Abstract
Background
Patients with Chuvash polycythemia, (homozygosity for the R200W mutation in the von Hippel Lindau gene (VHL)), have elevated levels of hypoxia inducible factors HIF-1 and HIF-2, often become iron-deficient secondary to phlebotomy, and have elevated estimated pulmonary artery pressure by echocardiography. The objectives of this study were to provide a comprehensive echocardiographic assessment of cardiovascular physiology and to identify clinical, hematologic and cardiovascular risk factors for elevation of tricuspid regurgitation velocity in children and adults with Chuvash polycythemia.
Design and Methods
This cross-sectional observational study of 120 adult and pediatric VHLR200W homozygotes and 31 controls at outpatient facilities in Chuvashia, Russian Federation included echocardiography assessment of pulmonary artery pressure (tricuspid regurgitation velocity), cardiac volume, and systolic and diastolic function, as well as hematologic and clinical parameters. We determined the prevalence and risk factors for elevation of tricuspid regurgitation velocity in this population and its relationship to phlebotomy.
Results
The age-adjusted mean ± SE tricuspid regurgitation velocity was higher in VHLR200W homozygotes than controls with normal VHL alleles (2.5±0.03 vs. 2.3±0.05 m/sec, P=0.005). The age-adjusted left ventricular diastolic diameter (4.8±0.05 vs. 4.5±0.09 cm, P=0.005) and left atrial diameter (3.4±0.04 vs. 3.2±0.08 cm, P=0.011) were also greater in the VHLR200W homozygotes, consistent with increased blood volume, but the elevation in tricuspid regurgitation velocity persisted after adjustment for these variables. Among VHLR200W homozygotes, phlebotomy therapy was associated with lower serum ferritin concentration, and low ferritin independently predicted higher tricuspid regurgitation velocity (standardized beta=0.29; P=0.009).
Conclusions
Children and adults with Chuvash polycythemia have higher estimated right ventricular systolic pressure, even after adjustment for echocardiography estimates of blood volume. Lower ferritin concentration, which is associated with phlebotomy, independently predicts higher tricuspid regurgitation velocity (www.clinicaltrials.gov identifier NCT00495638).
Keywords: pulmonary hypertension, tricuspid regurgitation velocity, ferritin, VHL, hypoxia inducible factor
Introduction
Chuvash polycythemia is a disorder that is endemic in the Chuvash region of Russia, although it is not limited to this geographical area. It is characterized by homozygosity for a 598C>T mutation of the von Hippel Lindau gene (VHL), which leads to an R200W substitution in VHL protein.1 The VHL protein and prolyl hydroxylase domain proteins are critical to the oxygen-related regulation of cellular hypoxia inducible factor (HIF)-α subunit levels. VHLR200W protein has impaired interaction with HIF-1α and HIF-2α,1 and this leads to increased levels of HIF-1 and HIF-2 under normoxic conditions,2 as well as elevated concentrations of products of hypoxia-induced genes, such as serum vascular endothelial growth factor and endothelin-1.3 The clinical features of Chuvash polycythemia include rubor, benign vertebral hemangiomas, varicose veins, lower systemic blood pressures, premature mortality related to cerebral vascular events, and peripheral thrombosis.4
Pulmonary hypertension develops in individuals exposed to chronic hypoxia.5 Data from cell lines and rodent models support the role of dysregulated HIF-1 and HIF-2 expression in the development of pulmonary hypertension.6,7 Pulmonary hypertension develops in a murine model of Chuvash polycythemia, and this finding seems to be related specifically to increased HIF-2 activity.2 Furthermore, late-onset pulmonary arterial hypertension has been reported in association with polycythemia due to an activating HIF-2A mutation,8 and hif2+/− mice are protected from pulmonary hypertension during chronic hypoxia.9 In keeping with these observations, we observed that right ventricular systolic pressure as estimated by echocardiography10 was higher in 14 adults with Chuvash polycythemia than in 14 controls.3 Other investigators have documented elevated pulmonary artery pressure and other changes in pulmonary vascular physiology in 3 individuals with Chuvash polycythemia.11
Iron is needed for the activity of proly hydroxylase domain proteins (PHDs), the principal negative regulators of HIF-α subunit levels.12–13 Therefore, iron deficiency could exacerbate the hypoxic response and its effects. It was recently reported that iron chelation promoted and iron infusion limited the increase in systolic pulmonary artery pressure resulting from exposure to acute hypoxia in 16 normal volunteers.14 It has also been shown that iron depletion exacerbated the increase in systolic pulmonary artery pressure associated with high altitude hypoxia in 11 subjects and iron infusion ameliorated this increase in 22 subjects.5 These observations pointed to the possibility that iron status may influence the expression of pulmonary hypertension in Chuvash polycythemia as well. Furthermore, iron deficiency is common in patients with idiopathic pulmonary hypertension15–17 and pulmonary hypertension secondary to cyanotic congenital heart disease,18 and is associated with reduced exercise capacity.16–17 Iron therapy improved exercise capacity in such patients.18
