Abstract
ATP-binding cassette transporter (and specially P-glycoprotein) activity is a well known prognostic factor in acute myeloid leukemia, but when compared to other molecular markers its prognostic value has not been well studied. Here we study relationships between this activity, fms-like tyro-sine kinase 3(FLT3/ITD), nucleophosmin(NPM1), CAAT-enhancer binding protein alpha(CEBPα), and brain and acute leukemia cytoplasmic protein (BAALC), in 111 patients with normal cytogenetics who underwent the same treatment, and evaluate its prognostic impact.
Independent factors for survival were age (P=0.0126), ATP-binding cassette transporter activity (P=0.018) and duplications in the fms-like tyrosine kinase 3 (P=0.0273). In the 66 patients without fms-like tyrosine kinase 3 duplication and without nucleophosmin mutation, independent prognostic factors for complete remission achievement and survival were age and ATP-binding cassette transporter activity.
In conclusion, ATP-binding cassette transporter activity remains an independent prognostic factor, and could assist treatment decisions in patients with no nucleophosmin mutation and no fms-like tyrosine kinase 3 duplication.
Keywords: acute myeloid leukemia, ABC transporter, FLT3, NPM1, CEBPA. BAALC
Introduction
The overall prognosis for adult acute myeloid leukemia (AML) remains poor. Cytogenetic analysis provides the major prognostic information,1 but more than 40% of patients have normal cytogenetics (CN-AML). In these cases, many molecular alterations with prognostic significance have been described to guide treatment. Mutations involving nucleophosmin (NPM1) and the CCAAT/enhancer binding protein alpha (CEBPA) have been included as references in the World Health Organization classification of AML, and examination for mutations of FLT3 is also strongly recommended.2 Moreover, many other molecular alterations and/or deregulation of gene expression have been identified and analyzed, such as brain and acute leukemia cytoplasmic protein (BAALC) expression.3–5 Unfortunately, their prognostic value has not been shown in all studies.6
In addition to these markers, the prognostic role of ABC proteins has been well characterized.7 Among them, the best known is ABCB1 (MDR1/Pgp) whose expression and activity8 have been associated with poor outcome. However, the role of other members of the ABC protein family has also been described.9,17
To our knowledge, relationships between ABC proteins and those molecular markers have not yet been explored, except for FLT3/ITD.10 Whether ABC protein activity at the time of diagnosis remains an independent prognosis factor in adult AML, despite the use of these new molecular markers, should be evaluated.
Here we explore the relationships between ABC protein activity, FLT3/ITD, NPM1, CEBPA, and BAALC expression, and evaluate whether ABC protein activity remains a prognostic factor and can be helpful for therapeutic decisions in 111 CN-AML patients who underwent the same treatment according to EORTC protocols.
Design and Methods
Patients and treatments
Bone marrow or blood samples from the time of diagnosis were obtained from 111 CN-AML patients after receiving their informed consent, and in accordance with local ethics committee approval (APHP, Formulary EORTC study n. 06931, n. 06991 and n. 06954). All patients treated in our institution between 1996 and 2008, included in either the EORTC AML-10 or AML-12 trials (for patients younger than 60 years) or in AML-13 trial (for patients older than 60 years) with available material were included. These treatments have already been described in detail.11–13 In the AML-10 and AML-12 protocols, patients with prior myelodysplasia or myeloproliferative disease were not included. The median follow-up time of patients who were still alive was five years. The availability of an HLA-matched donor was recorded for all young patients. Forty-two patients underwent allogeneic stem cell transplantation in first CR. For these patients, survival data have been censored at the time of transplantation.
FLT3/ITD, NPM1 mutations, CEBPA mutations and BAALC expression analyses
FLT3/ITD, NPM1 mutations and BAALC expression analyses have been previously described in detail14 and are available in the Online Supplementary Appendix.
Relative BAALC expression values were calculated with the comparative cycle threshold method using ABL as endogenous internal control, and one patient sample as positive control, whose expression was set at 1.
