Abstract
Background
The hypocellular variant of acute myeloid leukemia accounts for less than 10% of all cases of adult acute myeloid leukemia. It is defined by having less than 20 percent of cellular bone marrow in a biopsy at presentation. It is unclear in the literature whether the outcome of hypocellular acute myeloid leukemia differs from that of non-hypocellular acute myeloid leukemia.
Design and Methods
We retrospectively analyzed all the cases reported to be hypocellular acute myeloid leukemia between 2000 and 2009. A second pathology review was conducted and the diagnosis was confirmed in all cases.
Results
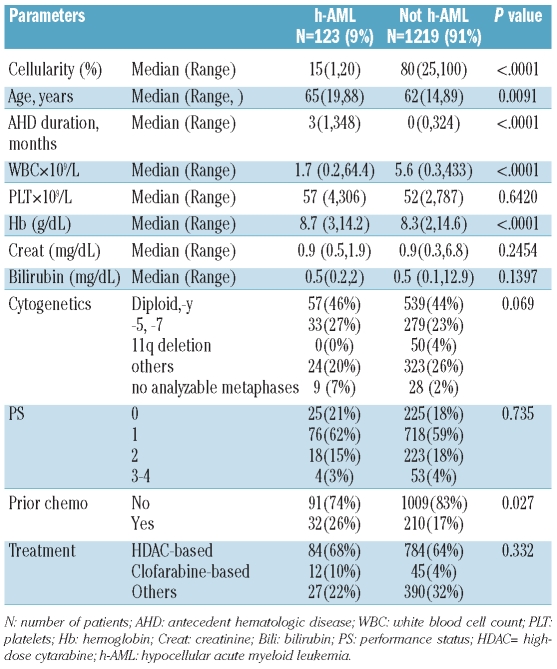
One hundred twenty-three (9%) patients were identified: patients with hypocellular acute myeloid leukemia were older than those with non-hypocellular acute myeloid leukemia (P=0.009) and more frequently presented with cytopenias (P<0.001). Forty-one patients with hypocellular acute myeloid leukemia had an antecedent hematologic disorder and 11 patients had received prior chemo-radiotherapy for non-hematopoietic neoplasms. On multivariate analysis, overall survival, remission duration and event-free survival were comparable to those of other patients with acute myeloid leukemia.
Conclusions
The outcome of hypocellular acute myeloid leukemia does not differ from that of non-hypocellular acute myeloid leukemia.
Keywords: hypocellular acute myeloid leukemia, outcome, prognosis
Introduction
Hypocellular acute myeloid leukemia (AML) is an infrequent entity. Its frequency ranges between 5–12% of all cases of AML.1,2 Published reports are based on small numbers of patients and do not include a comparison with other AML patients. Hypocellular AML is currently defined as AML with a bone marrow cellularity less than 20%, although in some earlier reports, cellularity less than 40% or 50% was considered to be hypocellular.2–5 Its diagnosis poses a challenge to hematopathologists because of the features in common between hypocellular AML and hypocellular myelodysplastic syndrome and aplastic anemia, including cytopenias and dysplasia; this is particularly the case in the few instances in which cellularity is extremely low. Guidelines were, therefore, proposed to facilitate the diagnosis.6,7
Information about additional clinical parameters is expected to help treating physicians. For instance, information about the frequency of preceding hematologic disorders is not well reported.1 As cytogenetics remain most prognostic for clinically relevant outcomes in AML,8 the distribution of cytogenetic abnormalities in hypocellular AML needs to be elucidated in larger cohorts of patients. Finally, there is a lack of data comparing survival and other clinically important outcomes between these patients and patients with AML that is not hypocellular.
In addition to cytogenetics, mutations have gained relevance to outcomes in AML and in particular internal tandem duplication (ITD) mutations in FLT3 have been linked to worse outcomes in normal karyotype AML. The frequency of these mutations ranges up to 30% in normal karyotype AML, but is unknown in patients with hypocellular AML.9,10 We undertook this study to assess the frequency of hypocellular AML among a large consecutive cohort of patients with AML. Here we describe the clinical features of this group and compare their clinical outcomes with those of other patients with AML.
