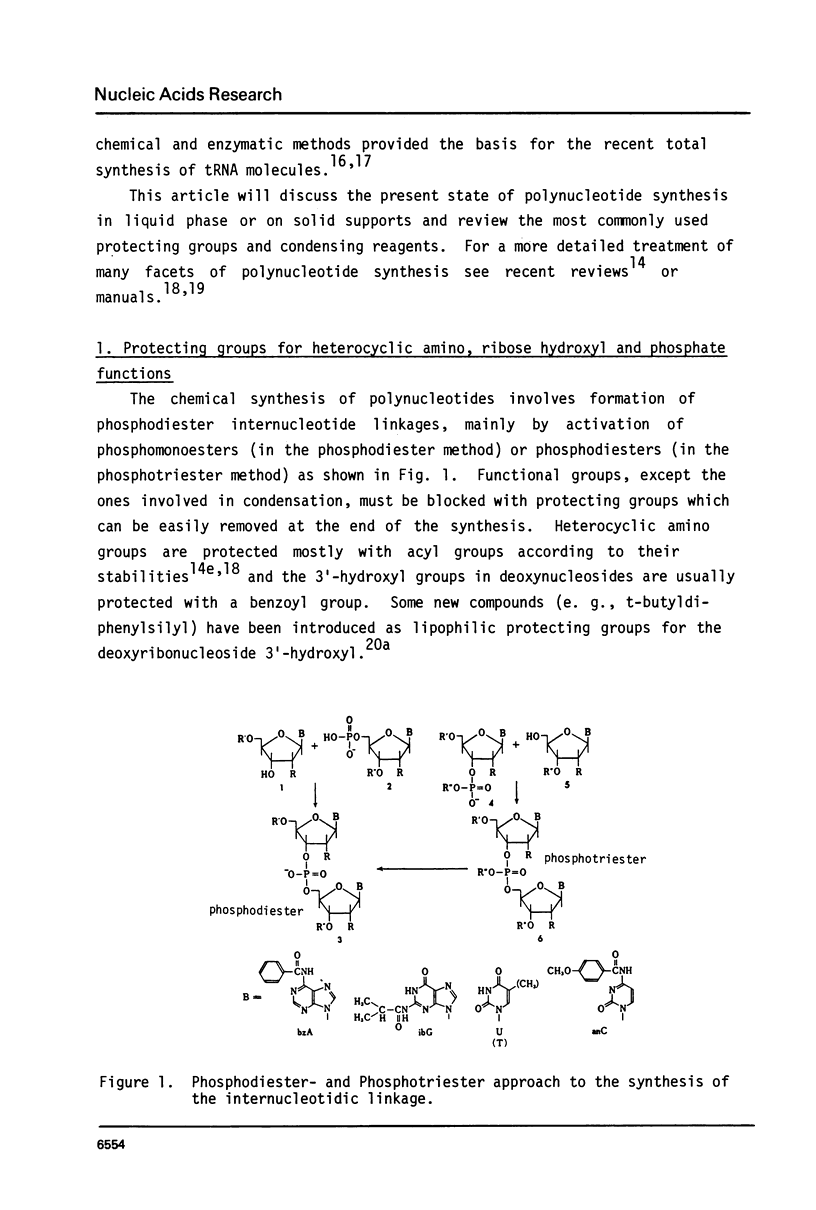
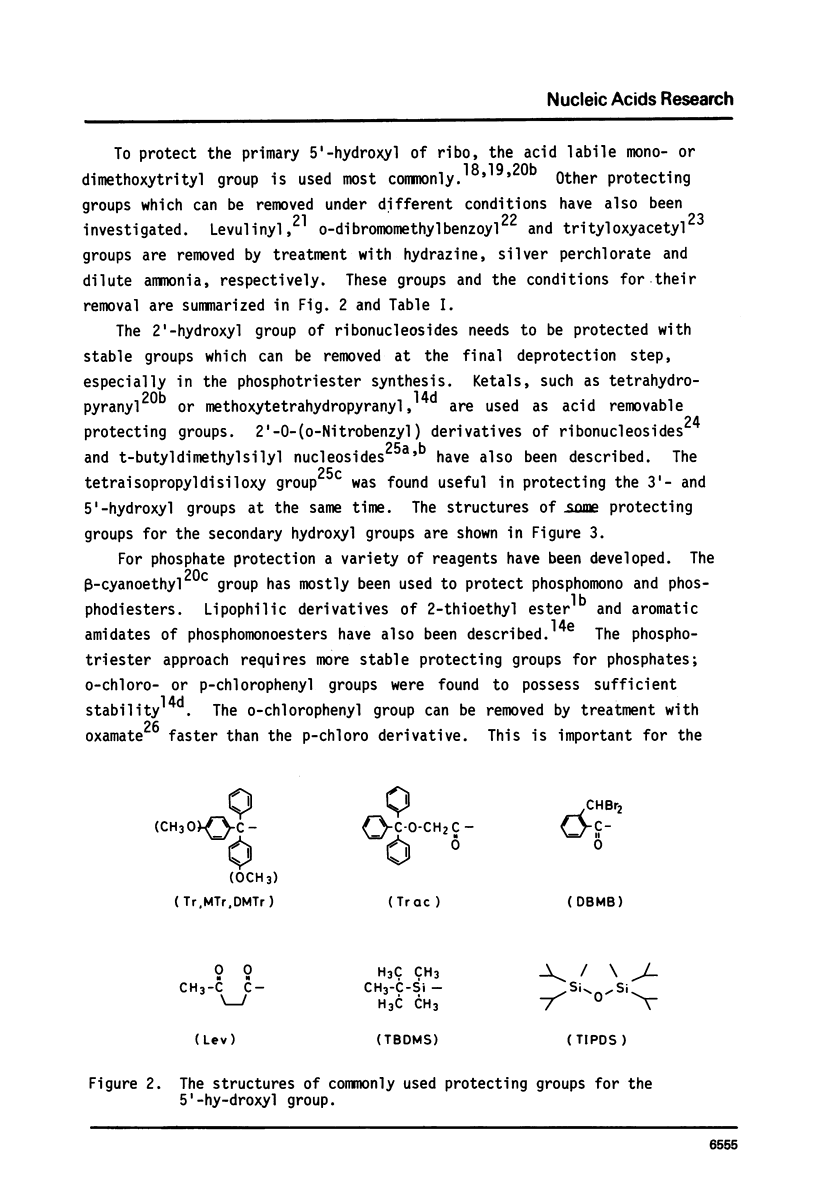
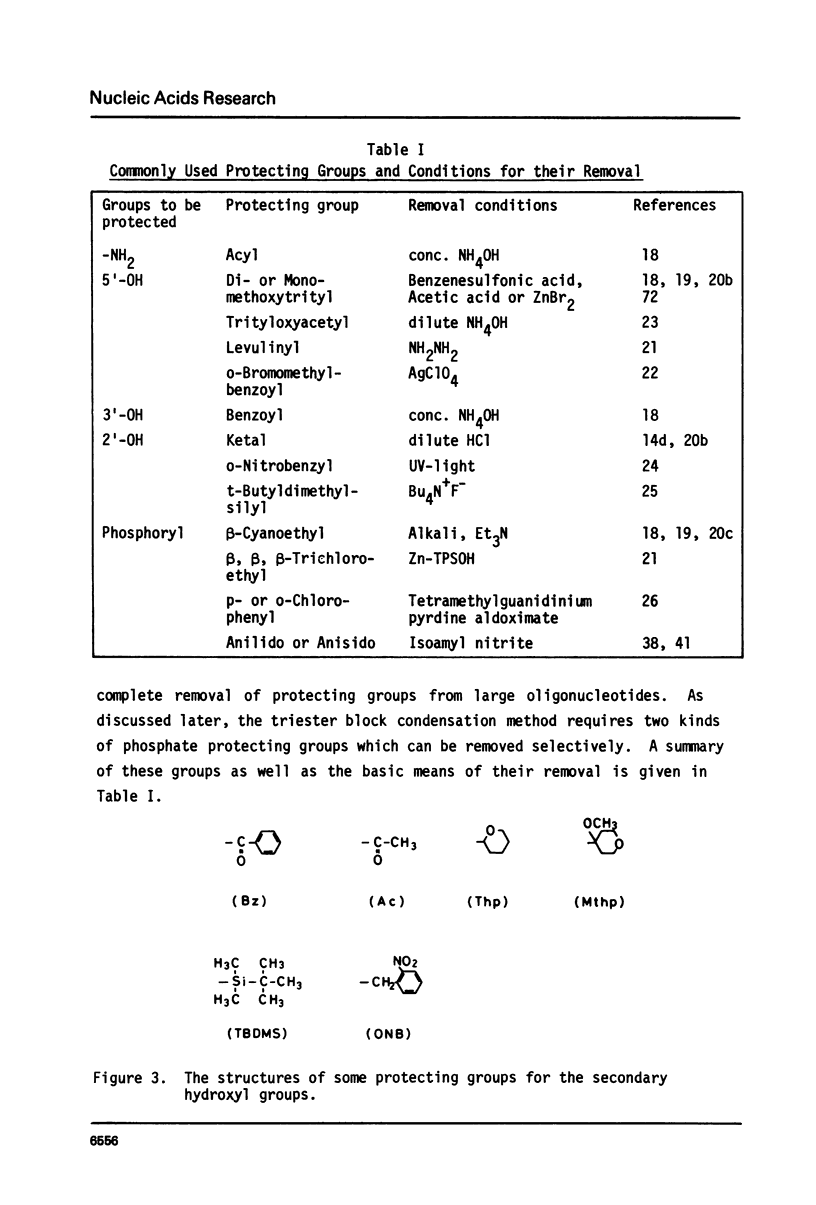
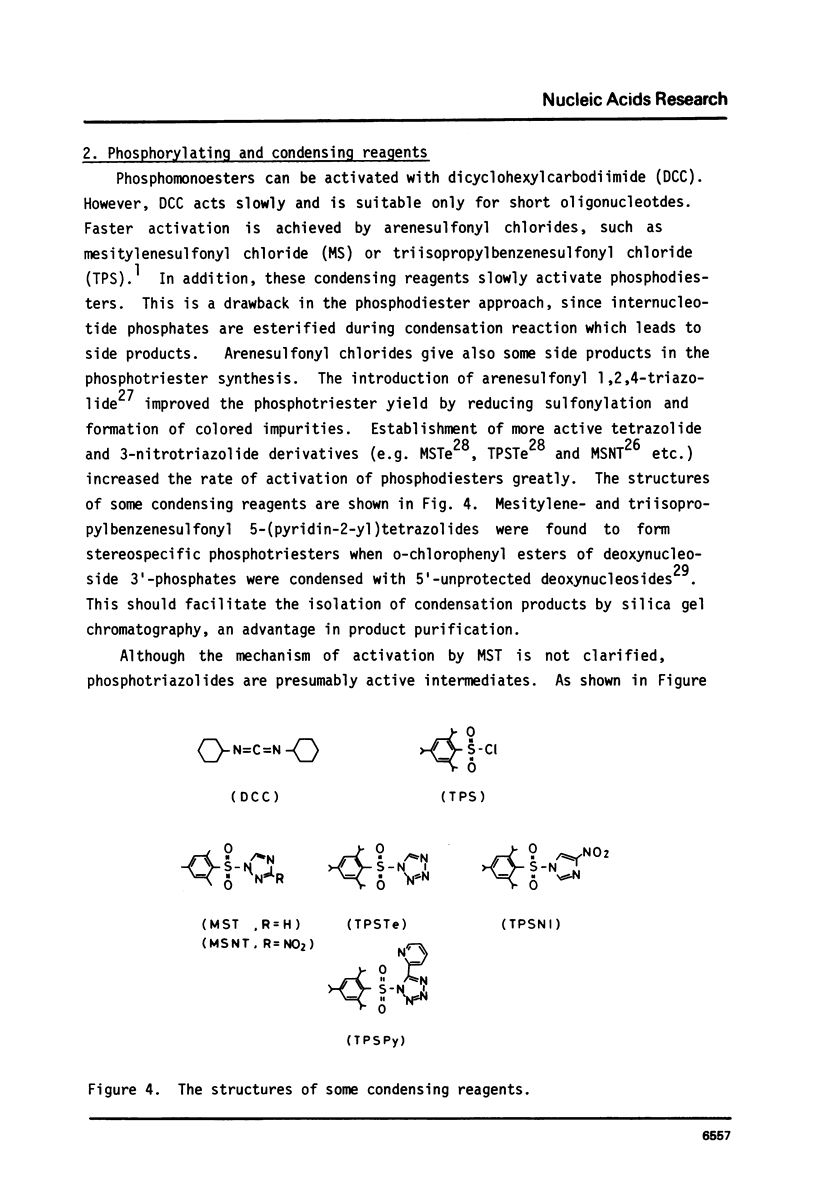
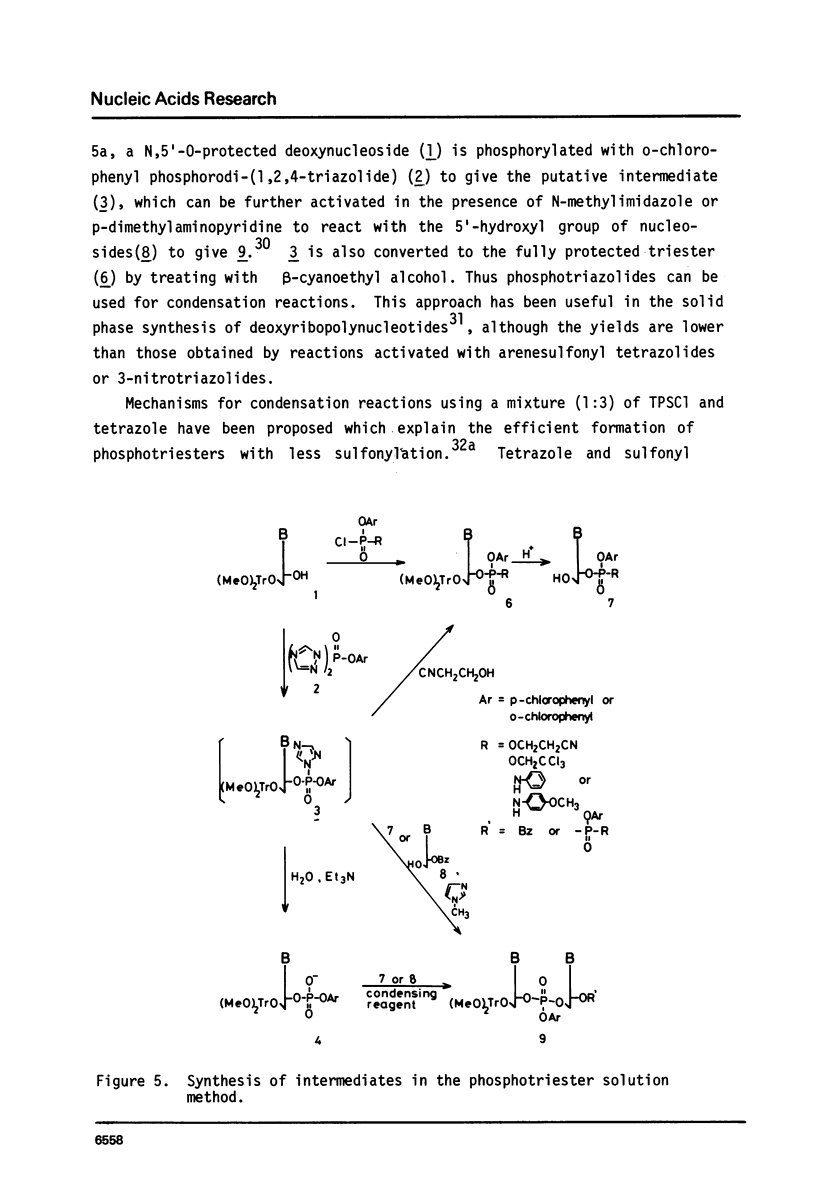
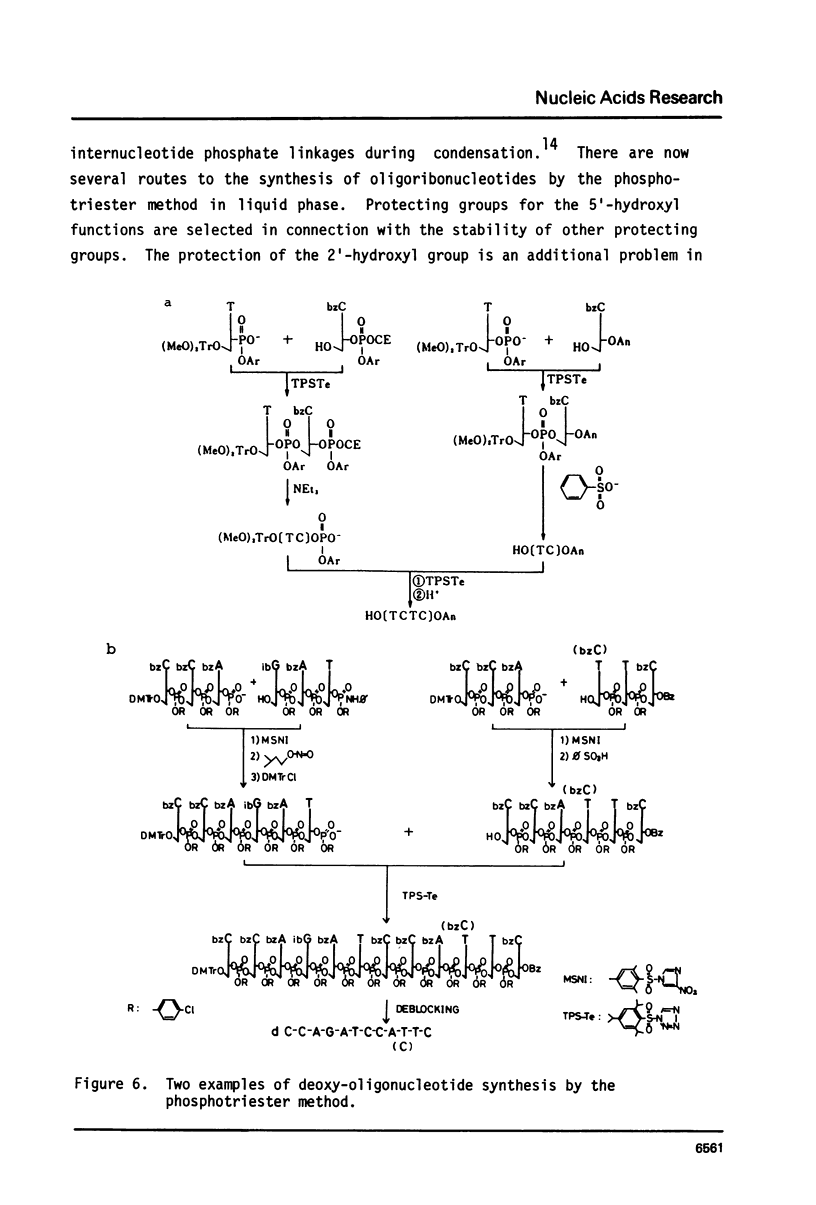
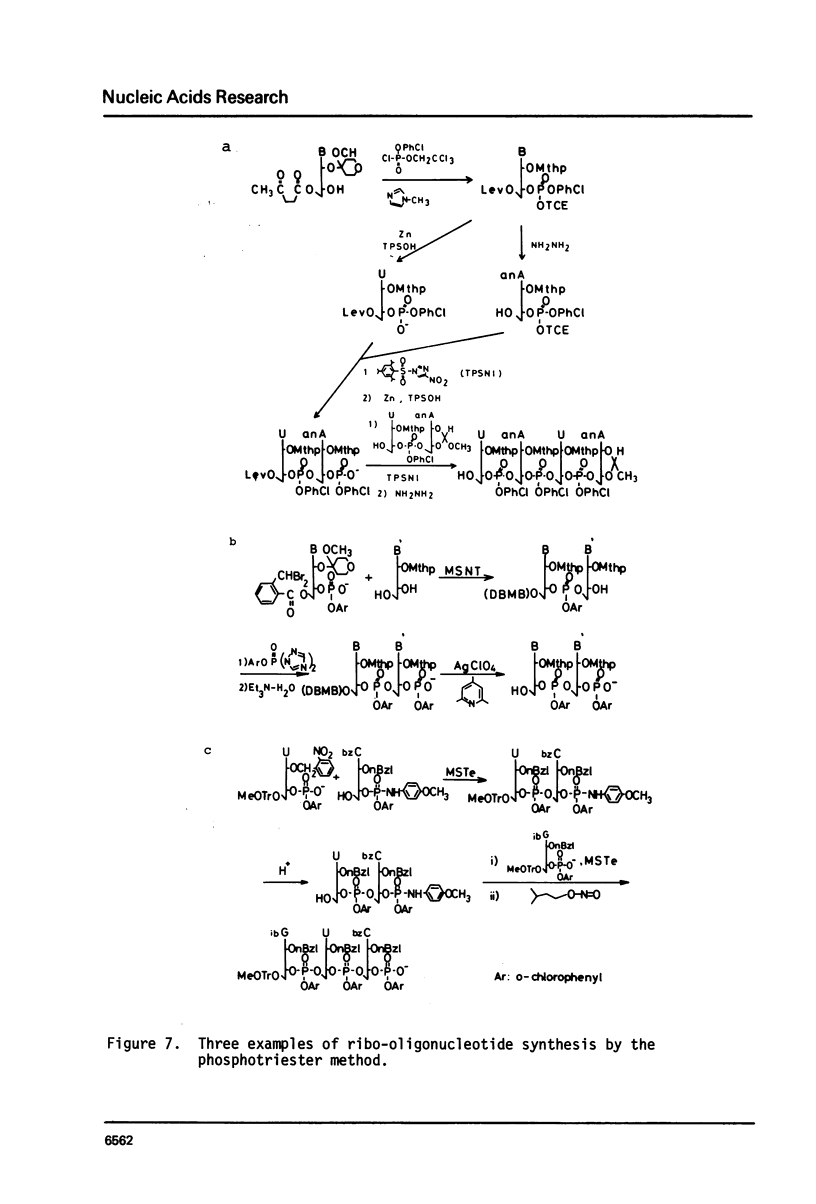
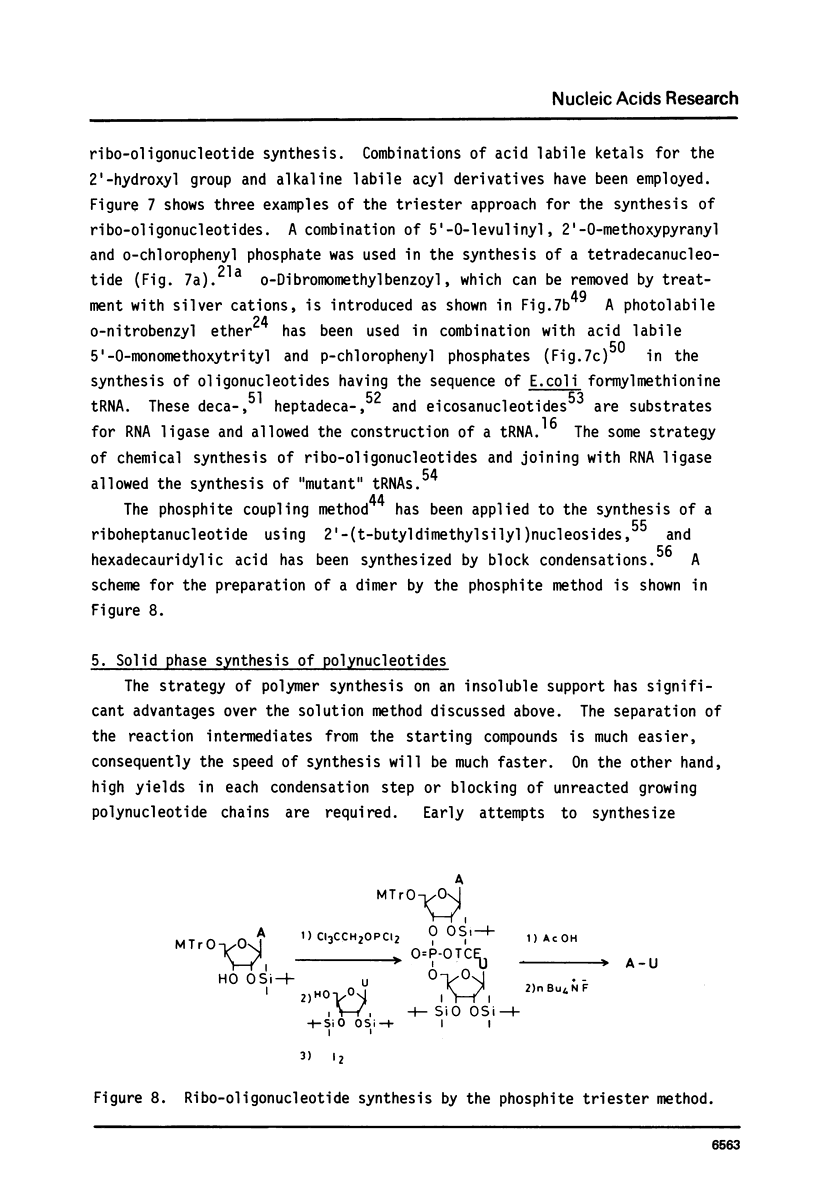
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Chemical synthesis of a self-complementary octanucleotide, dG-G-T-T-A-A-C-C by a modified triester method. Nucleic Acids Res. 1978 Aug;5(8):2809–2823. doi: 10.1093/nar/5.8.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal K. L., Yamazaki A., Cashion P. J., Khorana H. G. Chemical synthesis of polynucleotides. Angew Chem Int Ed Engl. 1972 Jun;11(6):451–459. doi: 10.1002/anie.197204511. [DOI] [PubMed] [Google Scholar]
- Alvarado-Urbina G., Sathe G. M., Liu W. C., Gillen M. F., Duck P. D., Bender R., Ogilvie K. K. Automated synthesis of gene fragments. Science. 1981 Oct 16;214(4518):270–274. doi: 10.1126/science.6169150. [DOI] [PubMed] [Google Scholar]
- Bahl C. P., Wu R., Brousseau R., Sood A. K., Hsiung H. M., Narang S. A. Chemical synthesis of versatile adaptors for molecular cloning. Biochem Biophys Res Commun. 1978 Apr 14;81(3):695–703. doi: 10.1016/0006-291x(78)91408-0. [DOI] [PubMed] [Google Scholar]
- Broka C., Hozumi T., Arentzen R., Itakura K. Simplications in the synthesis of short oligonucleotide blocks. Nucleic Acids Res. 1980 Nov 25;8(22):5461–5471. doi: 10.1093/nar/8.22.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. L., Belagaje R., Ryan M. J., Khorana H. G. Chemical synthesis and cloning of a tyrosine tRNA gene. Methods Enzymol. 1979;68:109–151. doi: 10.1016/0076-6879(79)68010-2. [DOI] [PubMed] [Google Scholar]
