, inhibits hemojuvelin-dependent hepcidin expression. Erythroid-regulator activity in hpx mice can be diminished by RBC transfusions or transferrin (TF) administration.
Footnotes
Funding: TBB and MDF are supported by NIH K99DK084122 and R01DK080011, respectively.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Fleming MD. The regulation of hepcidin and its effects on systemic and cellular iron metabolism. Hematology Am Soc Hematol Educ Program. 2008:151–8. doi: 10.1182/asheducation-2008.1.151. [DOI] [PubMed] [Google Scholar]
- 2.Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117(2):630–7. doi: 10.1182/blood-2010-05-287359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J. Clin Invest. 2005;115(8):2180–6. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J. Clin Invest. 2005;115(8):2187–91. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt PJ, Andrews NC, Fleming MD. Hepcidin induction by transgenic overexpression of Hfe does not require the Hfe cytoplasmic tail, but does require hemojuvelin. Blood. 2010;116(25):5679–87. doi: 10.1182/blood-2010-04-277954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch C. Regulators of iron balance in humans. Blood. 1994;84(6):1697–702. [PubMed] [Google Scholar]
- 7.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–11. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanno T, Miller JL. Iron Loading and Overloading due to Ineffective Erythropoiesis. Adv Hematol. 2010;2010:358283. doi: 10.1155/2010/358283. [DOI] [PMC free article] [PubMed] [Google Scholar]