Abstract
The recent identification of DNMT3A mutations in de novo acute myeloid leukemia prompted us to determine their frequency, patterns and clinical impact in a cohort of 98 patients with either therapy-related or secondary acute myeloid leukemia developing from an antecedent hematologic disorder. We identified 24 somatic mutations in 23 patients with a significantly higher frequency in secondary acute myeloid leukemia (35.1%) as compared to therapy-related acute myeloid leukemia (16.4%, P=0.0486). DNMT3A mutations were associated with a normal karyotype and IDH1/2 mutations, but did not affect survival. In contrast to de novo acute myeloid leukemia, most mutations did not affect arginine on position 882, but were predominantly found in the methyltransferase domain. All DNMT3A mutations identified in secondary acute myeloid leukemia were already present in the antecedent disorders indicating an early event. Reduction to homozygosity by uniparental disomy was observed in 2 patients with secondary acute myeloid leukemia during disease progression.
Keywords: acute myeloid leukemia, secondary and therapy-related AML, DNMT3A, mutation
Introduction
Epigenetic deregulation of gene expression is increasingly recognized as being fundamental for the pathogenesis of acute myeloid leukemia (AML). Although the mechanisms by which these changes contribute to leukemogenesis are still not completely understood, the identification of mutations in several genes involved in epigenetic regulation has given new insight into these mechanisms. Recently, whole genome and exome sequencing led to the identification of recurrent somatic mutations in DNMT3A (encoding DNA methyltransferase 3A) in about 20% of patients with de novo AML.1–3 DNMT3A is crucial for the methylation of unmodified DNA in CpG islands by converting cytosine to 5-methylcytosine which is associated with gene silencing.4,5 In vitro, enzymatic assays showed a reduction in DNA methylation by mutated DNMT3A and overexpression of the two most frequent DNMT3A mutants (R882H and R882C) promoted proliferation in cell culture experiments.3 Of clinical importance, DNMT3A mutations were associated with decreased overall survival.1,3,6 These results strongly suggest a prominent pathogenetic role of DNMT3A mutations in AML.
Although most patients are diagnosed with de novo AML, in about 20–30% of cases AML presents as a sequelae of a previous disease. This group includes patients in whom AML develops after chemo- and/or radiotherapy for a primary, most often malignant disease. Furthermore, patients suffering from myelodysplastic syndromes or myeloproliferative neoplasms (MDS/MPN) have a substantial risk of transformation to AML. Both therapy-related (t-AML) as well as secondary AML (sAML) following an antecedent MDS/MPN are associated with low survival rates following conventional therapy including hematopoietic stem cell transplantation.7–9 In some cases, t-AML and sAML share common cytogenetic and molecular alterations with poor risk de novo AML, such as loss of chromosome 5 and 7.10,11 However, in a substantial proportion of patients, genetic alterations responsible for the dismal clinical course have not been established.
To determine the frequencies, patterns and clinical impact of DNMT3A mutations in patients with t-AML and sAML, we carried out mutational analysis of all coding exons of DNMT3A in a cohort of 98 patients using direct DNA sequencing. Furthermore, specimens from the antecedent hematologic disorder in sAML patients with mutated DNMT3A provided fresh insight into the onset of DNMT3A mutations and their role in leukemic transformation.
Design and Methods
Patients, DNA isolation and sequencing
Thirty-seven patients with sAML and 61 patients with t-AML were included in this study. Diagnosis was made according to the French-American-British (FAB) classification. The majority of sAML patients had a history of antecedent MDS (n=27; 10 refractory anemia with excess blasts (RAEB); one RAEB in transformation; 16 refractory anemia) whereas the remaining sAML patients had a history of MPN (n=10; 5 primary myelofibrosis; one essential thrombocythemia; one polycythemia vera; 3 chronic myelomonocytic leukemia).
DNA was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions from diagnostic peripheral blood or bone marrow samples after Ficoll enrichment yielding more than 90% blast cells. In patients with MDS or MPN, DNA was isolated from bone marrow biopsy specimens without further enrichment. For analysis of constitutional material, DNA was either isolated from buccal swabs or biopsies of non-hematologic tissues. cDNA was synthesized using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and the RevertAidTM H Minus First strand cDNA Synthesis Kit (Fermentas Life Science Europe, Bremen, Germany). This study was approved by the institutional review board of the Medical University of Graz, Austria, and was conducted in accordance with the Declaration of Helsinki.