- Caruthers M. H., Beaucage S. L., Efcavitch J. W., Fisher E. F., Matteucci M. D., Stabinsky Y. New chemical methods for synthesizing polynucleotides. Nucleic Acids Symp Ser. 1980;(7):215–223. [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Majumdar A., Dunn R., Makabe O., RajBhandary U. L., Khorana H. G., Ohtsuka E., Tanaka T., Taniyama Y. O., Ikehara M. Bacteriorhodopsin: partial sequence of mRNA provides amino acid sequence in the precursor region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3398–3402. doi: 10.1073/pnas.78.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer F., Köster H. Synthesis of oligonucleotides on a polymeric carrier. Angew Chem Int Ed Engl. 1968 Jun;7(6):473–474. doi: 10.1002/anie.196804731. [DOI] [PubMed] [Google Scholar]
- Crea R., Kraszewski A., Hirose T., Itakura K. Chemical synthesis of genes for human insulin. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5765–5769. doi: 10.1073/pnas.75.12.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub G. W., van Tamelen E. E. Synthesis of oligoribonucleotides based on the facile cleavage of methyl phosphotriester intermediates. J Am Chem Soc. 1977 May 11;99(10):3526–3528. doi: 10.1021/ja00452a069. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R. J., Belagaje R., Brown E. L., Khorana H. G. The synthesis and cloning of two tyrosine suppressor tRNA genes with altered promoter sequences. J Biol Chem. 1981 Jun 25;256(12):6109–6118. [PubMed] [Google Scholar]