Concerning CEBPA mutations, all samples were first analyzed with genescan technology. All samples were analyzed a second time using High Resolution Melting technology and sequencing. Detailed methods are available in the Online Supplementary Appendix. As single CEBPA mutations had no prognostic impact in our patients, we only considered double CEBPA mutations in further statistical analyses, as already described.15
ABC transporter functional assay with JC1 probe
JC1 assay has already been described in detail.16 Briefly, cells were incubated with JC1 monomer with or without cyclosporine (CsA) then washed. Cell fluorescence was recorded using a FAC-SORT flow cytometer (Becton-Dickinson). Results were established in the whole blast cell population selected by CD45 antibody weak expression. JC1 uptake was expressed as D value ranging from 0 (no difference) to 1 (no overlap) generated by the Kolmogorov-Smirnov test, which was used to determine the differential distribution in the presence and in the absence of CsA. D of 0.6 or over was considered as high functionality.16 This assay was initially described to test ABCB1 activity, but it seems that it could actually evaluate the pooled activity of the whole ABC protein family (personal data, unpublished).
Statistical analysis
Associations between ABC protein functional assay and patients’ baseline characteristics were analyzed using Fisher, Mann-Whitney or Kruskall-Wallis tests. Complete remission (CR) was defined as recovery of morphologically normal bone marrow and normal blood count (i.e. neutrophil count ≥ 1×109/L and platelet count ≥ 100×109/L), with no evidence of extramedullary disease. Disease free survival (DFS) was measured from the date of CR until the date of relapse or death from any cause. Overall survival (OS) was measured from the date of diagnosis until the date of death from any cause. Estimated probabilities of DFS and OS were calculated using the Kaplan-Meier method and differences between survival distributions were evaluated by the log rank test. Proportional hazards models were constructed to determine whether high ABC protein functional assay was associated with outcome, when adjusting for other prognostic variables. Full models used variables with a P value of less than 0.2 in univariate analysis. For all analyses, results were considered as significant when P value was 0.05 or less. StatView software (version 5.0) was used for statistical analysis (SAS Institute, Inc., San Diego, CA, USA).
Results and Discussion
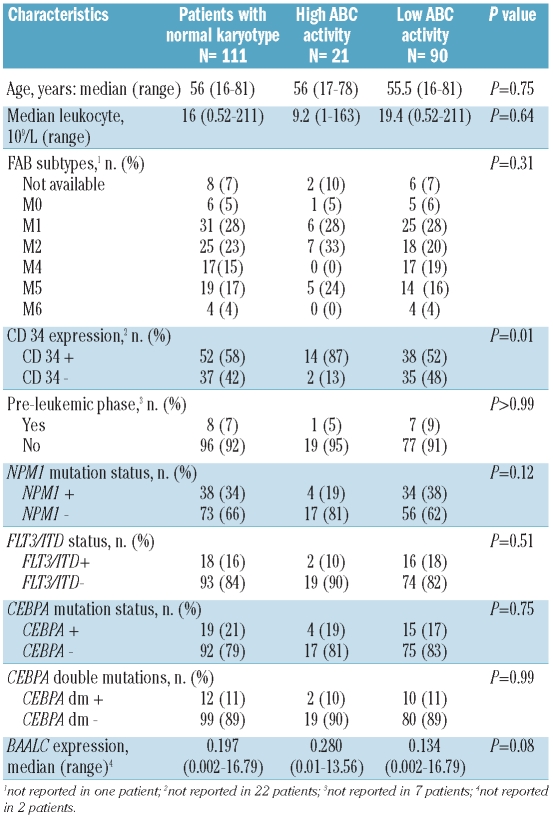
One hundred and eleven patients were enrolled. Main clinical and biological characteristics are summarized in Table 1. Twenty-one patients (19%) had high ABC protein activity. High ABC protein activity was associated with CD34 expression (P=0.01). There was also a trend in patients with high ABC protein activity to have higher BAALC expression (P=0.08) and NPM1 WT (P=0.12), but there was no difference in other variables (Table 1).
Table 1.
Comparison of clinical and biological variables according to ABC protein activity.
In the whole population, 79% of patients (88 of 111) achieved CR, 40%±5% achieved DFS, and 42%±5% achieved OS at five years. Survival curves for DFS and OS according to ABC protein activity are available in the Online Supplementary Appendix (Online Supplementary Figure S1). We used univariate analysis to evaluate the following parameters for CR, DFS and OS: age, leukocyte count, existence of a pre-leukemic phase, FLT3/ITD status, NPM1 mutational status, CEBPA mutational status, BAALC expression, ABC protein activity and type of anthracycline used. A multivariate model for CR, DFS and OS was used for all variables with a P value less than 0.2 in univariate analysis. Age was the only prognostic factor we found for CR (P=0.0009 univariate analysis; P=0.0056 multivariate analysis; data not shown).
