Abstract
The basolateral complex of the amygdala (BLA) modulates memory for emotional events, and direct activation of the BLA following a learning session can enhance subsequent memory. Yet optimal enhancement of episodic memory during emotional events would likely require that BLA activation occur close in time to the event and to be brief enough to target specific memories if some events are to be remembered better than others. In the present study, rats were given a novel object recognition memory task in which initial encounters with some of the objects were immediately followed by brief electrical stimulation of the BLA, and these objects were remembered better one day later as compared to objects for which the initial encounter was not followed by stimulation. The results indicated that BLA stimulation can enhance memory for individual events, a necessary ability for the BLA to modulate episodic memory effectively.
Keywords: basolateral amygdala, electrical stimulation, object recognition memory, memory enhancement, rat
The basolateral complex of the amygdala (BLA) has been shown to mediate enhancement of memory for emotional events (McGaugh, 2004). During these events, activation of the BLA can indirectly benefit memory by boosting arousal, attention, or perception (Phelps & LeDoux, 2005), and studies with experimental animals aimed at understanding its direct influence on memory have therefore followed a tradition of manipulating activity in the BLA only after the completion of a learning session so as to set aside the contributions of these other factors (e.g., Gold, Hankins, Edwards, Chester, & McGaugh, 1975; Kesner, 1982; McGaugh & Roozendaal, 2009). This approach has been particularly productive, and these studies have shown that sustained activation of the BLA following the completion of a learning session can enhance subsequent memory (Paré, 2003), even in instances such as object recognition memory in which emotional arousal ordinarily does not make an essential contribution (Roozendaal, Castello, Vedana, Barsegyan, & McGaugh, 2008). These effects on behavior are paralleled by findings indicating that BLA activation can alter synaptic plasticity in the hippocampus, both in studies of rats performing memory tasks (McIntyre et al., 2005), and in studies of long-term potentiation (LTP) induction in anesthetized rats (Akirav & Richter-Levin, 1999; Frey, Bergado-Rosado, Seidenbecher, & Pape, 2001; Ikegaya, Saito, & Abe, 1995). The efficacy of post-session manipulations, along with evidence of altered plasticity in other brain structures, has suggested that the BLA can enhance memory by modulating cellular processes related to memory consolidation in other brain regions (McGaugh, 2004).
The approach of manipulating activity in the BLA after a learning session has been important for distinguishing the contribution of the BLA to memory from its contribution to emotional arousal. However, the approach departs from the activation of the BLA that ordinarily occurs in emotional events in that, in experimental post-session manipulations, the BLA activation is removed from the time of the event and is typically rather long in duration due to the frequent use of pharmacological agents to activate the structure. Optimal enhancement of episodic memory during emotional events would likely require that BLA activation occur close in time to the event and to be brief enough to target specific memories if some events are to be remembered better than others. That is, it is difficult to understand how post-session manipulations of the BLA could prioritize memories of specific events in a way that would correspond to what is thought to occur in emotional encounters. Specifically, previous studies using post-session BLA manipulations have shown that the time window for modulating memory can stretch from several minutes up to an hour after the session (Gold, Hankins, & Rose, 1977, Vafaei, Jezek, Bures, Fenton, & Rashidy-Pour, 2007), but it remains unclear whether the amygdala can achieve the temporal specificity necessary to modulate memory over the course of seconds to minutes. In addition, it is unclear whether BLA stimulation facilitates plasticity in target structures generally or if the BLA can facilitate plasticity in a way that benefits some memories more than others.
Taken together, these considerations indicate that an important question is whether a briefer and more temporally-targeted activation of the BLA can modulate plasticity with a selectivity that allows for event-specific enhancement of memory. To address this question, rats in the present study were given a novel object recognition memory task in which initial encounters with some of the to-be-remembered objects were immediately followed by brief electrical stimulation of the BLA. When rats were tested one day later, memory for these objects was enhanced as compared to memory for objects not initially followed by stimulation.
