Abstract
Studies have questioned whether renal dysfunction in sickle cell disease is linked to hemolysis-associated vasculopathy. We have investigated renal function and markers of hemolysis in a cohort of 424 adult African-British patients with sickle cell disease. While significant associations were found in HbSS and HbSβ0 (sickle cell anemia) patients with and without controlling for covariates between hemolytic markers and albuminuria, the associations were not significant in patients with HbSC. Estimated glomerular filtration rate, a marker of renal function, correlated significantly with reticulocyte count and bilirubin. Alpha thalassemia, present in 34% of the sickle cell anaemia patients, had a protective effect against albuminuria in this group. Altogether, the incidence of hyperfiltration was 71% and microalbuminuria 37%, making nephropathy a common complication of sickle cell anemia.
Keywords: sickle cell disease, nephropathy, hemolysis, kidney
Introduction
Sickle cell nephropathy (SCN) is a highly prevalent and potentially life-threatening complication of sickle cell anemia (SCA). End-stage renal failure is present in around 11% of patients with SCA, and this increases markedly with age.1–2 As life expectancy continues to improve in patients with SCA,3 we can expect the proportion of those suffering from chronic organ damage, such as SCN, to increase.2
Hyperfiltration and hyposthenuria (inability to concentrate urine appropriately) are the earliest manifestations of SCN.4 It is thought that chronic ischemia secondary to repeated vaso-occlusive events in the microvasculature of the renal medulla results in a prostaglandin-induced increase in total renal plasma flow leading to the raised glomerular filtration rate (GFR) seen in childhood and early adulthood.4–5 Prolonged hyperfiltration eventually leads to glomerular sclerosis and the GFR decreases towards normal as the patient ages.4 In some, this decline is accelerated and continuous resulting in end-stage renal failure.6 The glomerular injury often also results in proteinuria, which may be severe enough to cause nephrotic syndrome in some patients.4 Microalbuminuria is an early manifestation of proteinuria in SCN and other non-sickle nephropathies; its presence is known to correlate with an increase in all-cause mortality.7
In addition to vaso-occlusion, chronic hemolysis is also a fundamental pathological process that occurs in SCA. Indeed, some have suggested that SCA can be split into two sub-phenotypes based on the relative dominance of one or other of these processes in individual patients.8–10 Pulmonary hypertension, a known complication in patients with the hemolytic sub-phenotype of SCD, is thought to be caused by vasculopathy related to the chronic nitric oxide depletion from continuing intravascular hemolysis.11 Although the markers of SCN are known to predict the presence of pulmonary hypertension,12 it is still not clear if both conditions share the same pathological process. In children with SCA, significant associations have been found between proteinuria and the hemolysis markers of low hemoglobin (Hb) and high lactate dehydrogenase (LDH).5,13 Similar studies in adults, however, have not replicated this finding, although an association between low hemoglobin levels and hyperfiltration have been reported.14–15 More recently, Maier-Redelsperger et al. utilized a marker of red cell survival derived from mature red blood cell hemoglobin (RBC Hb) and reticulocyte hemoglobin (RET Hb), and showed that the log (RBC Hb/RET Hb) was highly correlated with LDH, bilirubin and albuminuria.16
In the present study, we have carried out a retrospective investigation of the prevalence of albuminuria and hyperfiltration in a larger cohort of adults with SCD. We have also examined the associations between sickle cell nephropathy and markers of hemolysis, including RBC Hb/RET Hb to further clarify the pathogenesis of this increasingly important complication.