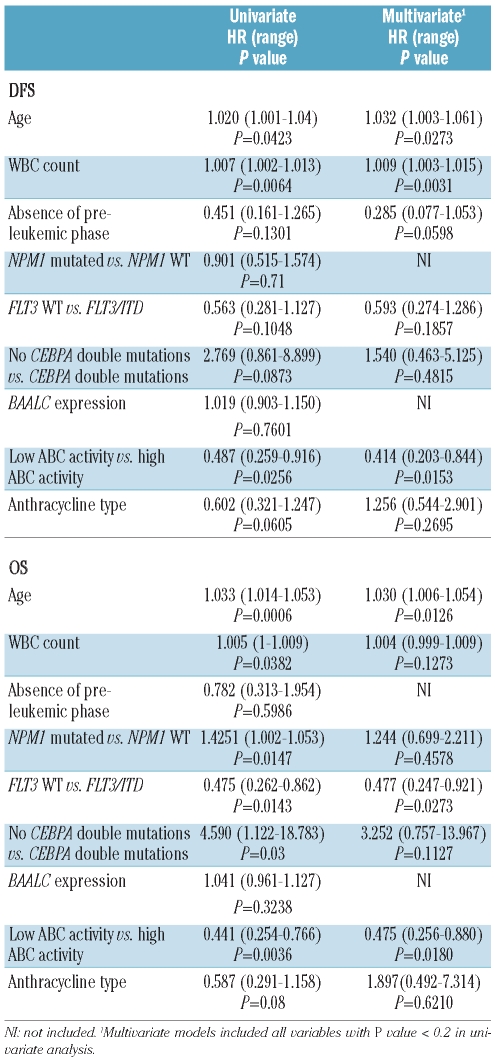
For DFS, in univariate analysis, age (P=0.0423), leukocyte count (P=0.0064), and ABC protein activity (P=0.0256) had significant prognostic value, but other variables did not. In our multivariate model, leukocyte count (P=0.0031), age (P=0.0273), and ABC protein activity (P=0.0153) were independent prognostic factors (Table 2).
Table 2.
Univariate and multivariate analyses for disease free survival and overall survival in the whole population.
For OS, in univariate analysis, age (P=0.0006), leukocyte count (P=0.0382), NPM1 mutation (P=0.0147), FLT3/ITD (P=0.0143), double CEBPA mutations (P=0.03) and ABC protein activity (P=0.0036) had significant prognostic value, but BAALC expression (P=0.32) did not (Table 2). In multivariate analysis, independent prognostic factors for OS were age (P=0.0126), ABC protein activity (P=0.0180) and FLT3/ITD (P=0.0273) (Table 2).
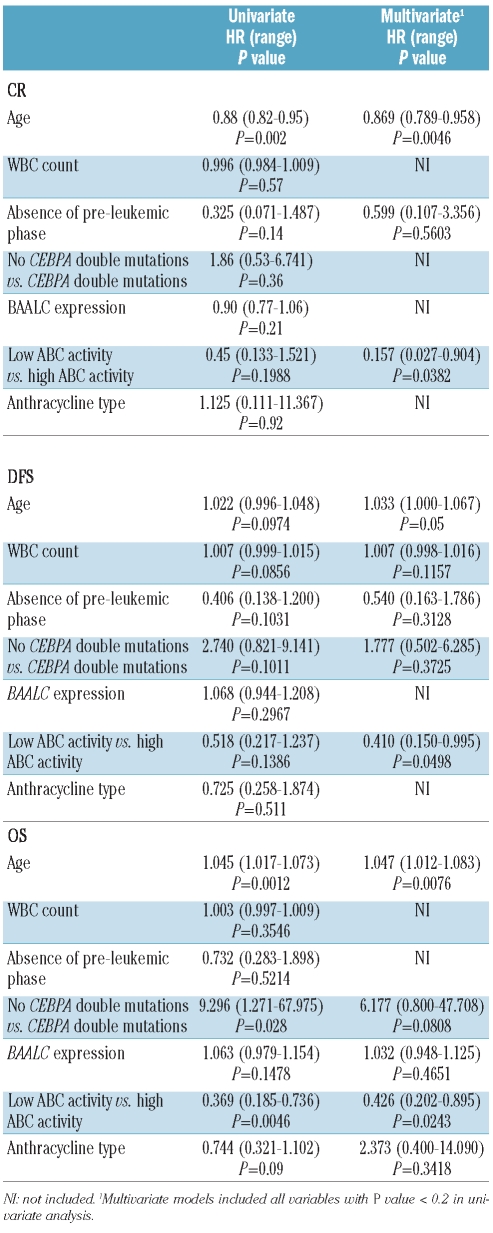
As treatment decisions in patients with both NPM1 WT and no FLT3/ITD are one of the major problems in CN-AML, we performed a second analysis in this subgroup (n=66). In these patients, 13 had high ABC protein activity. We evaluated the same variables as previously in univariate analysis. Results are shown in Table 3.