Design and Methods
All new cases of AML (according to World Health Organization criteria) seen at the University of Texas MD Anderson Cancer Center (MDACC) from 2000 to 2009 were reviewed. Patients with acute promyelocytic leukemia and core binding factor-associated AML were excluded from the analysis. Most patients were treated in clinical trials approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent document approved by the Institutional Review Board before entering any study.
According to the clinical practice of our institution bone marrow aspirate smears with core biopsies were performed on every patient at the time of presentation at our institution. Bone marrow aspirate smears were assessed by Wright-Giemsa stain followed by cytochemical analysis for myeloperoxidase and α-naphthyl butyrate esterase, as described previously.11 Four-color flow cytometry immunophenotypic analysis was performed on bone marrow aspirate specimens using a fluorescence-activated cell sorting (FACS) FACScan or FACSCalibur instrument, as described previously.11
Response was assessed according to previously published criteria. Briefly, complete remission was defined by the presence of less than 5% blasts in the bone marrow with recovery of peripheral blood counts.12 Induction-related or early mortality was defined as all deaths within 4 weeks of initiating therapy. Information on antecedent hematologic disorders13 or prior chemotherapy or radiotherapy was collected. An antecedent hematologic disorder was defined as an abnormality in blood counts for at least 1 month before presentation at the MDACC.
Cytogenetic analysis was assessed by G-banding with at least 20 metaphases counted. FLT3-ITD and codon 835 point mutations in the activation loop of the tyrosine kinase domain of FLT3 were assessed using genomic DNA extracted from bone marrow aspirate specimens by polymerase chain reaction assays followed by capillary electrophoresis, as described previously.11 Mutations in NRAS and KRAS, and KIT genes were assessed by polymerase chain reaction analysis followed by pyrosequencing, as described previously.14 A diagnosis of large granular lymphocytic leukemia was based on four-color flow cytometry for T-cell and natural killer-cell markers (CD3, CD4, CD8, CD56, and CD57). Determination of the paroxysmal nocturnal hemoglobinuria status was based on flow cytometry of both red and white blood cells for CD55/CD59 antigens.
Pathology review
All cases with hypocellular AML were reviewed by an independent pathologist. Cases with bone marrow biopsy cellularity of above 20% were excluded. Review included reading the peripheral blood smears, bone marrow aspirate smear and the bone marrow biopsies.
Statistical analysis
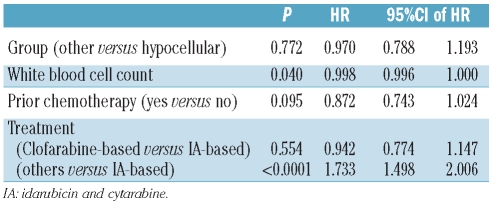
Wilcoxon’s rank sum test and Fisher’s exact test were used, as appropriate, to assess differences in patients’ characteristics. Overall survival and event-free survival were calculated from the time of diagnosis until death or an event, respectively. An event was defined as relapse after remission, failure to respond or death from any cause. The duration of complete remission was calculated from the time of achieving the complete remission until relapse. Kaplan-Meier methods were applied to generate overall survival, event-free survival and complete remission duration curves, while log-rank tests were used to assess the differences between groups. Additionally, univariate and multivariate Cox’s proportional hazard models were fitted to assess the impact of the diagnosis of hypocellular AML and other parameters on event-free survival, complete remission duration and overall survival. These parameters included age, history of antecedent hematologic disorder, white blood cell count, platelet count, hemoglobin, creatinine and bilirubin concentrations, history of exposure to chemotherapy, cytogenetics (grouped into diploid, -y/-5,-7, 11q/others) performance status and treatment (grouped as based on clofarabine, based on idarubicin + cytarabine, and targeted). The grouping of hypocellular AML versus other AML was retained in the final model irrespective of significance.