Congenital polycythemia is a rare disorder that occurs sporadically around the world. It is remarkable to find a large group of affected individuals with a single genotype and a homogeneous ethnic and environmental background, as is found in Chuvashia. We therefore took the opportunity to study this unique population. The objectives of this study were: i) to assess differences in tricuspid regurgitation velocity and other cardiovascular parameters in a large group of children and adults with Chuvash polycythemia relative to control subjects; and ii) to identify clinical, hematologic and cardiovascular risk factors for elevation of tricuspid regurgitation velocity and potential implications for management.
Design and Methods
Study design
We conducted a cross-sectional observational study of children and adults with the diagnosis of familial polycythemia in the Chuvash Republic of the Russian Federation (elevation <230 m) and controls matched by age and sex to every fourth polycythemia subject. Written informed consent was obtained from or on behalf of each participant. This study was approved by the Institutional Review Boards of Children’s National Medical Center, Howard University, and the National Institutes of Health,
Study participants
Subjects were not hospitalized and were at steady state (at least four weeks since last phlebotomy). History of smoking and phlebotomy therapy was recorded. Venous blood was drawn for complete blood count by an automated analyzer (Sysmex XT 2000i, Sysmex Corporation, Kobe, Hyogo, Japan) and genotyping for the Chuvash VHL mutation.
Echocardiograms
A pediatric (CS) and adult (VS) cardiologist blinded to the subject’s classification interpreted pediatric and adult studies, respectively. Echocardiography was performed according to the guidelines of the American Society of Echocardiography.19 For pediatric studies, diameters were compared to those of a large cohort of normal infants and children20 and expressed as a standard deviation below or above the mean for body surface area (z-score). Continuous-wave Doppler of the peak tricuspid regurgitation velocity was used to estimate the right ventricular to right atrial pressure gradient with the use of the modified Bernoulli equation in patients with measurable tricuspid regurgitation velocity.10;21 The absence of measurable tricuspid regurgitation velocity, which is found in normal practice in up to 1 in 3 patients, has no correlation with pulmonary artery pressure.10 Left ventricular diastolic function was assessed by calculating the velocity ratios of the peak Doppler mitral inflow E wave to both the inflow A wave,21–22 and the tissue Doppler E wave at the basilar segments of the left ventricular free wall and interventricular septum. Blood pressure was measured once at the start of each echocardiogram in the supine position to provide accurate correlation with echocardiography data, according to the policy of our echocardiography laboratory.
Biological markers
Plasma interleukins-6, 8 and 10, tumor necrosis factor-α, inter-feron-γ, basic fibroblastic growth factor, monocyte chemoattractant protein-1 (MCP-1), platelet-derived growth factor-BB (PDGF), CCL5 [chemokine (C-C motif) ligand 5], and vascular endothelial growth factor A (VEGF) were assayed using the Bio-Plex suspension array system (Bio-Rad, Hercules, CA, USA).23 Serum erythropoietin (R&D Systems, Minneapolis, MN, USA; reference range 3.3–16.6 IU/L) and ferritin (Ramco Laboratories Inc., Stafford, TX, USA; reference range 20–300 ng/mL) were measured with enzyme immunoassays, and NT-proBNP with an electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analysis
Skewed linear variables were transformed to provide the best approximation to normal. Linear variables were compared with the Student’s t-test or analysis of variance with adjustment for important co-variates. Categorical variables were compared with Pearson’s χ2 test. P<0.05 was considered to be significant. Multiple linear regression was used to assess independent associations with tricuspid regurgitation velocity. Variables with a P<0.2 in bivariate analysis were entered into the initial model and a stepwise approach was applied to select the final model. Analyses were performed with STATA 10.1 (StataCorp, College Station, TX, USA). A pathway analysis based on the findings from the multiple linear regression analysis was made to confirm the observed associations with TRV in VHLR200W homozygotes using AMOS 18.0 (IBM, Somers, NY, USA).
