Abstract
Translocation of the LYL1 oncogene are rare in T-cell acute lymphoblastic leukemia, whereas the homologous TAL1 gene is rearranged in approximately 20% of patients. Previous gene-expression studies have identified an immature T-cell acute lymphoblastic leukemia subgroup with high LYL1 expression in the absence of chromosomal aberrations. Molecular characterization of a t(7;19)(q34;p13) in a pediatric T-cell acute lymphoblastic leukemia patient led to the identification of a translocation between the TRB@ and LYL1 loci. Similar to incidental T-cell acute lymphoblastic leukemia cases with synergistic, double translocations affecting TAL1/2 and LMO1/2 oncogenes, this LYL1-translocated patient also had an LMO2 rearrangement pointing to oncogenic cooperation between LYL1 and LMO2. In hierarchical cluster analyses based on gene-expression data, this sample consistently clustered along with cases having TAL1 or LMO2 rearrangements. Therefore, LYL1-rearranged cases are not necessarily associated with immature T-cell development, despite high LYL1 levels, but elicit a TALLMO expression signature.
Keywords: T-ALL, pediatric, LYL1, LMO2, rearrangements
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is characterized by chromosomal rearrangements that activate several oncogenes, such as TAL1, LMO2, HOXA, TLX1 and TLX3, which predominantly occur in a mutually exclusive pattern. In our previous study, we used a supervised gene-expression profiling approach to cluster T-ALL patients with these chromosomal aberrations.1 Patients with HOXA, TLX1 and TLX3 abnormalities formed 3 separate T-ALL clusters. Patients with TAL1 and/or LMO2 rearrangements formed a single, fourth TAL-LMO cluster, explained by the fact that TAL1 and LMO2 participate in the same transcription complex and affect similar downstream pathways. Co-clustering of 45 additional patients who lack TAL1, LMO2, HOXA, TLX1 or TLX3 aberrations, led to the identification of 2 additional T-ALL genetic subgroups that are characterized by NKX2-1/NKX2-2 or MEF2C-activating rearrangements.1 The MEF2C-deregulated subgroup overlapped with the early thymic progenitor ALL (ETP-ALL) subgroup, as previously described by Dario Campana and coworkers.2 Nineteen of these 45 patient samples strongly co-clustered with TAL1- or LMO2-rearranged patients in supervised and unsupervised cluster analyses, pointing to a common pathogenic mechanism. These 19 cases were denoted as TALL-MO-likes, and we hypothesized that these patients might harbor rearrangements involving factors homologous to TAL1 or LMO2, or factors that participate in the TAL/LMO transcription complex. This hypothesis was confirmed when we identified translocations that involved LMO3,3 LMO1 or TAL2 in 3 of these TALLMO-like patients.4 A fourth patient had double translocations affecting TAL2 and LMO1 oncogenes.4 To identify aberrations in the remaining 15 TALLMO-like patients, we screened for T-cell receptor driven translocations for which the translocation partner was unknown.
Design and Methods
Patient material
Viably frozen diagnostic bone marrow or peripheral blood samples from 117 pediatric T-ALL patients was used.1,4 Clinical and immunophenotypic data were provided by the German Co-operative study group for childhood Acute Lymphoblastic Leukemia (COALL) and the Dutch Childhood Oncology Group (DCOG). The patients’ parents or their legal guardians provided informed consent to use leftover material for research purposes in accordance with the declaration of Helsinki, and the study was approved by the ethical committee of the Erasmus Medical Center. Leukemic cells were isolated and enriched from these samples as previously described.5 All resulting samples contained 90% or more leukemic cells, as determined morphologically by May-Grünwald-Giemsa-stained cytospins (Merck, Darmstadt, Germany). Cytospin slide preparation and DNA and RNA extraction were performed as previously described.5
Fluorescent in situ hybridization (FISH)
FISH analysis was performed on cytospin slides using the TCRalpha/delta and TCRbeta split signal probes according to the manufacturer’s protocol (DAKO, Glostrup, Denmark). Split signal FISH on the LYL1 locus was performed using the following BAC clones as previously described:6 RP11-352L7, RP11-356L15.
Ligation mediated PCR (LM-PCR) and real-time quantitative PCR (RQ-PCR)
LM-PCR for TRB@ breakpoint hotspots (TRB@D1 and TRB@D2), and RQ-PCR for LYL1 were performed as previously described.5,7,8 For LM-PCR, briefly, genomic DNA was digested with either one of four different restriction enzymes (PvuII, HincII, StuI, DraI) and ligated to adapters. Adaptor primers were then used in combination with TRB@ loci specific primers to amplify the breakpoint region in two PCR rounds. For the detection of the reciprocal LYL1-TRB@ breakpoint, the following specific primers located near LYL1 were used. First: 5′-CGG GCT GGA GGA GAG AAG-3′, nested: 5′-GTG GCT GAC GAC GTG TAA TTT-3′.