All coding exons including the splice sites of DNMT3A were amplified by PCR (primers and detailed PCR conditions are given in the Online Supplementary Table S1). Sequencing of both strands was performed with the ABI PRISM 3730 (Applied Biosystems, Foster City, CA, USA) and mutations were identified using SeqScape®Software v2.5 (Applied Biosystems, Foster City, CA, USA). Each identified mutation was confirmed at least once by an independent sequence analysis using genomic DNA. Analysis of mutations in IDH1/2 was performed as previously described.12
SNP array analysis in patients with homozygous DNMT3A mutations
Whole genome single nucleotide polymorphism (SNP) analysis was performed to determine copy number variations and regions with uniparental disomy in patients with homozygous DNMT3A mutations. For this purpose, Affymetrix human genome-wide SNP 6.0 arrays (Affymetrix, Santa Clara, CA, USA) were used according to the manufacturer’s protocol and results were analyzed in comparison to 60 HapMap individuals using Genotyping ConsoleTM Version 4.0.
Statistical analysis
Differences in characteristics of patients with or without DNMT3A mutations were calculated using a two-sided Fisher’s exact or Mann-Whitney test. The Kaplan-Meier method was applied to generate the survival curves and differences were assessed by log rank analysis. R 2.13.0 software (www.r-project.org) was used for the analyses.
Results and Discussion
Frequency and patterns of DNMT3A mutations in t- and sAML
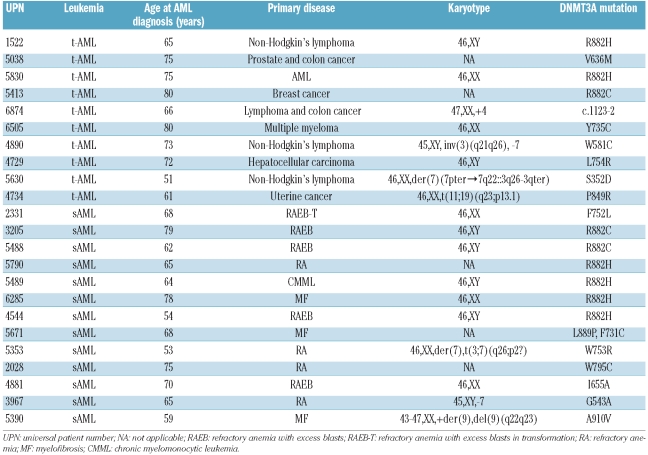
Of 98 t-AML and sAML samples in this study, a DNMT3A mutation was identified in a total of 23 (23.6%). One patient displayed two mutations (clinical details of patients with mutations are given in Table 1; a comparison of clinical characteristics in DNMT3A-mutated vs. non-mutated patients is given in the Online Supplementary Table S2). The frequency of DNMT3A mutations in this cohort is, therefore, similar to patients with de novo AML, which was reported to be 22.1%,1 20.5%3 and 17.8%.6 However, when assessing sAML and t-AML patients of our cohort separately, the percentage of patients with a DNMT3A mutation was higher in sAML (13 of 37, 35.1%) than in t-AML (10 of 61, 16.4%; P=0.0486). By analyzing patients for whom information on cytogenetics was available, the frequency of mutations was significantly higher in patients with a normal karyotype (11 of 25 patients, 44%) as compared to patients with cytogenetic aberrations (7 of 50, 14%; P=0.00841). Furthermore, as has been reported for de novo AML,1 mutations in the IDH1/2 gene were associated with mutated DNMT3A in our cohort of t-AML and sAML patients (5 of 23, 21.7% vs. 2 of 75, 2.7%, P=0.00731; Online Supplementary Table S2).
Table 1.
Clinical characteristics of t-AML and sAML patients with DNMT3A mutations.
The mutations identified in this study were made up of 23 missense and one splice site mutation located upstream of exon 10 (c.1123-2 A>G; Table 1 and Online Supplementary Figure S1). Although the hot spot mutation affecting arginine on position 882 was also the most frequent mutation in our study (3 in t-AML and 6 in sAML), the majority of mutations in DNMT3A (n=15) affected other amino acid residues (Table 1 and Online Supplementary Figure S1). This is in contrast to the findings in de novo AML, where according to all published studies1,3,6 116 out of 176 mutations were affecting R882 (P=0.01203 as compared to our cohort). These results suggest a somewhat different pattern of DNMT3A mutations in t-AML and sAML as compared to de novo AML. Since most of our identified mutations (S352N, W581C, V636M, I655A, F731C, Y735C, F752L, W753G, L754R, W795C, P849R, L889P, A910V) have not been reported previously, we tested constitutional DNA to prove the somatic nature of these mutations (Figure 1 and Online Supplementary Figure S1). In all but one case, in which we had no access to constitutional material, we were able to delineate the somatic origin of the mutations. In the remaining case, the affected amino acid is highly conserved among species and is not reported to constitute single nucleotide polymorphisms in public databases. Mutation of the acceptor splice site resulted in the use of an alternative 3’ splice site upstream, thereby inserting a 40-bp intronic sequence in between exon 9 and exon 10 in the patient’s mRNA (Online Supplementary Figure S1A, lower left panel). As a consequence, a premature stop codon is introduced that is predicted to lead to a truncated protein with 432 amino acids lacking the zinc finger and methyltransferase domains.