- Edge M. D., Green A. R., Heathcliffe G. R., Meacock P. A., Schuch W., Scanlon D. B., Atkinson T. C., Newton C. R., Markham A. F. Total synthesis of a human leukocyte interferon gene. Nature. 1981 Aug 20;292(5825):756–762. doi: 10.1038/292756a0. [DOI] [PubMed] [Google Scholar]
- Fritz H. J., Belagaje R., Brown E. L., Fritz R. H., Jones R. A., Lees R. G., Khorana H. G. High-pressure liquid chromatography in polynucleotide synthesis. Biochemistry. 1978 Apr 4;17(7):1257–1267. doi: 10.1021/bi00600a020. [DOI] [PubMed] [Google Scholar]
- Fukui T., Ishihama A., Ohtsuka E., Ikehara M., Fukuda R. Reverse transcriptase associated with avian sarcoma-leukosis viruses. III. Influence of primer chain length on the initiation of DNA synthesis. J Biochem. 1982 Jan;91(1):331–339. doi: 10.1093/oxfordjournals.jbchem.a133692. [DOI] [PubMed] [Google Scholar]
- Gait M. J., Sheppard R. C. Rapid synthesis of oligodeoxyribonucleotides. II. Machine-aided solid-phase syntheses of two nonanucleotides and an octanucleotide. Nucleic Acids Res. 1977 Dec;4(12):4391–4410. doi: 10.1093/nar/4.12.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Singh M., Sheppard R. C., Edge M. D., Greene A. R., Heathcliffe G. R., Atkinson T. C., Newton C. R., Markham A. F. Rapid synthesis of oligodeoxyribonucleotides. IV. Improved solid phase synthesis of oligodeoxyribonucleotides through phosphotriester intermediates. Nucleic Acids Res. 1980 Mar 11;8(5):1081–1096. doi: 10.1093/nar/8.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumport R. I., Uhlenbeck O. C. T4 RNA ligase as a nucleic acid synthesis and modification reagent. Gene Amplif Anal. 1981;2:313–345. [PubMed] [Google Scholar]