Design and Methods
The study group was drawn from a cohort of 484 patients with HbSS, HbS/β0 or HbSC who had been attending the specialist clinic at King’s College Hospital, London, since January 2006. Patients were excluded from the study group if they had a history of diabetes mellitus, HIV infection, malignancy or another cause of chronic kidney disease (CKD) or if they were on a blood transfusion program. Seventeen patients were on ACE (angiotensin converting enzyme) inhibitor or ARB (angiotensin II receptor blocker) therapy; these patients were also excluded from the study. Since 2006, urinary albumin/creatinine ratio (ACR) has been a routine measurement for patients attending the specialist clinic. All patients were routinely genotyped for α thalassemia using a PCR-based methodology on DNA extracted from peripheral blood.17
Hematologic and biochemical data were extracted from the electronic patient record (EPR) system into an anonymized database. Estimated glomerular filtration rates (eGFRs) were calculated using the 4-point Modification of Diet in Renal Disease (MDRD) formula. Bilirubin was always adjusted for uridine diphosphoglu-curonate glucuronosyltransferase 1A (UGT1A) genotype, a known genetic modifier of bilirubin levels in SCD.18 P<0.05 was considered significant. Data were managed and analyzed using Excel 2007 (Microsoft, Seattle, USA) and Prism 3 (GraphPad, San Diego, CA, USA). Regression analysis was performed in Stata 11 (Stata Corp). All analyses pooled all data from each individual allowing for dependency between multiple observations on the same individual using random effects models fitted either using generalized least squares or, when the dependent variable was either binary (logistic regression) or censored (interval regression), by maximum likelihood. ACR was found to be skewed and was, therefore, analyzed in the logarithm which rendered it more normally distributed. ACR was also analyzed as a dichotomous variable cut off at 4.5 mg/mmol (defined as microalbuminuria). Effect sizes were expressed as changes (or percentage changes or odds ratio (OR) depending on the dependent variable) per standard deviation (SD) of the dependent variable of interest. All analyses were repeated both with and without the presence of other covariates (age, sex, α thalassemia and UGT1A genotypes, white blood cell (WBC) count and hydroxycarbamide treatment). In order to capture the complex nature of the relationships with age, the age pattern was fitted as a cubic spline on 4 degrees of freedom (i.e. using four evenly spaced nodes).
Results and Discussion
After application of the various exclusion and inclusion criteria, the final study group consisted of 424 patients (253 HbSS, 7 HbSβ0 thalassemia, and 164 HbSC) of which 255 (60%) were female. Patients with HbSS and HbSβ0 thalassemia (HbSβ0) were analyzed as one group (total 260 and considered as SCA) but patients with HbSC were analyzed separately. The mean age of participants was 33 years for patients with HbSS/HbSβ0 (range 17–70, SD 11), and 39 years for patients with HbSC (range 17–80, SD 12).
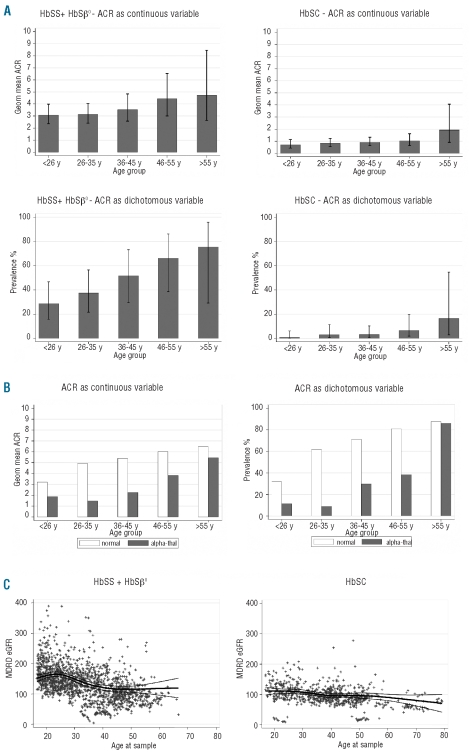
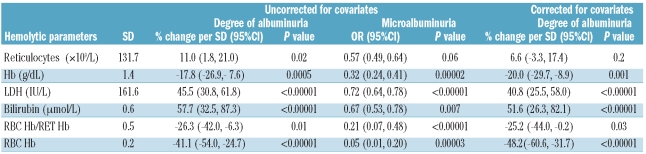
In the HbSS/HbSβ0 group, increasing age correlated with the degree of albuminuria (P=0.005, r2=0.06), and with the prevalence of microalbuminuria (Figure 1A). Table 1 describes the linear relationship of hemolytic markers with ACR and eGFR; a negative value (i.e. Hb, RBC Hb/RET Hb, and RBC Hb) denotes an inverse trend. When analyzed without controlling for covariates the degree of albuminuria was significantly predicted by all the variables relating to hemolysis, including RBC Hb/RET Hb (Table 1A). Bilirubin and LDH were the strongest predictors (P<0.00001% change/SD 57.7 and P<0.00001% change/SD 45.5, respectively). Reticulocyte count was the weakest predictor (P=0.02 % change/SD 11.0). RBC Hb, calculated by subtracting reticulocyte Hb (absolute reticulocyte count x reticulocyte Hb) from total Hb, was a stronger negative predictor of albuminuria than Hb (P<0.00001 %change/SD −41.1 vs. P=0.0005 %change/SD −17.8). When ACR was analyzed as a dichotomous variable (≥4.5mg/mmol referred to as microalbuminuria) the associations persisted; LDH remained the strongest predictor (P<0.00001, OR/SD 0.72) and reticulocyte count remained the weakest (P=0.06, OR/SD 0.57) (Table 1A). In the multivariate analysis, the correlation with reticulocyte count was lost, while other correlations remained significant (Table 1A).