Table 3.
Statistical analyses for CR, DFS and OS in patients with both NPM1 WT and no FLT3/ITD.
In multivariate models, including all parameters with P value less than 0.2 under univariate analysis, the only significant prognostic factors for achievement of CR, DFS and OS were age (P=0.0046, P=0.05 and P=0.0076, respectively) and ABC protein activity (P=0.0382, P=0.0498 and P=0.0243, respectively).
Many molecular alterations have been described and can be used to guide treatments in CN-AML, but identification of powerful markers is still needed to refine treatment strategy. Here, we evaluated the impact of the well-known ABC proteins and compared them to currently used molecular markers. ABC protein activity was evaluated with JC1 probe and cyclosporine. Using flow cytometry, this test, which is easily performed as part of daily routine, is highly reproducible and is more sensitive than the rhodamine-123 assay.16,18 JC1 assay was initially developed to test ABCB1 activity, but even though until now, it has not been fully studied it seems that it actually evaluates the pooled activity of the whole ABC protein family. Indeed, a recent study performed by our team has shown that the “D value” of JC1 assay was correlated to the number of ABC proteins expressed in blast cells. This might be linked to a lack of specifity of JC1, and to the broad spectrum of ABC proteins inhibited by CsA (personal data, unpublished). Consequently, it is likely that our results reflect the activity of at least a part of the ABC protein family, and not only ABCB1.
Patients with high ABC protein activity had higher CD34 expression and tended to have higher BAALC expression. When we studied a larger sample of 206 AML patients, including patients with abnormal cytogenetics, high ABC protein activity was significantly associated with NPM1 WT and higher BAALC expression (Online Supplementary Table S1). It is already known that CD34+ cells have a higher physiological ABC protein activity19 and in a gene expression study, high BAALC expression was associated with higher CD34 and ABCB1 expression.4 Our data confirm this association. Surprisingly, BAALC expression had no prognostic value in our study. This could be partially explained by the small size of our sample, but the high P value (P>0.2) associated with BAALC expression in all univariate analyses, when other variables are highly significant, seems to go against this hypothesis. The association between NPM1 WT and high ABC proteins’ activity has never been described to our knowledge, and might contribute to the poorer prognosis associated with NPM1 WT. Interestingly, in our patients, NPM1 mutational status had significant impact on OS in univariate analysis, but not in multivariate analysis, which could be partially explained by this association. Relationships between NPM1 and ABC proteins and between BAALC and ABC proteins should be investigated more deeply.
Around 20% of patients in the whole study population had high ABC protein activity; this includes patients with NPM1 WT and no FLT3/ITD. The independent prognostic factors we found for OS in the whole population were age, FLT3/ITD and ABC protein activity. In cases of NPM1 WT and no FLT3/ITD, ABC protein activity was the only independent prognostic factor we found, in addition to age. Therefore, we think that ABC protein activity should be assessed in patients with normal cytogenetics at the time of diagnosis, and could be extremely useful in guiding therapeutic decisions, at least in cases of NPM1 WT and no FLT3/ITD, due to the high frequency of high ABC protein activity, and to its high prognostic value. In all our analyses, we found no prognostic significance for single CEPBA mutations. When considering only double CEBPA mutations, as recently described,15 we found a significant prognostic value for OS in univariate analysis, but only a trend in our multivariate models. Although these results could be explained in part by the relatively small number of patients included, it underlines again that ABC protein activity seems to be a more powerful marker than the currently used molecular markers.
In conclusion, we showed that evaluating ABC protein activity at the time of diagnosis in CN-AML remains a valuable prognostic factor, especially in cases of NPM1 WT and no FLT3/ITD. Furthermore, ABC protein activity was the only independent prognostic factor in addition to age and to FLT3/ITD. We think that the JC1 test, which can easily be performed as part of daily routine, should be carried out in every adult patient with CN-AML, and could be helpful in refining treatment strategy. This has to be confirmed in larger prospective studies.