- Hsiung H. M., Sung W. L., Brousseau R., Wu R., Narang S. A. Synthesis of the human insulin gene. Part III. Chemical synthesis of 5'-phosphomonoester group containing deoxyribooligonucleotides by the modified phosphotriester method. Its application in the synthesis of seventeen fragments constituting human insulin C-chain DNA. Nucleic Acids Res. 1980 Dec 11;8(23):5753–5765. doi: 10.1093/nar/8.23.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. A., Fritz H. J., Khorana H. G. Use of the lipophilic tert-butyldiphenylsilyl protecting group in synthesis and rapid separation of polynucleotides. Biochemistry. 1978 Apr 4;17(7):1268–1278. doi: 10.1021/bi00600a021. [DOI] [PubMed] [Google Scholar]
- Katagiri N., Itakura K., Narang S. A. The use of arylsulfonyltriazoles for the synthesis of oligonucleotides by the triester approach. J Am Chem Soc. 1975 Dec 10;97(25):7332–7337. doi: 10.1021/ja00858a021. [DOI] [PubMed] [Google Scholar]
- Khorana H. G., Agarwal K. L., Büchi H., Caruthers M. H., Gupta N. K., Kleppe K., Kumar A., Otsuka E., RajBhandary U. L., Van de Sande J. H. Studies on polynucleotides. 103. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. J Mol Biol. 1972 Dec 28;72(2):209–217. doi: 10.1016/0022-2836(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Total synthesis of a gene. Science. 1979 Feb 16;203(4381):614–625. doi: 10.1126/science.366749. [DOI] [PubMed] [Google Scholar]
- Letsinger R. I., Finnan J. L., Heavner G. A., Lunsford N. B. Letter: Phosphite coupling procedure for generating internucleotide links. J Am Chem Soc. 1975 May 28;97(11):3278–3279. doi: 10.1021/ja00844a090. [DOI] [PubMed] [Google Scholar]