Results and Discussion
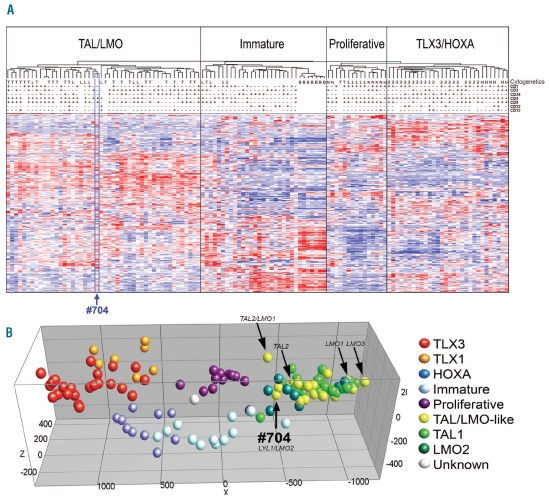
A FISH strategy was performed to identify novel TRB@-or TRAD@-driven oncogenic rearrangements in 15 TAL-LMO-like patients. These 15 cases strongly clustered in hierarchical cluster analyses with T-ALL cases having TAL1/2 and/or LMO1/2/3 rearrangements (Figure 1A and B). One sample (#704), from a 7-year old male patient, showed a TRB@ split signal pointing to a translocation that had not been revealed by karyotypic analysis (47, XY, +8[6]/46, XY[7]).
Figure 1.
Unsupervised and supervised hierarchical clustering of 117 pediatric T-ALL samples and 7 normal bone marrow samples. (A) Unsupervised hierarchical clustering of 117 pediatric T-ALL samples and 7 normal bone marrow samples (horizontal axis), according to microarray gene-expression (genes on vertical axis, gene names not shown).1 Red corresponds to high expression, blue to low expression. CD surface markers are shown as present (>25%, “+”), absent (<25% “−”) or not performed (white). Complete immunophenotype for #704: CD1−, CD2+, CD3−, CD4+, CD5−, CD7+, CD8+, cytoplasmatic CD3+, CD33−, CD14−, CD34−, CD71+, HLA_DR−, TDT+. Cytogentic abnormalities are shown as follows. T: SIL-TAL deletion or TAL1 translocation; L: LMO2 translocation/deletion; 1: TLX1 translocation; 3: TLX3 translocation; B: normal bone marrow; N: NKX2-1 translocation/inversion/duplication; M: MYB translocation; H: HOXA activating abberation (CALM-AF10, SET-NUP, HOXA inversion). Patient #704 is highlighted by a blue box. (B) Principal component analysis of supervised analyses of gene-expression data of 117 pediatric T-ALL samples.1 The position of the yellow dots representing LMO1, TAL2, LMO3, TAL2/LMO1 rearranged cases and sample #704 (LYL1/LMO2) are indicated by arrows.
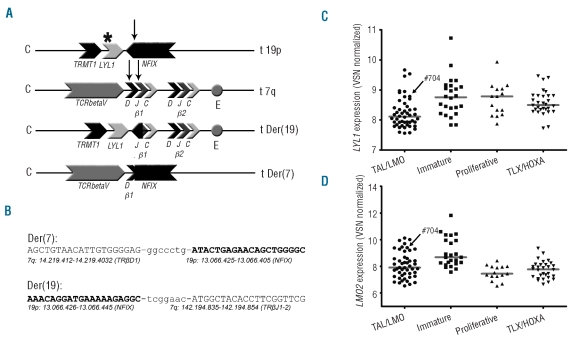
We then performed ligation-mediated PCR (LM-PCR) from two TRB@ translocation hotspots (TRB@D1 and TRB@D2) on DNA from this patient and identified a translocation between TRB@D1 and the last intron of the nuclear factor I/X (NFIX) gene for the derivative chromosome 7 (der(7)) (Figure 2A and B). The reciprocal breakpoint of the derivative chromosome 19 couples part of the last intron of the NFIX gene to an area between TRB@J1 and TRB@J2 (der(19); Figure 2A). The Lymphoid leukemia 1 (LYL1) gene is located 240bp centromeric of NFIX and is, therefore, placed under the influence of the TRB@ enhancer as a consequence of this translocation (Figure 2A). NFIX was not expressed in any of our patient samples based on microarray data (raw fluorescent intensities<50, 5 probesets; data not shown) indicating that changes in NFIX are not contributing to leukemogenesis. Positioning of the LYL1 gene under the influence of the TRB@ enhancer may explain the relatively high expression level of LYL1 in this patient (Figure 2C). FISH analysis of the LYL1 locus on the remaining 14 TALLMO-like patients revealed no additional LYL1 rearrangements.