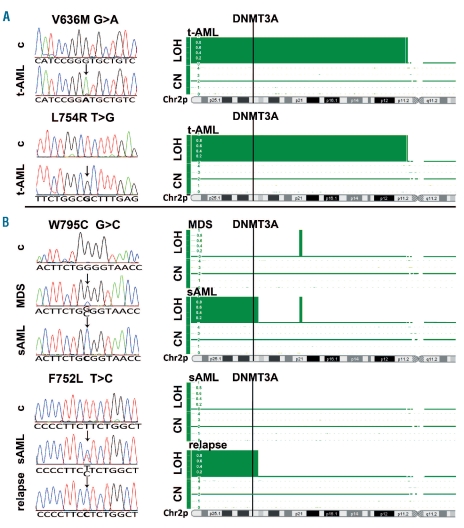
Figure 1.
Uniparental disomy of 2p23.3 results in homozygous DNMT3A mutations in distinct t-AML and sAML patients. (A) Electropherograms of two homozygous DNMT3A mutations in t-AML samples showing no mutation in the corresponding constitutional material. SNP-array analysis revealed a copy-neutral loss of heterozygosity of 2p11-2pter that harbors the DNMT3A gene locus in both t-AML samples. (B) Electro-pherograms of one patient (upper panel) with a somatic heterozygous mutation in MDS who developed a homozygous W795C mutation during the progression to sAML. A second patient (lower panel) showed a somatic heterozygous mutation at sAML diagnosis, but displayed a homozygous F752L mutation at the time of sAML relapse after autologous stem cell transplantation. Corresponding SNP-array analysis demonstrated a copy-neutral loss of heterozygosity of 2p23.3-2pter in both samples with the homozygous mutations, but not in the samples with heterozygous DNMT3A mutations. c: constitutional material; LOH: loss of heterozygosity; CN: copy number; Chr2p: short arm of chromosome 2.
DNMT3A mutations occur early in MDS/MPN and reduction to homozygosity is occasionally associated with disease progression
Two recent studies reported an incidence of 8 and 10% of DNMT3A mutations in MDS and MPN, respectively,13,14 substantially lower than the percentage of mutated samples in our sAML cohort (35.1%). One explanation for this discrepancy might be the acquisition of DNMT3A mutations during MDS/MPN progression resulting in transformation to sAML. To test this hypothesis, we analyzed DNA from bone marrow biopsies at initial diagnosis of MDS/MPN in 10 patients with mutated sAML samples. In all patients, the corresponding mutation could already be detected at that time indicating that DNMT3A mutations are an early event in the pathogenesis of these malignancies. Interestingly, we identified one MDS patient with a heterozygous mutation that became homozygous during progression to sAML (Figure 1B, upper panel). Another patient, who was still heterozygous for the mutation at sAML diagnosis displayed homozygosity for the mutation at the time of sAML relapse after autologous stem cell transplantation (Figure 1B, lower panel). Remarkably, this patient also showed a homozygous IDH2 mutation at relapse of the sAML, which was recently reported by our group.12 These results indicate that in some MDS/MPN patients, progression of disease is associated with the loss of the DNMT3A wild-type allele.
Two patients with t-AML also showed a homozygous DNMT3A mutation (Figure 1A) which, together with the 2 sAML patients, is a substantial number considering that homozygous DNMT3A mutations have rarely been documented in AML.1,3 To identify the mechanisms leading to homozygosity of mutant DNMT3A, we performed copy-number variation analysis and heterozygosity mapping in all 4 patients using Affymetrix human genome-wide SNP 6.0 arrays. We detected a copy-neutral loss of heterozygosity of 2p23.3-2pter in both sAML patients and a copy-neutral loss of heterozygosity of 2p11-2pter in both t-AML patients (Figure 1C). Since the DNMT3A gene is located on 2p23.3, these results implicate uniparental disomy (UPD) of the region carrying the mutated DNMT3A allele in all these patients. Except for the patient who also showed a homozygous IDH2 mutation and UPD of the involved region on 15q as well as a deletion of 7q,14 UPD of 2p was the only detected aberration in the remaining 3 patients using SNP array analysis.