Results
VHL genotyping
A total of 123 Chuvash polycythemia subjects (26 aged 21 years or younger) and 35 controls (11 aged 21 years or younger) were enrolled. On genotyping, 120 of the subjects with a clinical diagnosis of Chuvash polycythemia proved to be VHLR200W homozygotes, one to be a VHLR200W heterozygote, and two to be VHL wild type. Thirty-one of the controls were genotyped to be VHL wild type and 4 to be VHLR200W heterozygotes. Only the 120 subjects with the diagnosis of Chuvash polycythemia who were VHLR200W homozygotes and 31 controls who were VHL wild type are included in this report.
Comparison of VHLR200W homozygotes and VHL wild-type controls
Demographics and clinical variables (Table 1)
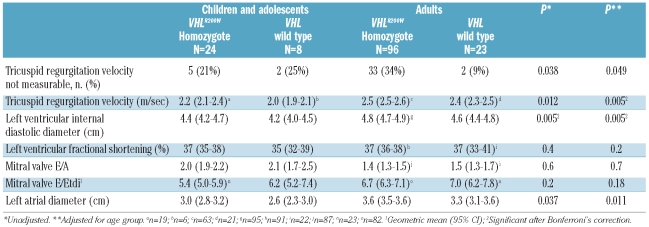
Table 1.
Comparison of demographic and clinical variables in VHLR200W homozyogotes and VHL wild-type controls. Results are in geometric mean (95% CI) unless otherwise indicated.
The hemoglobin concentrations and serum erythropoietin concentrations were higher in the VHLR200W homozygotes than controls, while systolic blood pressures were lower (Table 1) in agreement with previously published data.4;24 Phlebotomy had been performed in 73% of the VHLR200W homozygotes; 83% of adults and 29% of children and adolescents. Serum ferritin concentrations were lower in the VHLR200W homozygotes, consistent with the frequent history of phlebotomy. The geometric mean serum ferritin concentrations were below the lower limit of the reference range in both the pediatric and the adult VHLR200W homozygotes.
Echocardiographic variables (Table 2)
Table 2.
Echocardiography variables in VHLR200W homozygotes and VHL wild-type controls. Results are in arithmetic mean (95% CI) unless otherwise indicated.
The tricuspid regurgitation velocity was measurable in 27 (87.1%) of the VHL wild-type controls and 82 (68.3%) of the VHLR200W homozygotes. No patients or control subjects were seen to have a dilated inferior vena cava. Among the VHLR200W homozygotes, the mean±SD hemoglobin concentration was 17.7±3.2 g/dL among those with a measurable tricuspid regurgitation velocity compared to 18.5±2.3 g/dL in those without a measurable tricuspid regurgitation velocity (P=0.16).
The tricuspid regurgitation velocity was higher in VHLR200W homozygotes than controls (Table 2). Among combined adult and pediatric subjects, the mean ± SE age-adjusted tricuspid regurgitation velocity was 2.5±0.03 m/sec in 82 VHLR200W homozygotes compared to 2.3±0.05 m/sec in 27 controls (P=0.005), and this difference persisted after adjustment for gender and hemoglobin concentration (P=0.007). The tricuspid regurgitation velocity was greater than 3.0 m/sec in 8 (9.8%) of the VHLR200W homozygotes and no controls. A velocity of 3.0 m/sec is above the 95% confidence interval of values in the general population25 and above the value recommended for further investigation in persons with dyspnea.26
There was no significant difference in left ventricular shortening fraction, mitral valve E/A ratio and ratio of the mitral valve inflow Doppler to tissue Doppler E wave (E/Etdi) between VHLR200W homozygotes and control subjects, suggesting no alterations in left ventricular systolic or diastolic function. Left ventricular diastolic and left atrial diameters were higher in VHLR200W homozygotes, consistent with increased blood volume and flow. Among adult and pediatric subjects combined, the age-adjusted left ventricular internal diastolic diameter was 4.8±0.05 cm in 119 VHLR200W homozygotes versus 4.5±0.09 cm in 31 controls (P=0.005), and the left atrial diameter was 3.4±0.04 cm in VHLR200W homozygotes versus 3.2±0.08 cm in controls (P=0.011). The increase in tricuspid regurgitation velocity persisted after adjustment for these variables.