- Letsinger R. L., Lunsford W. B. Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc. 1976 Jun 9;98(12):3655–3661. doi: 10.1021/ja00428a045. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]
- Markham A. F., Edge M. D., Atkinson T. C., Greene A. R., Heathcliffe G. R., Newton C. R., Scanlon D. Solid phase phosphotriester synthesis of large oligodeoxyribonucleotides on a polyamide support. Nucleic Acids Res. 1980 Nov 25;8(22):5193–5205. doi: 10.1093/nar/8.22.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Cheng D. M., Dreon N., Jayaraman K., Kan L. S., Leutzinger E. E., Pulford S. M., Ts'o P. O. Preparation of a decadeoxyribonucleotide helix for studies by nuclear magnetic resonance. Biochemistry. 1980 Sep 30;19(20):4688–4698. doi: 10.1021/bi00561a023. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Arentzen R., Huang T., Itakura K. Solid-phase synthesis of polynucleotides. IV. Usage of polystyrene resins for the synthesis of polydeoxyribonucleotides by the phosphostriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5507–5517. doi: 10.1093/nar/8.22.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Hasegawa A., Tomiyama M., Miyake T. Chemical synthesis of 27-desamidosecretin gene by the polymer support method. Nucleic Acids Symp Ser. 1981;(10):197–200. [PubMed] [Google Scholar]