Results
Between January 2000 and December of 2009, 1342 patients were newly diagnosed with AML at the MDACC based on WHO criteria.15 One hundred and twenty-three (9%) patients were diagnosed with hypocellular AML based on a bone marrow cellularity of less than 20%. The characteristics of the patients with hypocellular AML and non-hypocellular AML are presented in Table 1. The median age of the patients with hypocellular AML was 65 years (range, 19–88 years) and this group had a median bone marrow cellularity of 15% (range, 4–20%). The median white blood cell count was 1.7×109/L (range, 0.2 ×109/L - 64.4×109/L). Seven patients had a white count greater than 10×109/L and three of them had prior myeloproliferative neoplasm even though the diagnostic bone marrow for AML did not show significant fibrosis. Only four patients (3%) had an Eastern Co-operative Oncology Group performance status of 3 or more, which reflects a fairly functional cohort. Forty-six percent of the group (57 patients) had a diploid karyotype (or –Y), 28% (33 patients) had chromosome 5 and/or 7 abnormalities and none had 11q deletions (metaphase recovery for cytogenetic analysis was inadequate in 9 patients). Among patients with hypocellular AML, 41 had an antecedent hematologic disorder: the diagnoses of these disorder were myelodysplastic syndrome (n=18), non-Hodgkin’s lymphoma (n=16), Hodgkin’s disease (n=2), multiple myeloma (n=2) and myeloproliferative neoplasm (n=3). Eleven patients had prior exposure to chemotherapy for non-hematopoietic neoplasms (breast cancer, n=5; prostate cancer, n=4, endometrial carcinoma, n=1; osteosarcoma, n=1).
Table 1.
Patients’ characteristics.
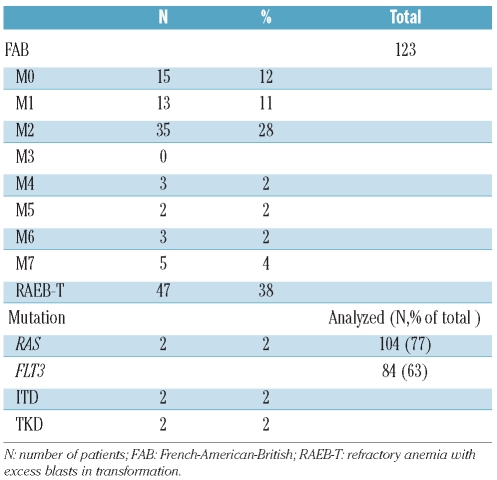
AML M2 according to the French-American-British (FAB) classification, was the most common subtype, accounting for 22% of cases, followed by M0 and M1 at 12% and 9%, respectively (Table 2). Interestingly 34 (28%) patients had a bone marrow blast percentage between 20–29% (i.e. refractory anemia with excess blasts in transformation according to the FAB classification). From the molecular perspective, NRAS and KRAS gene mutation analysis (codons 12, 13 and 61) was done in 104 (85%) patients, of whom only two (2%) were positive for a RAS mutation. On the other hand, of the 84 (63%) patients who underwent FLT3 gene testing for ITD or TKD mutations, two (2%) were positive for FLT3-ITD and another two (2%) were positive for an FLT3-TKD mutation. As for large granular lymphocytic leukemia, flow cytometry was performed in 40 patients, two of whom were positive for a clonal T-cell expansion. None of the 15 cases checked for paroxysmal nocturnal hemoglobinuria was positive.
Table 2.
Patients’ distribution by FAB classification and mutation analysis.