In a pediatric subgroup analysis, the 19 VHLR200W homozygotes had a higher mean tricuspid regurgitation velocity than the 6 VHL wild-type subjects with measurable tricuspid regurgitation velocity: mean±SD of 2.2±0.1 m/sec versus 2.0±0.3 m/sec (P=0.017). There were no significant differences in measurements of chamber size or left ventricular systolic and diastolic function between VHLR200W homozygote children and controls, but the low sample size means that perhaps there was not sufficient power to detect any such differences.
Clinical findings according to histories of phlebotomy therapy or smoking in VHLR200W homozygotes
Chuvash polycythemia patients with a history of phlebotomy were older than those without (40±14 vs. 25±19 years; P<0.0001) and had lower serum ferritin concentrations (geometric mean [SD range] values of 10 [3–32] vs. 17 [6–46] μg/L; P=0.035) and higher values for systolic blood pressure (114±16 vs. 105±16 mm Hg; P=0.003), diastolic blood pressure (79±10 vs. 71±11 mm Hg; P=0.0002), serum erythropoietin (50 [21–132] vs. 28 [10–75] U/L; P=0.005) and tricuspid regurgitation velocity (2.6±0.3 vs. 2.4±0.4 m/sec.; P=0.023). However, hemoglobin concentration (17.9±2.9 vs. 18.1±3.1 g/dL; P=0.7) was not significantly lower.
VHLR200W homozygotes with a smoking history had higher diastolic blood pressure (80±10 vs. 76±11 mm Hg; P=0.034) and lower platelet counts (202 ±96×109/L vs. 240 ± 78×109/L; P=0.022) compared to VHLR200W homozygotes with no smoking history, but there was no significant difference in tricuspid regurgitation velocity. There was a significant difference in tricuspid regurgitation velocity between VHLR200W non-smoking homozygote adults and non-smoking control adults (2.6±0.3 vs. 2.4±0.2 m/sec, P=0.009).
Potential predictors of higher tricuspid regurgitation velocity in VHLR200W homozygotes
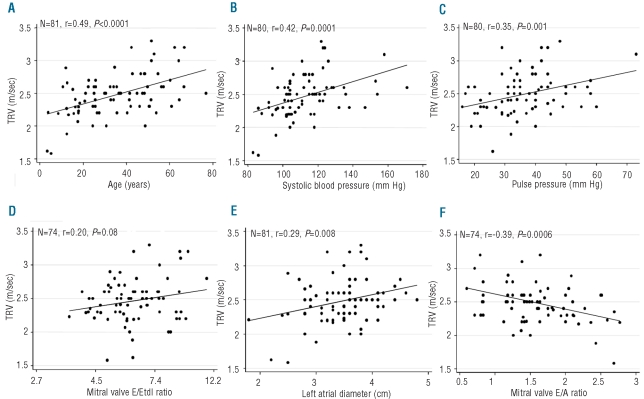
Figure 1 shows the univariate relationships of age, systolic blood pressure, pulse pressure, E/Etdi ratio, left atrial diameter, and E/A ratio with tricuspid regurgitation velocity. Increasing age, higher values for systemic systolic blood pressure, systemic pulse pressure, E/Etdi ratio, left atrial diameter and lower values for E/A ratio were associated with a higher velocity. There was no significant correlation between left ventricular internal diastolic diameter and tricuspid regurgitation velocity (n=81, r=0.17, P=0.13). Figure 2 shows the relationships of phlebotomy history, hemoglobin, and serum concentrations of erythropoietin and ferritin with tricuspid regurgitation velocity. There were trends for associations of phlebotomy, higher erythropoietin and lower serum ferritin with higher tricuspid regurgitation velocity, but there was no relationship between hemoglobin and higher velocity. There were no significant relationships between biomarkers of inflammation or vasculogenesis with tricuspid regurgitation velocity.