- Miyoshi K., Huang T., Itakura K. Solid-phase synthesis of polynucleotides. III. Synthesis of polynucleotides with defined sequences by the block coupling phosphotriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5491–5505. doi: 10.1093/nar/8.22.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Itakura K. Solid-phase synthesis of polynucleotides: V. Synthesis of oligodeoxyribonucleotides by the phosphomonotriazolide method. Nucleic Acids Symp Ser. 1980;(7):281–291. [PubMed] [Google Scholar]
- Miyoshi K., Miyake T., Hozumi T., Itakura K. Solid-phase synthesis of polynucleotides. II. Synthesis of polythymidylic acids by the block coupling phosphotriester method. Nucleic Acids Res. 1980 Nov 25;8(22):5473–5489. doi: 10.1093/nar/8.22.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery D. L., Hall B. D., Gillam S., Smith M. Identification and isolation of the yeast cytochrome c gene. Cell. 1978 Jul;14(3):673–680. doi: 10.1016/0092-8674(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Narang S. A., Hsiung H. M., Brousseau R. Improved phosphotriester method for the synthesis of gene fragments. Methods Enzymol. 1979;68:90–98. doi: 10.1016/0076-6879(79)68008-4. [DOI] [PubMed] [Google Scholar]
- Neilson T., Werstink E. S. Synthesis of the anticodon loop of Escherichia coli methionine transfer ribonucleic acid. J Am Chem Soc. 1974 Apr 3;96(7):2295–2297. doi: 10.1021/ja00814a074. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Fujiyama K., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XL. Synthesis of an eicosaribonucleotide corresponding to residues 35-54 of tRNAfMet from E. coli. Nucleic Acids Res. 1981 Jul 24;9(14):3503–3522. doi: 10.1093/nar/9.14.