Figure 2.
Schematic overview of LYL1-TRB@ translocation and breakpoint sequences. (A) Schematic overview of breakpoint loci on germline chromosomes 19 and 7, and the der(19) and der(7). Arrows indicate approximate breakpoint locations; *: approximate breakpoint of previously described LYL1 translocation;9 c: centromeric side; t: telomeric side of chromosal region. (B) Reciprocal breakpoint sequences of the t(7;19)(q34;p13). In caps sequences corresponding to chromosomal regions as described below, in non-caps; randomly inserted nucleotides. LYL1 (C) and LMO2 (D) expression according to VSN normalized array data in the four subgroups as shown in Figure 1A.
Various research groups including ours have reported that LYL1 and LMO2 are highly expressed in T-ALL patients with an immature immunophenotype,10–12 despite the fact that LMO2 rearrangements that are also associated with ectopic LMO2 expression are exclusively associated with the TALLMO subgroup which has a more advanced immunophenotype.1,13 In another study,2 immature T-ALL cases were described with an early thymic progenitor expression profile that was associated with poor prognosis, and were denoted as ETP-ALL cases. Based on combined expression profiling and molecular-cytogenetic analyses, we recently identified an immature T-ALL subset that was predominantly characterized by rearrangements that activate the MEF2C oncogene.1 This subset could also be predicted by the ETP-ALL profile. For these immature, ETP-ALL cases, MEF2C has been shown to directly activate expression of LYL1, LMO2 and HHEX1 that may explain the high LYL1 expression in immature T-ALL cases. So far, we and others have been unable to reveal LYL1 rearrangements in these immature, ETP-ALL cases.1,2,10 In line with this, the single reported T-ALL case with an LYL1 translocation had a mature (CD3+, CD1−, CD4+, CD8+ and CD34−) immunophenotype.14
LYL1 is a basic helix-loop-helix (bHLH) transcription factor that shows 82% amino acid homology in the bHLH domain with TAL1.15 TAL1 and LYL1 also show overlapping expression patterns in hematopoietic development16 and in some pathways they can exert identical functions.17 The strong co-clustering of this patient sample (#704) along with TAL1-rearranged T-ALL cases indicates that LYL1 rearrangements elicit a similar expression profile as TAL1 rearrangements during T-cell oncogenesis.
Using array-CGH, we further identified a small del(11)(p12p13) near the LMO2 locus in this patient #70413 (data not shown), accompanied by ectopic LMO2 expression (Figure 2D). No copy number changes were found at the TAL1 locus. LMO2 rearrangements (translocations or del(11)(p12p13)) occur in approximately 9% of pediatric T-ALL13 and have been exclusively associated with the TALLMO subgroup.1,13 The identification of an LYL1 translocation, as well as an LMO2 rearrangement in this TALLMO-like patient implies that LYL1 and LMO2 synergize in T-cell oncogenesis. Other incidental cases harbor TAL1/2 as well as LMO1/2 aberrations,4 and 2 additional cases out of 55 TALLMO patients (including the TALLMO-like patients) as present in our T-ALL cohort (n=117) had combined rearrangements of TAL and LMO family members: one case had an SIL-TAL1 deletion and the LMO2-activating del(11)(p12p13), and one had a TAL2/TRB@ translocation in combination with an LMO1/TRAD@ translocation.4 This points to strong synergistic effects between these oncogenic family members in line with their participation in similar transcriptional complexes.18–20 Lmo1/Lmo2 and Tal1 have also been shown to synergize to T-cell leukemogenesis in mice studies.18,21–23
To conclude, we suggest that LYL1 rearranged cases are not part of the immature, ETP-ALL subgroup, but belong to the TALLMO subgroup. LYL1 translocations fulfill a TAL1-like role that can synergize with LMO2 aberrations in T-cell oncogenesis.