Figure 1.
Potential clinical and echocardiographic predictors of tricuspid regurgiation velocity. Relationships of tricuspid regurgitation velocity (TRV) with (A) age, (B) systolic blood pressure, (C) pulse pressure, (D) mitral valve E/Etdi ratio, (E) left atrial diameter, and (F) E/A ratio in VHLR200W homozygotes.
Figure 2.
Hemoglobin, erythropoietin and ferritin as potential predictors of tricuspid regurgitation velocity. Relationships of tricuspid regurgitation velocity (TRV) with (A) hemoglobin concentration, (B) history of phlebotomy, (C) serum erythropoietin concentration and (D) serum ferritin concentration in VHLR200W homozygotes.
Independent predictors of elevated tricuspid regurgitation velocity (Table 3)
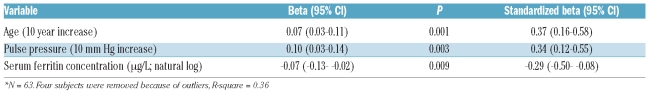
Table 3.
Independent predictors of tricuspid regurgitation velocity by multiple linear regression among VHLR200W homozygotes.*
In multiple linear regression analysis, only higher age (P=0.001), higher pulse pressure (P=0.0003) and lower serum ferritin concentration (P=0.009) were independent predictors of higher tricuspid regurgitation velocity (Table 3). Although there was a significant negative correlation between serum concentrations of erythropoietin and ferritin (n=93, r=−0.31, P=0.003), the effect of ferritin could not be replaced by erythropoietin in this multiple linear regression analysis (P=0.16). According to this model, a decline in the serum ferritin concentration from 75 μg/L to 10 μg/L (representing a decrease of two natural logs) would be associated with a 0.14 m/sec increase in tricuspid regurgitation velocity if age and pulse pressure were constant. In these analyses, gender was not significantly related to tricuspid regurgitation velocity.
Pathway analysis of tricuspid regurgitation velocity in VHLR200W homozygotes
The results of the multiple linear regression analysis of tricuspid regurgitation velocity were confirmed in a pathway analysis (Figure 3). In this analysis, older age (P<0.001) and higher pulse pressure (P<0.001) are directly associated with higher tricuspid regurgitation velocity, but higher serum ferritin (P=0.009) is directly associated with lower TRV. Older age is also directly associated with higher pulse pressure (P=0.021) and with a history of phlebotomy (P<0.001). In turn, history of phlebotomy is directly associated with lower serum ferritin (P=0.05).
Figure 3.
Pathway analysis of tricuspid regurgitation velocity (TRV) in VHLR200W homozygotes. Older age (standardized β=0.42; P<0.001) and higher pulse pressure (standardized β=0.31; P<0.001) are directly associated with higher TRV, but higher serum ferritin (standardized beta=−0.25; P=0.009) is directly associated with lower TRV. Older age is also directly associated with higher pulse pressure (standardized beta=0.21; P=0.021) and with a history of phlebotomy (standardized b=0.37; P <0.001). In turn, history of phlebotomy is directly associated with lower serum ferritin (standardized β=−0.20; P=0.05). This pathway fits the data (root mean square error approximation=0.0, 90% CI: 0.00–0.08) and is supported by data (χ2=1.4, P=0.8).
Discussion
This is the largest report of echocardiographic findings in subjects with Chuvash polycythemia, a unique homogenous population with congenital upregulation of hypoxia sensing. It is also the first report to include children and provide a more complete assessment of cardiovascular physiology by echocardiography. We confirmed previous findings based on smaller numbers of patients3,27 that Chuvash polycythemia is associated with lower systolic blood pressure and higher tricuspid regurgitation velocity than control subjects. We also found that, in contrast to previous reports,3 the left atrial and left ventricular internal diastolic diameters were larger in patients with Chuvash polycythemia compared to controls. This is consistent with the known increase in blood volume associated with polycythemic states. However, the increase in tricuspid regurgitation velocity with Chuvash polycythemia persisted after adjusting by linear regression for the left ventricular internal diastolic diameter, the left atrial diameter or the hemoglobin concentration. Other important findings were that no significant differences in echocardiographic measurements of left ventricular systolic or diastolic function were observed.