Figure 1.
(A) ACR as a continuous (upper panels) and dichotomous (lower panels) variable vs. age in HbSS + HbSβ0 group (left), and HbSC group (right) panels. Bar charts: error bars=95% confidence interval. The geometric (geom) mean was derived by anti-logging the fitted values of the interval regression of log(ACR) on age group (as categorical variable). When ACR was analyzed as a dichotomous variable, a cut-off value of ≥4.5mg/mmol was applied (referred to as microalbuminuria). (B) Effect of α-thalassemia status in HbSS + HbSβ0 on ACR with age as a continuous (left: P<0.0005, 95%CI −1.22,−0.38) and dichotomous variable (right panel; P=0.001, 95%CI −3.01,−0.82). The geometric (geom) mean was derived by anti-logging the fitted values of the interval regression of log(ACR) on age group (as categorical variable), the binary variable for alpha-thal (indicating the presence of one or more deletions) and their interaction. When ACR was analyzed as a dichotomous variable, a cut-off value of ≥4.5mg/mmol was applied (referred to as microalbuminuria). (C) Distribution of MDRD eGFR according to genotype and age in HbSS + HbSβ0 and HbSC group. Thick line represent cubic spline fit; thin lines represent 95% confidence interval of fit.
Table 1A.
Relationship of hemolytic parameters to ACR in HbSS + HbSβ0 group.
Alpha genotype was available in 208 patients of the HbSS/HbSβ0 group; 119 (57%) had normal α genotype (αα/αα), 88 (42%) patients had alpha trait (68 αα/α- and 20 α-/α-) and one (<1%) had extra α globin genes (ααα/αα). The patient with extra α globin genes was excluded from this analysis. Among the 207 patients of all ages, the presence of α-thalassemia trait was seen to be protective for the degree (P<0.0005, 95% CI −1.22, −0.38) and prevalence of microalbuminuria, i.e. when albuminuria was treated as a dichotomous variable (P=0.001, 95% CI −3.01, −0.82) (Figure 1B). Thirty-four of 260 SCA patients were on hydroxycarbamide (HC) for a minimum of six months during the study period. HC has a small but significant predictive value for degree of albuminuria (P=0.05, 95%CI 0, 232) but not presence (P = 0.1, 95% CI −0.22, 2.76) of microalbuminuria. There were no significant difference in the eGFR between the HC group and non-HC group (data not shown).
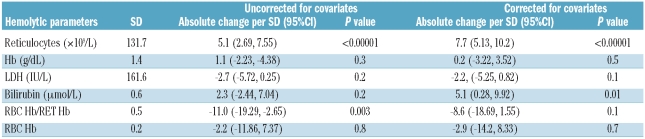
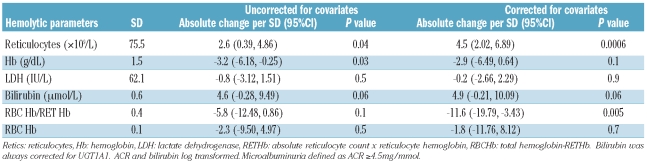
One hundred and eighty-five of 260 (71%) of the HbSS/HbSβ0 group had at least one eGFR of 140 ml/min/1.73m2 or over during the study period. Overall, significant negative correlation was seen between eGFR and age (P<0.0005, r2= 0.22) (Figure 1C) although eGFR appears positively correlated in the under-25 age group. Reticulocyte count was the strongest predictor of eGFR when uncorrected (P<0.00001, absolute effect per SD 5.1) and when corrected for covariates (P<0.00001 absolute effect per SD 7.7) (Table 1B). Bilirubin only became a significant predictor after controlling for covariates (P<0.01 effect per SD 5.1) (Table 1B).
Table 1B.
Relationship of hemolytic parameters to eGFR in HbSS + HbSβ0 group.