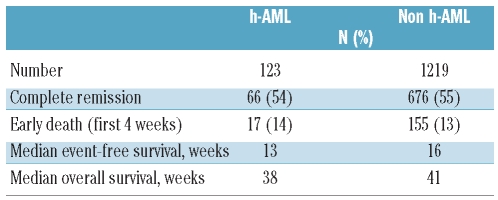
Two-thirds of the patients (84 patients, 68%) received high-dose cytarabine-based regimens, 12 (10%) patients received intravenous clofarabine-based treatments and 9 (7%) patients received a hypomethylating agent as induction. Seventeen patients received miscellaneous targeted therapies. This distribution of treatment options is remarkably similar to that of the patients with non-hypocellular AML, as clofarabine- and hypomethylating agent-based regimens are being tested in older patients with AML at our institute. Sixty-six (54%) patients of the whole group with hypocellular AML achieved complete remission or complete remission with incomplete recovery of platelet count (CR/CRp). However, 36/57 (63%) patients in the diploid cytogenetic group achieved CR/CRp compared to 41% (14/33) of the patients with monosomy 5/monosomy 7. The median complete remission duration for all patients with hypocellular AML was 49 weeks. Seventeen (14%) patients died within the first 4 weeks of initiating therapy. Among patients with non-hypocellular AML, the complete remission rate was 55% (676/1219), the median complete remission duration was 41 weeks and the mortality rate in the first month was 13% (155/13). The median time to CR/CRp was 33 days in both the hypocellular AML and non-hypocellular AML groups.
When comparing the two cohorts (AML and hypocellular AML), patients with hypocellular AML were older (P=0.009) and more likely to have had an antecedent hematologic disorder (P<0.0001). In addition, patients with hypocellular AML had lower white blood cell counts (P<0.0001) and hemoglobin concentrations (P<0.0001) at presentation. More patients with hypocellular AML received prior chemotherapy for other malignancies (26% versus 18%, P<0.027).
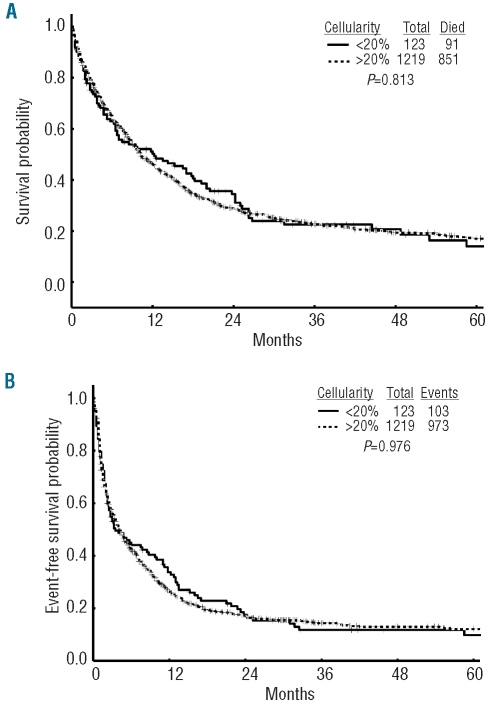
The median overall survival, event-free survival, and complete remission duration for the hypocellular AML group were 38, 13, and 49 weeks, respectively (Table 3) and were not different from those of patients with non-hypocellular AML (Figure 1). Fifty-five (45%) patients with hypocellular AML were treated with idarubicin and cytarabine and the CR/CRp rate in this cohort was 66% with a median overall survival and event-free survival of 60 weeks and 35 weeks, respectively. Twelve (10%) patients were treated with intravenous clofarabine with or without cytarabine and their complete remission rate was 83% with a median overall survival not reached and an event-free survival of 47 weeks (statistically not better than the cohort treated with idarubicin + cytarabine). The cohort treated with clofarabine regimens was, however, older than the cohort treated with idarubicin + cytarabine (P=0.04) as older patients were enrolled into studies of these regimens. On log-rank testing and multivariate analysis (Table 4), hypocellular AML was not associated with a difference in overall survival (P=0.81) (Figure 1A), event-free survival (P=0.98) (Figure 1B) or complete remission duration (P=0.62) compared to other patients with AML.
Table 3.
Clinical outcomes of patients with hypocellular (h-AML) and non h-AML patients.
Figure 1.
(A) Overall survival of patients with hypocellular acute myeloid leukemia (h-AML) and non h-AML patients. (B) Event-free survival of h-AML and non h-AML patients.
Table 4.
Multivariate Cox’s proportional hazards model for overall survival among all patients (N=1342).
Discussion
Hypoplastic AML can be challenging for both the pathologist and the treating physician. This entity is seen more frequently in older patients and patients tend to have more profound presenting cytopenias. The limited published information about the clinical and prognostic parameters of this entity makes specific clinical recommendations difficult.