Among the subjects with Chuvash polycythemia, we found that age, systemic pulse pressure, and reduced iron stores as reflected in lower serum ferritin were independent predictors of higher tricuspid regurgitation velocity. Systemic pulse pressure, which reflects systemic arterial stiffness, may also reflect pulmonary artery stiffness and has been associated with increased pulmonary pressure in a population study.28 Our finding of an association between low serum ferritin and higher tricuspid regurgitation velocity represents the first clinical evidence of modulation of a congenital disorder of oxygen sensing by iron status.
Elevation of tricuspid regurgitation velocity by echocardiography is common in hemolytic hematologic disorders such as sickle cell anemia, thalassemia and paroxysmal nocturnal hemoglobinuria,29–33 and potential contributing mechanisms include a hemolysis-induced vasculopathy,34 volume overload, and diastolic dysfunction.29,35 In contrast to sickle cell disease, we found no evidence that subjects with Chuvash polycythemia had significant hemolysis or diastolic dysfunction. However, there was an inverse correlation between tricuspid regurgitation velocity and E/A ratio, suggesting a possible role of diastolic function. As noted above, the elevation in tricuspid regurgitation velocity in Chuvash polycythemia subjects persisted after adjustment for measurements reflecting volume overload.
PHDs are enzymes that require oxygen as a substrate and that serve to hydroxylate HIF-1α and HIF-2α on specific proline residues; this proline hydroxylation is required for the interaction of HIF-1α and HIF-2α with VHL.12,36 The VHL protein is the recognition component of an E3 ubiquitin-protein ligase complex that mediates pro-teasomal degradation of HIF-1α and HIF-2α under normoxic conditions.37 Thus, HIF-1 and HIF-2 levels rise in response to hypoxia and this leads to increased expression of erythropoietin38 and other hypoxia-induced genes.36 We have postulated that increased levels of HIFs during normoxia and elevated concentrations of products of hypoxia-induced genes may play a role in the development of pulmonary vascular changes in subjects with Chuvash polycythemia.3 In keeping with this possibility, pulmonary hypertension in a murine model of Chuvash polycythemia seems to be mediated by elevated HIF-2.6 Likewise, high altitude dwellers of Tibet39 and the Andes mountains,40 continuously exposed to high altitude hypoxia, may develop pulmonary hypertension, and the pulmonary hypertension appears to be mediated by elevated HIF-1 and HIF-2 levels.6,41 Conversely, partial deficiency of HIF-2 expression protects against hypoxia-induced pulmonary hypertension in mice.9
It has recently been suggested that inactivation of PHDs may promote the detrimental effects of augmented HIF-1 and HIF-2 levels.13 PHDs (at least 3 isoforms with tissue specific expressions) are oxygen- and iron-dependent enzymes and their reduced activity inhibits the degradation of the alpha subunits of HIF-1 and HIF-2.6,12,36,41 Decreased PHD activity could result in increased levels of erythropoietin as well as other HIF-controlled genes, as suggested by the report of exacerbation of high altitude pulmonary hypertension by desferrioxamine administration recently published by Smith and colleagues.5 Similarly, in the Chuvash polycythemia subjects, iron deficiency would be predicted to inactivate PHDs leading to decreased degradation of HIF-1α and HIF-2α and further augmentation of the increased HIF activity that occurs due to the genetic loss of VHL function.1,12 Consistent with this model, in cultured cells, iron chelating drugs induce the alpha subunits of HIF,42–43 likely via PHD inhibition, and apparently overriding a possible confounding effect of iron in augmenting HIF-2α translation by inhibiting iron response proteins.44
Although the use of tricuspid regurgitation velocity to assess right ventricular systolic pressure by echocardiography has been well validated, there are several pitfalls. The absence of a tricuspid regurgitation velocity signal does not exclude the patient from having an elevated pressure.45 Additionally, if proper alignment cannot be obtained, a true tricuspid regurgitation velocity may be underestimated. Higher hemoglobin concentration may directly contribute to elevated pulmonary artery pressure in patients with chronic emphysema,46 and it is conceivable that an expanded blood volume contributed to elevated estimated systolic pulmonary artery pressure in the patients with Chuvash polycythemia in this study. Higher hemoglobin and increased blood viscosity may result in fewer patients with measurable tricuspid regurgitation among VHLR200W homozygotes. However, the higher tricuspid regurgitation velocity in VHLR200W homozygotes compared to controls, and the relationship of lower ferritin with higher tricuspid regurgitation velocity persisted after adjusting for surrogates of volume expansion: hemoglobin concentration, left ventricular internal diastolic diameter and left atrial diameter.
