POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal component, and skin changes) is a paraneoplastic disorder associated with an underlying plasma cell dyscrasia. Associated features to this syndrome are sclerotic bone lesions, elevated levels of vascular endothelial growth factor (VEGF), Castleman’s disease, endocrinopathies, papilledema, peripheral edema, effusions, ascites, thrombocytosis and fatigue.1 The pathogenesis of the POEMS syndrome is not well understood, but it is likely that proangiogenic2 (such as VEGF) and proinflammatory cytokines3 (such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1 and interleukin (IL)-6) play an important role in the pathogenesis of this disease.
Therapy for POEMS syndrome has not been standardized. So far, many different strategies have been used, including radiotherapy, chelating agents and corticoids, immunoglobulins, plasmapheresis, and high doses of chemotherapy followed by peripheral blood stem cell transplant (SCT).4 Monoclonal antibodies, like rituximab (anti-CD20) or bevacizumab (anti-VEGF), have also been used. Recently, immunomodulatory drugs (IMiDs), such as thalidomide or lenalidomide, have emerged as therapeutic options in these patients because they are powerful drugs against malignant plasma cells which reduce the production of proinflammatory and proangiogenic cytokines. Both drugs have shown efficacy in improving the clinical condition of patients with POEMS syndrome.5–7 Lenalidomide has the additional advantage of being associated with a much lower risk of peripheral neuropathy than thalidomide. Similarly, concerns about exacerbating neuropathy also arise when other new therapeutic agents, such as bortezomib, are taken into consideration.
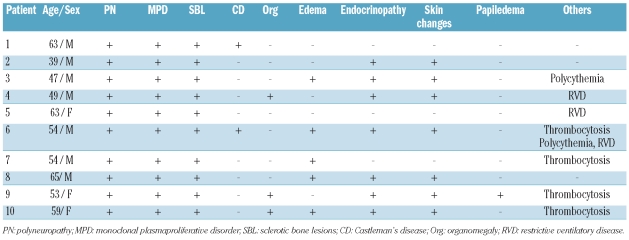
We present the clinical outcome of 10 patients with POEMS syndrome who were treated at different centers in Spain with lenalidomide as salvage therapy from 2007 to 2010. This restrospective study was approved by the MD Anderson Cancer Center Madrid Scientific Committee (Institutional Review Board). Age at entry ranged from 39 to 63 years and patients’ condition fulfilled published diagnostic criteria.1 Main clinical features of POEMS syndrome are described in Table 1. All patients had motor-sensory polyneuropathy, predominantly affecting the lower limbs, monoclonal plasmaproliferative disorder and sclerotic bone lesions. Of note, neurological symptoms led to a restrictive ventilatory alteration in 2 cases (ns. 4 and 5), and confined patients to a wheelchair or to bed in 7 cases (ns. 3, 4, 5, 6, 7, 8 and 10).
Table 1.
Patients’ clinical characteristics.
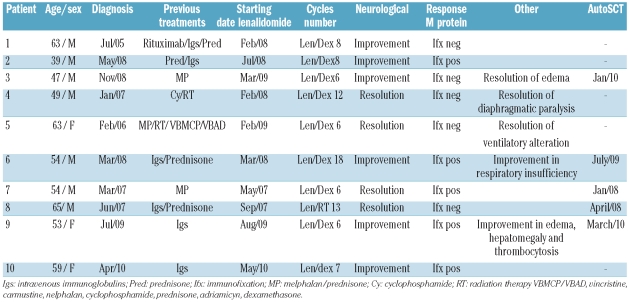
All patients had received previous treatments to lenalidomide therapy (Table 2), including 4 cases who had received chemotherapy (melphalan-containing regimens, cyclophosphamide or polychemotherapy treatment), one who was treated with rituximab, and 6 who had received immunoglobulin treatment. Local radiation therapy was administered in 2 patients for sclerotic lesions. Median follow up of 4 (range 1–36) months showed minimal or no clinical improvements in response to these therapies.
Table 2.
Treatment and response.
All but 3 patients received salvage treatment with combined therapy of lenalidomide 25mg/day for 21 days in 28-day cycles with dexamethasone (40 mg/week). Two patients (case ns. 3 and 10) received the prior scheme with a lower dose of lenalidomide (15 mg/day) and another patient was treated with lenalidomide alone at 25 mg/day (case n. 8). Median number of lenalidomide courses was 7.5 (range 6–18): 4 patients received 6 cycles, 2 patients received 8 cycles, and 4 patients received 7, 12, 13 and 18 cycles, respectively. All patients showed clinical improvement after lenalidomide treatment, even those who later underwent an SCT (Table 2). Due to the delayed responses observed with treatments like melphalan, some effect of the previous treatment could have contributed to the responses observed (case ns. 3, 4 and 5). Clinical resolution of neuropathy was observed in 4 cases and in the remaining patients a significant improvement in neurological symptoms was reported. None of the patients confined to a wheelchair needed further administration of the study treatment and all but 3 regained a fully independent walk; these 3 patients needed support with crutches or assistance. With respect to the M-component, a negative immunofixation was reached in 5 patients.