Method
Subjects
Thirteen adult male Long-Evans rats were tested. Four of these rats were excluded from data analysis after histological inspection revealed that the stimulating electrodes missed their target locations in the basolateral complex of the amygdala. Rats were individually housed (12h light/dark cycle; testing during light phase) with free access to water and were placed on a restricted diet such that they maintained at least 90% of their free-feeding weight. All procedures involving rats were approved by the Institutional Animal Care and Use Committee at Emory University.
Surgery
Stereotaxic surgery was performed after rats were deeply anesthetized with isoflurane and given buprenorphine as an analgesic. Twisted, bipolar stimulating electrodes (platinum, 0.075 mm diameter, teflon insulation; Plastics One, Roanoke, VA) were aimed bilaterally at the basolateral complex of the amygdala (3.7 mm posterior, 5.2 mm lateral, and 9.2 mm ventral to bregma; Paxinos & Watson, 2007). For 24 hours following surgery, rats were given additional subcutaneous injections of buprenorphine every 8–12 hours as an analgesic and then were allowed an additional 4–6 days to recover.
Electrical Stimulation
During testing, unilateral (half left, half right) electrical stimulation of the BLA was generated by a current generator (S88X Dual Output Square Pulse Stimulator; Grass Technologies, West Warwick, RI) and a stimulus isolator (SIU-BI Stimulus Isolation Unit; Grass Technologies), which sent a constant current of 20 µA in 8 trains of 4 pulses (each pulse = 500 µs biphasic square wave; pulse frequency = 50 Hz; train frequency = 8 Hz) for 1 sec with each press of a hand-held trigger. The electrical stimulation was intended to resemble 50 Hz gamma bursts superimposed on an 8 Hz theta wave.
Novel Object Recognition Memory Task
Rats performed a recognition memory task that was based on rats' spontaneous preference for exploring novel objects more so than repeated objects (Ennaceur & Delacour, 1988). In general, rats encountered new and repeated objects as they completed clockwise laps on a circular track (outside diameter = 91.5 cm; track width = 7 cm). Objects were attached to the outside edge of the track on small platforms. The objects were randomly selected from a collection of plastic, wood, metal, or ceramic junk objects that were typically larger than 10 cm3 but smaller than 2000 cm3. Rats were rewarded with a small piece of chocolate on a central runway for completing each lap, irrespective of object exploration. Rats were trained to complete laps for two weeks prior to surgery and were retrained for two weeks following recovery, during which time they were exposed to objects that were not used during testing.
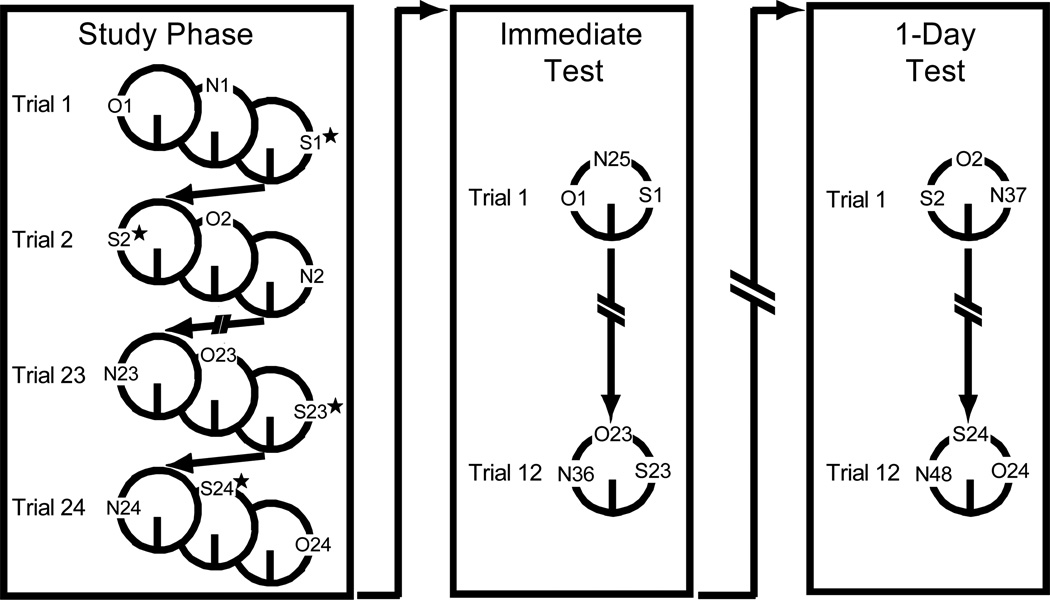