Intuitively patients with hypocellular AML would be expected to have more prolonged cytopenia after induction therapy but the median time to CR/CRp in our series was not significantly different from that in the patients with non-hypocellular AML.
The frequency of hypocellular AML has been reported to be between 5–7% among all patients with AML. This is based mainly on older reports, in which the definition of AML required 30% of myeloblasts and the cellularity cutoffs used to define hypocellular AML varied, ranging from marrow cellularity of less than 20% to less than 50%, while the current definition restricts it to less than 20%. We, therefore, expected the true frequency of this entity to be even lower. However, in our analysis, the frequency of hypocellular AML turned out to be 9% of all AML cases.
Interestingly, nearly half the cases of hypocellular AML were composed of secondary AML (either with an antecedent hematologic disorder or prior history of chemotherapy or radiotherapy). The marrow hypoplasia also brings the additional difficulty of differentiating these cases from hypocellular myelodysplastic syndromes and aplastic anemia with marrow dysplasia. Several attempts have, therefore, been made to establish guidelines for this difficult diagnosis; some proposals included a count of at least 100 cells on the peripheral blood film if possible, performing a 500-cell differential from bone marrow aspirate if possible, and performing iron staining for assessment of ring sideroblasts.7 As for the bone marrow biopsy, assessment for abnormal localization of immature precursors (ALIP) with immunostaining for CD34/CD117, flow cytometry for paroxysmal nocturnal hemoglobinuria screening and standard cytogenetics and interphase fluorescence in situ hybridization are suggested for the initial work-up. Even complying with the above mentioned suggestions, the concordance rate for diagnosis of hypocellular AML was only 57%.6 Our report is also subject to some of the same limitations.
Our study tries to shed light on the cytogenetic and molecular characteristics of hypocellular AML. In this comparison, no difference was noted in the cytogenetic abnormalities between patients with hypocellular AML and those with non-hypocellular AML. However, from the molecular aspect, there was a lower frequency of RAS and FLT3 mutations (2% and 4%, respectively) in patients with hypocellular AML. Indeed, this frequency is more consistent with the low frequency of molecular mutations in patients with myelodysplastic syndromes.10,16 The low frequency of these mutations is not unexpected as FLT3- and RAS-mutated myeloid malignancies are expected to be more proliferative. In addition, 2/40 patients analyzed for large granular lymphocytic leukemia tested positive which again is more similar to the reported frequency in patients with myelodys-plastic syndromes. Unfortunately very few of our patients were screened for paroxysmal nocturnal hemoglobinuria; none was positive.
Few differences were evident from the comparison of patients with hypocellular AML and the other patients with AML. Presenting cytopenias (leukopenia and anemia) were more frequent among hypocellular AML and so was prior exposure to chemotherapy. On the other hand, overall survival, complete remission duration and event-free survival were not significantly different in the two groups of patients. One possible explanation, put forward in prior reports, is that the more indolent and slowly progressing nature of hypocellular AML offsets the possible increased morbidity and mortality from the intense chemotherapy.2 Patients included in this analysis underwent age-appropriate induction therapy and results suggest that appropriately aggressive therapy and supportive care delivered to these patients may play a role in equalizing their outcome with that of patients with non-hypocellular AML. For example, the complete remission rate of 54% in our cohort is better than historical rates, such as the 25% (2/8) in the report by Needleman.2 Lastly better supportive care in the contemporary period may have contributed to an improved outcome in patients with hypocellular AML.
Interestingly, 21 (17%) patients older than 60 years of age in the hypocellular AML group were treated with clofarabine or a hypomethylating agent with comparable clinical outcomes to a younger cohort treated with idarubicin + cytarabine. This is potentially of interest based on improved survival data with hypomethylating agents in patients with myelodysplastic syndromes.17–19 A preliminary report of improved outcome of older patients with AML (not necessarily hypocellular AML) treated with a regimen of clofarabine and low-dose cytarabine alternating with decitabine may also expand therapeutic options for patients with hypocellular AML as rates of prolonged cytopenias are expected to be low with such regimens.20
In summary, this report describes a cohort of patients with hypocellular AML characterized by low bone marrow cellularity, prominent cytopenias, older age, a high percentage of antecedent hematologic disorders or prior chemotherapy/radiotherapy and a low frequency of proliferative mutations. Better understanding of disease biology and more comprehensive molecular characterization of disease is likely to result in better therapeutic options.