It would be interesting to determine whether iron deficiency influences pulmonary artery pressure in patients with other types of polycythemia in whom pulmonary hypertension does not appear to be related to up-regulated hypoxia sensing. However, our study was limited to subjects with a VHL mutation. Smith et al. speculated that the cardiopulmonary abnormalities in Chuvash polycythemia result directly from dysregulation of the VHL pathway rather than from the hematologic manifestations of the disease, based on findings from 12 subjects with Chuvash polycythemia and 3 with other forms of polycythemia.27
The independent association of elevated tricuspid regurgitation velocity with low serum ferritin concentration in the present study, along with the recently reported association of high altitude pulmonary hypertension with iron deficiency,5 may have implications for the clinical management of polycythemic patients. In the present study, a history of phlebotomy was the primary predictor of iron deficiency as indicated by a low serum ferritin concentration. It is possible that the relative reduction of red cell mass and oxygen carrying capacity to “normal” from very high levels with phlebotomy therapy might also give rise to relatively lower oxygen delivery to tissue, inducing HIF-1 and HIF-2 compared to the polycythemic state. Thus, the findings of this study warrant reassessment of phlebotomy therapy in polycythemic patients, which induces iron deficiency. We propose that a longitudinal study of Chuvash polycythemia patients before and after phlebotomy should be carried out to further assess the effect of phlebotomy therapy on right ventricular systolic pressure.
Footnotes
Funding: supported in part by grants ns. 2 R25 HL003679-08 and 1 R01 HL079912-02 from NHLBI, by Howard University GCRC grant n. 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD, USA, and by the intramural research program of the National Institutes of Health.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32(4):614–21. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 2.Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117(12):3879–89. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushuev VI, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin D, Gaskin PR, et al. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91(6):744–9. [PubMed] [Google Scholar]
- 4.Gordeuk VR, Sergueeva AI, Miasnikova GY, Okhotin D, Voloshin Y, Choyke PL, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103(10):3924–32. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 5.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA. 2009;302(13):1444–50. doi: 10.1001/jama.2009.1404. [DOI] [PubMed] [Google Scholar]
- 6.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120(3):827–39. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Involvement of hypoxia-inducible factor 1 in pulmonary pathophysiology. Chest. 2005;128(6 Suppl):592S–4S. doi: 10.1378/chest.128.6_suppl.592S. [DOI] [PubMed] [Google Scholar]
- 8.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358(2):162–8. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, et al. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111(10):1519–27. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359–65. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 11.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, et al. Mutation of the von Hippel-Lindau gene alters human cardiopulmonary physiology. Adv Exp Med Biol. 2008;605:51–6. doi: 10.1007/978-0-387-73693-8_9. [DOI] [PubMed] [Google Scholar]
- 12.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Minamishima YA, Kaelin WG., Jr Reactivation of hepatic EPO synthesis in mice after PHD loss. Science. 2010;329(5990):407. doi: 10.1126/science.1192811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, et al. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol. 2008;586(Pt 24):5999–6005. doi: 10.1113/jphysiol.2008.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soon E, Treacy CM, Toshner MR, MacKenzie-Ross R, Manglam V, Busbridge M, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66(4):326–32. doi: 10.1136/thx.2010.147272. [DOI] [PubMed] [Google Scholar]