Figure 1 shows a schematic of the task design. After completing all 24 trials of the Study Phase, rats immediately began testing for half of the repeated objects on the Immediate Test (mean delay between the initial encounter with an object during study and its repetition during test was 72 mins; range = 40–110 mins). The next day, rats were tested on the remaining half on the 1-Day Test. On each trial of the study phase, rats were presented with three objects, for one of which exploration was immediately (1–3 sec) followed by stimulation of the BLA. On each trial of both tests, rats were presented with 3 objects: a repeated object whose exploration during the Study Phase was followed by BLA stimulation ("Stimulation"), a repeated object whose exploration during the Study Phase was not followed by BLA stimulation ("No Stimulation"), and a novel object ("New"). No stimulation was delivered on either test. Duplicates were used for repeated objects. Objects were randomly assigned to the conditions, and the locations of objects were counterbalanced across trials. During the Study Phase only, two experimenters participated in testing each rat, one who tested the rat and was unaware of group assignment of objects and one who observed the Study Phase from behind a curtain and pressed the trigger to deliver electrical stimulation. An additional session was run in which the procedure was identical to the standard procedure except that the current generator did not deliver electrical stimulation following trigger presses.
Figure 1.
Schematic of the novel object recognition memory task. Rats encountered three groups of objects in each phase: Stimulation objects (denoted by an “S”), for which brief electrical stimulation was delivered to the BLA immediately after a rat disengaged from exploration during the Study Phase only and which were repeated during one test, “No Stimulation” objects (denoted by an “O”), which were also repeated during one test, and “New” objects (denoted by an “N”), which were not repeated. Objects presented on the Immediate Test were not included on the 1-Day Test. Objects within a trial were presented on the same lap during the Immediate Test and during the 1-Day Test but were presented on separate laps during the Study Phase in order to better isolate the influence of amygdala stimulation to a particular object. Rats completed a lap on an empty track between each trial on all three phases (see Method for details).
Histology
At the end of testing, small marking lesions were made at the tips of the electrodes before rats were euthanized. Brain sections were stained for acetylcholinesterase to facilitate identification of structures in the BLA. Localization of stimulating electrodes was verified by a second rater who was unaware of rat identity.
Data Analysis
Frame-by-frame (30 frames/sec) analysis of digital video was used to record times when a rat initiated or terminated exploration of an object. A rat was considered to be exploring an object only if the rat was within 2 cm of the object and was showing evidence of active investigation (e.g., sniffing and directed attention). If a rat did not examine all 3 objects of a trial during the Study Phase, the trial was discarded and those objects were not included on either of the tests. Five videos of the 1-Day Test were rescored by a blinded observer, and the median exploration times were well correlated between observers (correlation coefficients of 0.989, 0.992, and 0.996, for Stimulation, No Stimulation, and New objects, respectively).