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Fujiyama K., Tanaka T., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XXXIX. Chemical synthesis of heptadeca- and hexadecaribonucleotides corresponding to the 3'-terminus of the tRNAfMet of E. coli. Chem Pharm Bull (Tokyo) 1981 Oct;29(10):2799–2806. doi: 10.1248/cpb.29.2799. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Shibahara S., Ikehara M. Studies on deoxyribonucleic acids and related compounds. III. Synthesis of oligodeoxyribonucleotides complementary to the anticodon loop of Escherichia coli tRNAfmet by an improved triester method. Chem Pharm Bull (Tokyo) 1981 Dec;29(12):3440–3448. doi: 10.1248/cpb.29.3440. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. IX. Ribooligonucleotide synthesis using a photosensitive o-nitrobenzyl protection at the 2'-hydroxyl group. Nucleic Acids Res. 1974 Oct;1(10):1351–1357. doi: 10.1093/nar/1.10.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Tanaka T., Miyake T., Markham A. F., Nakagawa E., Wakabayashi T., Taniyama Y., Nishikawa S., Fukumoto R. Total synthesis of a RNA molecule with sequence identical to that of Escherichia coli formylmethionine tRNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5493–5497. doi: 10.1073/pnas.78.9.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Pardi A., Itakura K. DNA conformation, dynamics, and interactions in solution. Science. 1982 May 7;216(4546):581–590. doi: 10.1126/science.6280281. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Dickerson R. E., Boyer H. W., Riggs A. D., Itakura K. Chemical synthesis of restriction enzyme recognition sites useful for cloning. Science. 1977 Apr 8;196(4286):177–180. doi: 10.1126/science.847463. [DOI] [PubMed] [Google Scholar]