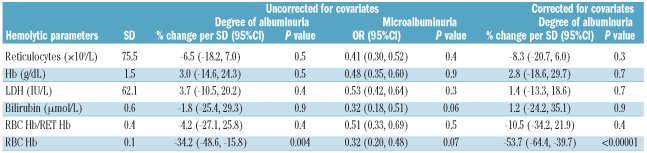
Forty-one of 164 (26%) HbSC had at least one sample with an ACR of 4.5 mg/mmol or over (i.e. microalbuminuria). There was no significant association between degree of albuminuria and age, although there was an increase in the prevalence of microalbuminuria in this group with age (Figure 1A). Only RBC-Hb was a significant predictor of degree of albuminuria without (P=0.004, % change/SD −34.2%) and with (P<0.0001, % change/SD −53.7%) controlling for covariates (Table 2A). Alpha genotype status was known in 130 of 164 (79%) of HbSC patients, 41 (32%) had alpha trait (39 αα/α- and 2α/α-) and the rest (89) had normal α genotype (αα/αα). The presence of alpha thalassemia trait had no influence on the development of albuminuria in the HbSC population (P=0.2, 95%CI −0.18, 0.89). Thirty-nine of 164 patients (24%) had at least one eGFR of 140 ml/min/1.73m2 or over during the study period. Estimated GFR was significantly associated with reticulocyte count in both simple and multiple regression analysis (Table 2B) although less strongly than in the SCA population. This is likely to be explained by the more homogeneous HbSC population with less variation in hemolysis.
Table 2A.
Relationship of hemolytic parameters to ACR in HbSC group.
Table 2B.
Relationship of hemolytic parameters to eGFR in HbSC group.
Sickle cell nephropathy (SCN) is an important cause of morbidity and mortality in patients with SCD.19 It is characterized by hyperfiltration at a young age, followed by a gradual reduction in GFR and worsening proteinuria, in some cases culminating in end-stage renal failure, which itself has a markedly increased risk of death.4 We have demonstrated a high prevalence of SCN amongst a UK sickle population, with a combined incidence of hyperfiltration (as defined by an eGFR of 140 ml/min/1.73 m2 or over) of 71%, and of microalbuminuria (ACR of ≥4.5 mg/mmol) of 37%, making SCN one of the most common complications of SCA.
We have also demonstrated associations between both degree and prevalence of albuminuria and all markers of increased hemolysis, including the new parameter of red cell survival described by Maier-Redelsperger et al.16 In addition, an association was found between hyperfiltration and high reticulocyte counts, another marker for hemolysis.
Co-inheritance of alpha thalassemia, has a protective effect against albuminuria in SCA patients with a negative association between number of deleted α-genes and the degree of albuminuria and prevalence of microalbuminuria. A previous study by Nebor et al. showed that alpha thalassemia was associated with a delayed age-of-onset of albuminuria in SCA patients but an association between albuminuria and LDH could not be shown.20
Levels of hemolysis vary amongst patients with SCA, and the existence of a sub-phenotype of patients with markedly increased hemolysis has been postulated.8 Patients with high levels of hemolysis have increased mortality, and increased rates of specific sickle complications including pulmonary hypertension, priapism and leg ulcers.21 Our data suggest that SCN is also associated with the hemolytic sub-phenotype of SCA. Asnani et al., however, suggest that higher rates of hemolysis were not associated with albuminuria in Jamaicans with SCD, but this study analyzed both HbSS and HbSC patients, who have quite different disease severity, as a group.22
There are several potential limitations to this study. There is the potential confounding factor between hemolytic and vaso-occlusive mechanisms of renal damage and the lack of a reliable measurement of vascular occlusion to control for. Further studies with larger cohorts are required to describe more accurately the association between hemolysis and SCN. As this study is clinic-based, there is also a risk of sampling bias towards patients who have more severe disease. Another limitation is the reliance on estimations of GFR by the MDRD formula. MDRD eGFR relies on measurements of serum creatinine. This molecule is maximally secreted by the proximal tubule in patients with SCA and is known to over-estimate GFR at high ranges. However, Haymann et al. have measured GFR in a proportion of their patients with SCA using accurate radio-labeled isotope techniques. They found that eGFR using the MDRD formula was a robust predictor of hyper-filtration when compared to the actual GFR.15
Although our data have confirmed the downward trend of eGFR and upward trend of ACR as patients age, the exact temporal and pathological relationship between these two factors has not been fully investigated in individual patients, and further prospective studies are needed. The use of angiotensin-converting enzyme (ACE) inhibiting medications has been shown to reduce levels of proteinuria in small-scale trials and may prove to be beneficial in delaying the onset of CKD.23 In view of the seriousness of this complication, large-scale randomized controlled trials of therapies to halt progression of SCN are urgently required.