Footnotes
Funding: this work was supported in part by Tumorothèque Leucémies, Hôpital Saint-Antoine, Paris, in part by Inserm, and in part by Université Pierre et Marie Curie.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 2.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 3.Baldus CD, Tanner SM, Ruppert AS, Whitman SP, Archer KJ, Marcucci G, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B Study. Blood. 2003;102(5):1613–8. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 4.Langer C, Radmacher MD, Ruppert AS, Whitman SP, Paschka P, Mrozek K, et al. High BAALC expression associates with other molecular prognostic markers, poor outcome, and a distinct gene-expression signature in cytogenetically normal patients younger than 60 years with acute myeloid leukemia: a Cancer and Leukemia Group B (CALGB) study. Blood. 2008;111(11):5371–9. doi: 10.1182/blood-2007-11-124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcucci G, Maharry K, Whitman SP, Vukosavljevic T, Paschka P, Langer C, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2007;25(22):3337–43. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 6.Mrozek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood. 2007;109(2):431–48. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbach D, Legrand O. ABC transporters and drug resistance in leukemia: was P-gp nothing but the first head of the Hydra? Leukemia. 2007;21(6):1172–6. doi: 10.1038/sj.leu.2404692. [DOI] [PubMed] [Google Scholar]
- 8.Broxterman HJ, Sonneveld P, Feller N, Ossenkoppele GJ, Wahrer DC, Eekman CA, et al. Quality control of multidrug resistance assays in adult acute leukemia: correlation between assays for P-glycoprotein expression and activity. Blood. 1996;87(11):4809–16. [PubMed] [Google Scholar]
- 9.Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94(3):1086–99. [PubMed] [Google Scholar]
- 10.Marzac C, Teyssandier I, Calendini O, Perrot JY, Faussat AM, Tang R, et al. Flt3 internal tandem duplication and P-glycoprotein functionality in 171 patients with acute myeloid leukemia. Clin Cancer Res. 2006;12(23):7018–24. doi: 10.1158/1078-0432.CCR-06-0641. [DOI] [PubMed] [Google Scholar]
- 11.Maurillo L, Buccisano F, Spagnoli A, Del Poeta G, Panetta P, Neri B, et al. Monitoring of minimal residual disease in adult acute myeloid leukemia using peripheral blood as an alternative source to bone marrow. Haematologica. 2007;92(5):605–11. doi: 10.3324/haematol.10432. [DOI] [PubMed] [Google Scholar]
- 12.Mandelli F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol. 2009;27(32):5397–403. doi: 10.1200/JCO.2008.20.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amadori S, Suciu S, Jehn U, Stasi R, Thomas X, Marie JP, et al. Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood. 2005;106(1):27–34. doi: 10.1182/blood-2004-09-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang R, Hirsch P, Fava F, Lapusan S, Marzac C, Teyssandier I, et al. High Id1 expression is associated with poor prognosis in 237 patients with acute myeloid leukemia. Blood. 2009;114(14):2993–3000. doi: 10.1182/blood-2009-05-223115. [DOI] [PubMed] [Google Scholar]
- 15.Green CL, Koo KK, Hills RK, Burnett AK, Linch DC, Gale RE. Prognostic significance of CEBPA mutations in a large cohort of younger adult patients with acute myeloid leukemia: impact of double CEBPA mutations and the interaction with FLT3 and NPM1 mutations. J Clin Oncol. 2010;28(16):2739–47. doi: 10.1200/JCO.2009.26.2501. [DOI] [PubMed] [Google Scholar]
- 16.Legrand O, Perrot JY, Simonin G, Baudard M, Marie JP. JC-1: a very sensitive fluorescent probe to test Pgp activity in adult acute myeloid leukemia. Blood. 2001;97(2):502–8. doi: 10.1182/blood.v97.2.502. [DOI] [PubMed] [Google Scholar]
- 17.Marzac C, Garrido E, Tang R, Fava F, Hirsch P, De Benedictis C, et al. ATP Binding Cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts, and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011 May 23; doi: 10.3324/haematol.2010.031823. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhnel JM, Perrot JY, Faussat AM, Marie JP, Schwaller MA. Functional assay of mul-tidrug resistant cells using JC-1, a carbocyanine fluorescent probe. Leukemia. 1997;11(7):1147–55. doi: 10.1038/sj.leu.2400698. [DOI] [PubMed] [Google Scholar]
- 19.Marie JP, Brophy NA, Ehsan MN, Aihara Y, Mohamed NA, Cornbleet J, et al. Expression of multidrug resistance gene mdr1 mRNA in a subset of normal bone marrow cells. Br J Haematol. 1992;81(2):145–52. doi: 10.1111/j.1365-2141.1992.tb08199.x. [DOI] [PubMed] [Google Scholar]