Clinical impact of DNMT3A mutations in t-AML and sAML
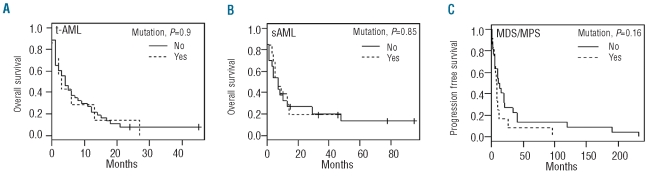
In de novo AML, DNMT3A mutations are associated with an adverse prognosis.1,3,6 However, while uni- and multivariate analysis identified well-known risk factors for poor survival in our cohort, such as cytogenetic risk and white blood cell counts at AML diagnosis, the presence of DNMT3A mutations did not affect overall survival in t-AML and sAML patients (Figure 2A and B). Since t-AML and sAML are known to be associated with poor survival as compared to de novo AML and many of these patients display poor risk cytogenetics, it may not be surprising that DNMT3A mutations do not further add to the poor prognosis in this cohort of patients. Mutated patients showed a shorter interval between MDS/MPN diagnosis and transformation to sAML (median time to progression 7.5 vs. 12 months), which is in accordance with the findings of Walter et al. who reported a more rapid progression to AML in MDS patients with DNMT3A mutations.13 However, in our cohort the difference was not statistically significant (P=0.17).
Figure 2.
Survival analysis of 82 t-AML and sAML patients with respect to their DNMT3A mutational status. (A) The overall survival of t-AML patients with DNMT3A mutations (n=7) as compared to non-mutated patients (n=42). (B) The overall survival of sAML patients with DNMT3A mutations (n=13) as compared to patients without DNMT3A mutations (n=20). (C) Kaplan-Meier plot of time to progression to sAML with respect to the DNMT3A mutation status in patients diagnosed with MDS/MPN.
In summary, this study demonstrated DMNT3A mutations in about one sixth of patients with t-AML. In sAML, even one out of 3 patients was carrying a mutation in DNMT3A. The respective mutations occurred early in the myelodysplastic or myeloproliferative phase of the disease and reduction to homozygosity by uniparental disomy of the genetic region involved was found in some patients during disease progression. DNMT3A mutations did not constitute an additional adverse risk factor in these poor prognostic subtypes of AML.
Footnotes
Funding: this study was supported by Leukämiehilfe Steiermark.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363(25):2424–33. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita Y, Yuan J, Suetake I, Suzuki H, Ishikawa Y, Choi YL, et al. Array-based genomic resequencing of human leukemia. Oncogene. 2010;29(25):3723–31. doi: 10.1038/onc.2010.117. [DOI] [PubMed] [Google Scholar]
- 3.Yan XJ, Xu J, Gu ZH, Pan CM, Lu G, Shen Y, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43(4):309–15. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 4.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 5.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286–98. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 6.Thol F, Damm F, Lüdeking A, Winschel C, Wagner K, Morgan M, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29(21):2889–96. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- 7.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22(12):2510–1. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 8.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–45. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 10.Sill H, Olipitz W, Zebisch A, Schulz E, Wölfler A. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162(4):792–805. doi: 10.1111/j.1476-5381.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauritzson N, Albin M, Rylander L, Billström R, Ahlgren T, Mikoczy Z, et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976–1993 and on 5098 unselected cases reported in the literature 1974–2001. Leukemia. 2002;16(12):2366–78. doi: 10.1038/sj.leu.2402713. [DOI] [PubMed] [Google Scholar]
- 12.Pichler MM, Bodner C, Fischer C, Deutsch AJ, Hiden K, Beham-Schmid C, et al. Evaluation of mutations in the isocitrate dehydrogenase genes in therapy-related and secondary acute myeloid leukaemia identifies a patient with clonal evolution to IDH2 R172K homozygosity due to uniparental disomy. Br J Haematol. 2011;152(5):669–72. doi: 10.1111/j.1365-2141.2010.08404.x. [DOI] [PubMed] [Google Scholar]
- 13.Walter MJ, Ding L, Shen D, Shao J, Grillot M, McLellan M, et al. Recurrent DNMT3A mutations in patients with myelodysplastic syndromes. Leukemia. 2011;25(7):1153–8. doi: 10.1038/leu.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stegelmann F, Bullinger L, Schlenk RF, Paschka P, Griesshammer M, Blersch C, et al. DNMT3A mutations in myeloproliferative neoplasms. Leukemia. 2011;25(7):1217–9. doi: 10.1038/leu.2011.77. [DOI] [PubMed] [Google Scholar]