In conclusion, we have shown associations between raised hemolytic markers and the presence of both albuminuria and glomerular hyperfiltration in patients with the more severe form of SCD, HbSS and HbSβ0 thalassemia. We have also demonstrated a protective effect of α thalassemia against the onset of albuminuria, which is in keeping with the reduced hemolysis associated with this genotype. Our data emphasize the progressive nature of renal impairment in SCA. Raised markers of hemolysis may prove to be clinically useful in identifying those at increased risk of developing sickle cell nephropathy.
Acknowledgments
We thank Claire Steward for help in preparation of the manuscript.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–76. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 2.Serjeant GR, Higgs DR, Hambleton IR. Elderly survivors with homozygous sickle cell disease. N Engl J Med. 2007;356(6):642–3. doi: 10.1056/NEJMc066547. [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease: Life expectancy and risk factors for early death. New England Journal of Medicine. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Scheinman JI. Sickle cell disease and the kidney. Nat Clin Pract Nephrol. 2009;5(2):78–88. doi: 10.1038/ncpneph1008. [DOI] [PubMed] [Google Scholar]
- 5.Becton LJ, Kalpatthi RV, Rackoff E, Disco D, Orak JK, Jackson SM, et al. Prevalence and clinical correlates of microalbuminuria in children with sickle cell disease. Pediatr Nephrol. 2010;25(8):1505–11. doi: 10.1007/s00467-010-1536-8. [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol. 2000;63(4):205–11. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander N, Higgs D, Dover G, Serjeant GR. Are there clinical phenotypes of homozygous sickle cell disease? Br J Haematol. 2004;126(4):606–11. doi: 10.1111/j.1365-2141.2004.05025.x. [DOI] [PubMed] [Google Scholar]
- 9.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol. 1991;36(2):122–30. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 10.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84(9):618–25. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–95. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 13.Gurkan S, Scarponi KJ, Hotchkiss H, Savage B, Drachtman R. Lactate dehydrogenase as a predictor of kidney involvement in patients with sickle cell anemia. Pediatr Nephrol. 2010;25(10):2123–7. doi: 10.1007/s00467-010-1560-8. [DOI] [PubMed] [Google Scholar]
- 14.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228–35. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 15.Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol. 2010;5(5):756–61. doi: 10.2215/CJN.08511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier-Redelsperger M, Levy P, Lionnet F, Stankovic K, Haymann JP, Lefevre G, et al. Strong association between a new marker of hemolysis and glomerulopathy in sickle cell anemia. Blood Cells Mol Dis. 2010;45(4):289–92. doi: 10.1016/j.bcmd.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Clark BE, Thein SL. Molecular diagnosis of haemoglobin disorders. Clin Lab Haematol. 2004;26(3):159–76. doi: 10.1111/j.1365-2257.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 18.Vasavda N, Menzel S, Kondaveeti S, Maytham E, Awogbade M, Bannister S, et al. The linear effects of alpha-thalassaemia, the UGT1A1 and HMOX1 polymorphisms on cholelithiasis in sickle cell disease. Br J Haematol. 2007;138(2):263–70. doi: 10.1111/j.1365-2141.2007.06643.x. [DOI] [PubMed] [Google Scholar]
- 19.Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med. 1991;115(8):614–20. doi: 10.7326/0003-4819-115-8-614. [DOI] [PubMed] [Google Scholar]
- 20.Nebor D, Broquere C, Brudey K, Mougenel D, Tarer V, Connes P, et al. Alpha-thalassemia is associated with a decreased occurrence and a delayed age-at-onset of albuminuria in sickle cell anemia patients. Blood Cells Mol Dis. 2010;45(2):154–8. doi: 10.1016/j.bcmd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Taylor JG, 6th, Nolan VG, Mendelsohn L, Kato GJ, Gladwin MT, Steinberg MH. Chronic hyper-hemolysis in sickle cell anemia: association of vascular complications and mortality with less frequent vasoocclu-sive pain. PLoS One. 2008;3(5):e2095. doi: 10.1371/journal.pone.0002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asnani MR, Fraser RA, Reid ME. Higher rates of hemolysis are not associated with albuminuria in Jamaicans with sickle cell disease. PLoS One. 2011;6(4):e18863. doi: 10.1371/journal.pone.0018863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med. 1992;326(14):910–5. doi: 10.1056/NEJM199204023261402. [DOI] [PubMed] [Google Scholar]