Footnotes
Funding: this work was supported by a grant from the Dutch Cancer Society (KWF-EMCR 2006-3500).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Homminga I, Pieters R, Langerak AW, de Rooi JJ, Stubbs A, Verstegen M, et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19(4):484–97. doi: 10.1016/j.ccr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–56. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonis M, Klous P, Homminga I, Galjaard RJ, Rijkers EJ, Grosveld F, et al. High-resolution identification of balanced and complex chromosomal rearrangements by 4C technology. Nat Methods. 2009;6(11):837–42. doi: 10.1038/nmeth.1391. [DOI] [PubMed] [Google Scholar]
- 4.Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood. 2008;111(9):4668–80. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Grotel M, Meijerink JP, Beverloo HB, Langerak AW, Buys-Gladdines JG, Schneider P, et al. The outcome of molecular-cytogenetic subgroups in pediatric T-cell acute lymphoblastic leukemia: a retrospective study of patients treated according to DCOG or COALL protocols. Haematologica. 2006;91(9):1212–21. [PubMed] [Google Scholar]
- 6.Van Vlierberghe P, Beverloo HB, Buijs-Gladdines J, van Wering ER, Horstmann M, Pieters R, et al. Monoallelic or biallelic LMO2 expression in relation to the LMO2 rearrangement status in pediatric T-cell acute lymphoblastic leukemia. Leukemia. 2008;22(7):1434–7. doi: 10.1038/sj.leu.2405063. [DOI] [PubMed] [Google Scholar]
- 7.Przybylski GK, Dik WA, Wanzeck J, Grabarczyk P, Majunke S, Martin-Subero JI, et al. Disruption of the BCL11B gene through inv(14)(q11.2q32.31) results in the expression of BCL11B-TRDC fusion transcripts and is associated with the absence of wild-type BCL11B transcripts in T-ALL. Leukemia. 2005;19(2):201–8. doi: 10.1038/sj.leu.2403619. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 9.Cleary ML, Mellentin JD, Spies J, Smith SD. Chromosomal translocation involving the beta T cell receptor gene in acute leukemia. J Exp Med. 1988;167(2):682–7. doi: 10.1084/jem.167.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1(1):75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 11.Soulier J, Clappier E, Cayuela JM, Regnault A, Garcia-Peydro M, Dombret H, et al. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL) Blood. 2005;106(1):274–86. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 12.van Grotel M, Meijerink JP, van Wering ER, Langerak AW, Beverloo HB, Buijs-Gladdines JG, et al. Prognostic significance of molecular-cytogenetic abnormalities in pediatric T-ALL is not explained by immunophenotypic differences. Leukemia. 2008;22(1):124–31. doi: 10.1038/sj.leu.2404957. [DOI] [PubMed] [Google Scholar]
- 13.Van Vlierberghe P, van Grotel M, Beverloo HB, Lee C, Helgason T, Buijs-Gladdines J, et al. The cryptic chromosomal deletion del(11)(p12p13) as a new activation mechanism of LMO2 in pediatric T-cell acute lymphoblastic leukemia. Blood. 2006;108(10):3520–9. doi: 10.1182/blood-2006-04-019927. [DOI] [PubMed] [Google Scholar]
- 14.Smith SD, Morgan R, Gemmell R, Amylon MD, Link MP, Linker C, et al. Clinical and biologic characterization of T-cell neoplasias with rearrangements of chromosome 7 band q34. Blood. 1988;71(2):395–402. [PubMed] [Google Scholar]
- 15.Mellentin JD, Smith SD, Cleary ML. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- 16.Giroux S, Kaushik AL, Capron C, Jalil A, Kelaidi C, Sablitzky F, et al. lyl-1 and tal-1/scl, two genes encoding closely related bHLH transcription factors, display highly overlapping expression patterns during cardiovascular and hematopoietic ontogeny. Gene Expr Patterns. 2007;7(3):215–26. doi: 10.1016/j.modgep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Souroullas GP, Salmon JM, Sablitzky F, Curtis DJ, Goodell MA. Adult hematopoietic stem and progenitor cells require either Lyl1 or Scl for survival. Cell Stem Cell. 2009;4(2):180–6. doi: 10.1016/j.stem.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, et al. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 1994;13(20):4831–9. doi: 10.1002/j.1460-2075.1994.tb06809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, et al. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16(11):3145–57. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Omari K, Hoosdally SJ, Tuladhar K, Karia D, Vyas P, Patient R, et al. Structure of the leukemia oncogene LMO2: implications for the assembly of a hematopoietic transcription factor complex. Blood. 2011;117(7):2146–56. doi: 10.1182/blood-2010-07-293357. [DOI] [PubMed] [Google Scholar]
- 21.Larson RC, Lavenir I, Larson TA, Baer R, Warren AJ, Wadman I, et al. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J. 1996;15(5):1021–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Draheim KM, Hermance N, Yang Y, Arous E, Calvo J, Kelliher MA. A DNA-binding mutant of TAL1 cooperates with LMO2 to cause T cell leukemia in mice. Oncogene. 2011;30(10):1252–60. doi: 10.1038/onc.2010.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aplan PD, Jones CA, Chervinsky DS, Zhao X, Ellsworth M, Wu C, et al. An scl gene product lacking the transactivation domain induces bony abnormalities and cooperates with LMO1 to generate T-cell malignancies in transgenic mice. EMBO J. 1997;16(9):2408–19. doi: 10.1093/emboj/16.9.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]