- 16.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37(6):1386–91. doi: 10.1183/09031936.00100510. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58(3):300–9. doi: 10.1016/j.jacc.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 18.Tay EL, Peset A, Papaphylactou M, Inuzuka R, Alonso-Gonzalez R, Giannakoulas G, et al. Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the Eisenmenger syndrome. Int J Cardiol. 2011;151(3):307–12. doi: 10.1016/j.ijcard.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 19.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19(12):1413–30. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen MD, Du W, Skeens ME, Humes RA. Regression Equations for Calculation of Z Scores of Cardiac Structures in a Large Cohort of Healthy Infants, Children, and Adolescents: An Echocardiographic Study. J Am Soc Echocardiogr. 2008;21(8):922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 23.Niu X, Nouraie M, Campbell A, Rana S, Minniti CP, Sable C, et al. Angiogenic and inflammatory markers of cardiopulmonary changes in children and adolescents with sickle cell disease. PLoS One. 2009;4(11):e7956. doi: 10.1371/journal.pone.0007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordeuk VR, Prchal JT. Vascular complications in Chuvash polycythemia. Semin Thromb Hemost. 2006;32(3):289–94. doi: 10.1055/s-2006-939441. [DOI] [PubMed] [Google Scholar]
- 25.Bossone E, Rubenfire M, Bach DS, Ricciardi M, Armstrong WF. Range of tricuspid regurgitation velocity at rest and during exercise in normal adult men: implications for the diagnosis of pulmonary hypertension. J Am Coll Cardiol. 1999;33(6):1662–6. doi: 10.1016/s0735-1097(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 27.Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, et al. Mutation of von Hippel-Lindau tumour suppressor and human cardiopulmonary physiology. PLoS Med. 2006;3(7):e290. doi: 10.1371/journal.pmed.0030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dham N, Ensing G, Minniti C, Campbell A, Arteta M, Rana S, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104(5):713–20. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 31.Hill A, Rother RP, Wang X, Morris SM, Jr, Quinn-Senger K, Kelly R, et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2010;149(3):414–25. doi: 10.1111/j.1365-2141.2010.08096.x. [DOI] [PubMed] [Google Scholar]
- 32.Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris CR, Vichinsky EP. Pulmonary hypertension in thalassemia. Ann NY Acad Sci. 2010;1202:205–13. doi: 10.1111/j.1749-6632.2010.05580.x. [DOI] [PubMed] [Google Scholar]
- 34.Kato GJ, Taylor JG. Pleiotropic effects of intravascular haemolysis on vascular homeostasis. Br J Haematol. 2010;148(5):690–701. doi: 10.1111/j.1365-2141.2009.08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472–9. doi: 10.1016/j.jacc.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda ) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 37.Safran M, Kaelin WG., Jr HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111(6):779–83. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, et al. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133(2):223–34. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge RL, Ma RY, Bao HH, Zhao XP, Qi HN. Changes of cardiac structure and function in pediatric patients with high altitude pulmonary hypertension in Tibet. High Alt Med Biol. 2009;10(3):247–52. doi: 10.1089/ham.2009.0001. [DOI] [PubMed] [Google Scholar]
- 40.Reeves JT, Grover RF. Insights by Peruvian scientists into the pathogenesis of human chronic hypoxic pulmonary hypertension. J Appl Physiol. 2005;98(1):384–9. doi: 10.1152/japplphysiol.00677.2004. [DOI] [PubMed] [Google Scholar]
- 41.Formenti F, Beer PA, Croft QP, Dorrington KL, Gale DP, Lappin TR, et al. Cardiopulmonary function in two human disorders of the hypoxia-inducible factor (HIF) pathway: von Hippel-Lindau disease and HIF-2alpha gain-of-function mutation. FASEB J. 2011;25(6):2001–11. doi: 10.1096/fj.10-177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SS, Bae I, Lee YJ. Flavonoids-induced accumulation of hypoxia-inducible factor (HIF)-1alpha/2alpha is mediated through chelation of iron. J Cell Biochem. 2008;103(6):1989–98. doi: 10.1002/jcb.21588. [DOI] [PubMed] [Google Scholar]
- 43.Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J Biol Chem. 2002;277(42):40112–7. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14(5):420–6. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 45.Pashankar FD, Carbonella J, Bazzy-Asaad A, Friedman A. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121(4):777–82. doi: 10.1542/peds.2007-0730. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura A, Kasamatsu N, Hashizume I, Shirai T, Hanzawa S, Momiki S, et al. Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration. 2000;67(5):502–6. doi: 10.1159/000067463. [DOI] [PubMed] [Google Scholar]