Footnotes
Funding: this work was partly supported by a grant from the American Society of Clinical Oncology, Career Development Award (GB).
Prior publication: part of the data was presented at the 46th annual meeting of the American Society of Clinical Oncology (ASCO) June 4–8th 2010
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Tuzuner N, Cox C, Rowe JM, Bennett JM. Hypocellular acute myeloid leukemia: the Rochester (New York) experience. Hematol Pathol. 1995;9(3–4):195–203. [PubMed] [Google Scholar]
- 2.Needleman SW, Burns CP, Dick FR, Armitage JO. Hypoplastic acute leukemia. Cancer. 1981;48(6):1410–4. doi: 10.1002/1097-0142(19810915)48:6<1410::aid-cncr2820480624>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Berdeaux DH, Glasser L, Serokmann R, Moon T, Durie BG. Hypoplastic acute leukemia: review of 70 cases with multivariate regression analysis. Hematol Oncol. 1986;4(4):291–305. doi: 10.1002/hon.2900040406. [DOI] [PubMed] [Google Scholar]
- 4.Tomonaga M. Hypocellular acute leukemia. Rinsho Ketsueki. 1995;36(5):457–64. [PubMed] [Google Scholar]
- 5.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 6.Bennett JM, Orazi A. Diagnostic criteria to distinguish hypocellular acute myeloid leukemia from hypocellular myelodysplastic syndromes and aplastic anemia: recommendations for a standardized approach. Haematologica. 2009;94(2):264–8. doi: 10.3324/haematol.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai K, Kohno T, Chen YX, Tsushima H, Mori H, Nakamura H, et al. Diagnostic criteria for hypocellular acute leukemia: a clinical entity distinct from overt acute leukemia and myelodysplastic syndrome. Leuk Res. 1996;20(7):563–74. doi: 10.1016/0145-2126(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 9.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 10.Bacher U, Haferlach T, Kern W, Haferlach C, Schnittger S. A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica. 2007;92(6):744–52. doi: 10.3324/haematol.10869. [DOI] [PubMed] [Google Scholar]
- 11.Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations. Am J Clin Pathol. 2004;122(3):348–58. doi: 10.1309/5DGB-59KQ-A527-PD47. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Estey EH, Pierce S, Keating MJ. Identification of a group of AML/MDS patients with a relatively favorable prognosis who have chromosome 5 and/or 7 abnormalities. Haematologica. 2000;85(3):246–9. [PubMed] [Google Scholar]
- 14.Zuo Z, Chen SS, Chandra PK, Galbincea JM, Soape M, Doan S, et al. Application of COLD-PCR for improved detection of KRAS mutations in clinical samples. Mod Pathol. 2009;22(8):1023–31. doi: 10.1038/modpathol.2009.59. [DOI] [PubMed] [Google Scholar]
- 15.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 16.Constantinidou M, Chalevelakis G, Economopoulos T, Koffa M, Liloglou T, Anastassiou C, et al. Codon 12 ras mutations in patients with myelodysplastic syndrome: incidence and prognostic value. Ann Hematol. 1997;74(1):11–4. doi: 10.1007/s002770050248. [DOI] [PubMed] [Google Scholar]
- 17.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20(10):2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 19.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodys-plastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 20.Faderl S, Ravandi F, Garcia-Manero G, Huang X, Jabbour E, Kadia T, et al. Frontline therapy for older patients (pts) with acute myeloid leukemia (AML): clofarabine plus low-dose cytarabine induction followed by prolonged consolidation with clofarabine plus low-dose cytarabine alternating with decitabine. ASH Annual Meeting Abstracts. 2010;116(21):336. [Google Scholar]