Treatment tolerance was optimal and only 2 patients (case ns. 1 and 9) developed episodes of respiratory infections that led to a transient discontinuation of therapy; therapy was restarted (with lower doses in case n. 1) after clinical resolution. In all cases for whom PBSC harvest was programmed (n=5), a sufficient number of CD34+ cells was collected. No particular toxicity was observed after the PBSC autologous transplantation performed in 5 patients; melphalan 200mg/m2 was used as conditioning regimen in 4 cases and 100 mg/m2 in one other case. Transplant could have contributed clinically to the favorable outcome of the patients.
Our data indicate that lenalidomide is an effective treatment for POEMS syndrome. In fact, several POEMS syndrome cases treated with lenalidomide have demonstrated favorable responses.5–11 Dispenzieri and co-workers were the first to suggest the efficacy of the lenalidomide and dexamethasone combination for POEMS syndrome after its administration led to a marked improvement in one patient. This improvement was particularly evident at a neurological level with recovery of the ability to walk; improvements in functional status, VEGF and IL-6 levels, skin changes and anasarca were also observed.5 Similarly, another patient was able to walk further and climb stairs without assistance after 4 cycles of this therapy.9 Also, an unusual case of POEMS syndrome with Kappa restriction showed improved symptoms and the resolution of the associated neuropathy6 after lenalidomide treatment.
These data agree with the favorable outcomes shown in the 10 patients described here. These patients experienced systemic improvement, especially of the polyneuropathic condition, after receiving lenalidomide. All patients achieved improved extremity mobility and strength, and patients confined to a wheelchair or bed-bound (n=7) regained the ability to walk. This is one of the most relevant advances in terms of the quality of life of the patients, since one of the main characteristics of this syndrome is the fact that peripheral nerves are compromised. In addition, other improvements included the disappearance of or a decrease in monoclonal component, at the level of bone capture, edema and achromatosis, as well as a reduction in organomegalia.
The favorable responses shown in the 10 cases presented show the efficacy of lenalidomide in recovery from the neurological damage associated with POEMS syndrome, both at sensory and motorary levels. Neurological damage is, in fact, one of the more devastating manifestations of the progression of this disease. Therefore, these observations support the use of the immunomodulatory agent lenalidomimde in patients with POEMS syndrome. The treatment-related adverse events associated with lenalidomide, reported for 2 of the patients, were largely predictable and manageable.
Acnowledgments
The authors appreciate the contribution of Dr G. Ramirez,1 Dr A. Mendizabal2 and Dr J. Prieto3 who contributed with one case to the series. (1Hospital Virgen de la Victoria, Malaga, 2Hospital Txagorritxu, Vitoria and 3Hospital San Pedro de Alcántara. Cáceres, Spain).
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Dispenzieri A. POEMS syndrome. Blood Rev. 2007;21(6):285–99. doi: 10.1016/j.blre.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe O, Maruyama I, Arimura K, Kitajima I, Arimura H, Hanatani M, et al. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve. 1998;21(11):1390–7. doi: 10.1002/(sici)1097-4598(199811)21:11<1390::aid-mus5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Gherardi RK, Bélec L, Soubrier M, Malapert D, Zuber M, Viard JP, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood. 1996;87(4):1458–65. [PubMed] [Google Scholar]
- 4.Dispenzieri A, Moreno-Aspitia A, Suarez GA, Lacy MQ, Colon-Otero G, Tefferi A, et al. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood. 2004;104(10):3400–7. doi: 10.1182/blood-2004-05-2046. [DOI] [PubMed] [Google Scholar]
- 5.Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide Therapy in a Patient with POEMS Syndrome. Blood. 2007;110(3):1075–6. doi: 10.1182/blood-2007-03-082354. [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Tageja N, Arabi H, Penumetcha R. Lenalidomide therapy in a rare case of POEMS syndrome with kappa restriction. South Med J. 2009;102(10):1092–3. doi: 10.1097/SMJ.0b013e3181b2f7d8. [DOI] [PubMed] [Google Scholar]
- 7.Kuwabara S, Misawa S, Kanai K, Sawai S, Hattori T, Nishimura M, et al. Thalidomide reduces serum VEGF levels and improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79(11):1255–7. doi: 10.1136/jnnp.2008.150177. [DOI] [PubMed] [Google Scholar]
- 8.Jaccard A, Abraham J, Recher C, Dulery R, Guichard I, Haroche J, et al. Lenalidomide therapy in nine patients with POEMS syndrome [abstract]. Proceedings of the 51st Annual Meeting and Exposition of the American Society of Hematology; 2009; December 5–8; New Orleans, LA. Abstract #3872. [Google Scholar]
- 9.Kuehne R, Goede J, Benz R, Stüssi G, Renner C. Immune modulatory drugs in POEMS syndrome [abstract]. Onkologie. Proceedings of the Joint Annual Conference of the German, Austrian, and Swiss Societies for Hematology and Oncology; 2008; pp. 186–7. Abstract # P538. [Google Scholar]
- 10.Ramirez G, Campos A, Rosell A, Queipo de Llano MP, García-Sánchez R, García-Delgado R, et al. Patient suffering from POEMS syndrome treated with lenalidomide plus dexamethasone [abstract] Proceedings of the 14th Congress of the European Hematology Association2009; June 4–7Berlin, GermanyAbstract #1631. [Google Scholar]
- 11.Patel T, Moreno-Aspitia A. Alternative treatment for POEMS syndrome: A Lazarus response to lenalidomide [abstract]. Proceedings of the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008b; December 6–9; San Francisco, CA. Abstract #5205. [Google Scholar]