- Seth A. K., Jay E. A study of the efficiency and the problem of sulfonation of several condensing reagents and their mechanisms for the chemical synthesis of deoxyoligoribonucleotides. Nucleic Acids Res. 1980 Nov 25;8(22):5445–5459. doi: 10.1093/nar/8.22.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Sumi S., Hasegawa A., Nishizawa T., Miyoshi K., Wakisaka S., Miyake T., Misoka F. Production in Escherichia coli of biologically active secretin, a gastrointestinal hormone. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2475–2479. doi: 10.1073/pnas.79.8.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Oshima T., Ohsue K., Ono T., Oikawa S., Takano I., Noguchi T., Kangawa K., Minamino N., Matsuo H. Expression in Escherichia coli of chemically synthesized gene for a novel opiate peptide alpha-neo-endorphin. Nucleic Acids Res. 1982 Mar 11;10(5):1741–1754. doi: 10.1093/nar/10.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Letsinger R. L. Syringe method for stepwise chemical synthesis of oligonucleotides. Nucleic Acids Res. 1982 May 25;10(10):3249–3260. doi: 10.1093/nar/10.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Uesugi S., Yano J., Yano E., Ikehara M. Synthesis and properties of the dinucleoside monophosphates containing adenine S-cyclonucleosides and adenosine. Factors determining the stability and handedness of the stacking conformation in a dinucleoside monophosphate. J Am Chem Soc. 1977 Mar 30;99(7):2313–2323. doi: 10.1021/ja00449a049. [DOI] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]