To assess memory for Stimulation and No Stimulation objects during the Immediate Test and 1-Day Test, a discrimination index was calculated to evaluate the extent to which a rat explored New objects more so than either the repeated Stimulation objects or repeated No Stimulation objects on each test. Specifically, a discrimination index was obtained for each rat by dividing the median New object exploration time by the sum of the median New object and median repeated object exploration time [for Stimulation objects: New/(New + Stimulation); for No Stimulation objects: New/(New + No Stimulation)]. This discrimination index resulted in a number for which 0.50 represented no memory and higher numbers represented better memory for repeated objects. Discrimination index scores were averaged across rats and the means were compared to a baseline of 0.50 with a one-sample t-test. Discrimination index scores for Stimulation and No Stimulation objects were compared with paired-samples t-tests. Additionally, a stimulation by test interaction was evaluated with a 2X2 repeated measures ANOVA.
Results
Histology revealed that the tips of the both the left and right stimulating electrodes were located in the BLA (3 in the lateral nucleus, 12 in the basal nucleus, and 3 in the accessory basal nucleus, all between 3.3 mm and 4.4 mm posterior to bregma, Paxinos & Watson, 2007) for the 9 rats included in the data analysis. None of the rats showed signs of stress (vocalizations, defecation, or freezing) or seizures in response to electrical stimulation.
During the Study Phase, all objects were novel, and electrical stimulation was delivered to the BLA only after rats disengaged from exploration. As a result, rats spent a similar amount of time exploring the Stimulation (mean sec ± SEM = 3.39 ± 0.62), No Stimulation (3.27 ± 0.67), and New objects (2.95 ± 0.48).
Figure 2A shows the discrimination index scores for the Immediate Test and the 1-Day Test. For the Immediate Test, the scores for Stimulation and No Stimulation objects were both significantly above baseline (mean ± SEM: Stimulation = 0.64 ± 0.03, t(8) = 4.52, p < 0.01; No Stimulation = 0.63 ± 0.03, t(8) = 3.98, p < 0.01) and were similar (Stimulation vs. No Stimulation: t(8) = 0.29, p > 0.1). In contrast, for the 1-Day Test, the discrimination index was significantly above baseline for only the Stimulation objects (mean ± SEM; Stimulation = 0.67 ± 0.04, t(8) = 3.83, p < 0.01; No Stimulation = 0.51 ± 0.05; t(8) = 0.26, p > 0.1), and the discrimination index differed significantly between the groups of repeated objects (Stimulation vs. No Stimulation: t(8) = 2.85, p < 0.05). Further, a 2X2 repeated measures ANOVA showed a significant stimulation by test interaction (F(1,8) = 5.06, p = .05). These results suggested that objects were remembered well on the Immediate Test regardless of BLA stimulation but that objects were remembered on the 1-Day Test only when initial encounters with those objects during the Study Phase had been followed by BLA stimulation.
Figure 2.
Performance on recognition memory tests shown as a discrimination index (n=9). A. Rats remembered repeated objects well during the Immediate Test, but remembered repeated objects during the 1-Day Test only if exploration of those objects during the Study Phase had been followed by brief electrical stimulation of the BLA. B. Sham Stimulation did not significantly alter memory on either the Immediate Test or the 1-Day Test as compared to No Stimulation. The dashed line indicates chance performance. Error bars show SEM. * = p < 0.05, ** = p < 0.01.
To address the possibility that factors other than the BLA stimulation (e.g., experimenter's expectations) might have influenced the results, a session was conducted in which, unbeknownst to the experimenter placing the objects and handling the rats, the current generator was disconnected from the cable leading to the rat. Figure 2B shows that there was no benefit of sham stimulation on the 1-Day Test (mean sec ± SEM: sham Stimulation = 0.40 ± 0.07 vs. sham No Stimulation = 0.51 ± 0.09, t(8) = 1.89, p = 0.1), suggesting that non-specific factors did not account for the memory-enhancing effect of BLA stimulation. Indeed, memory for sham Stimulation objects was significantly less than for actual Stimulation objects on the 1-Day Test (sham Stimulation vs. Stimulation: t(8) = 3.02, p < 0.05).
Discussion
The results of the present study indicated that electrical stimulation of the BLA led to enhanced memory for individual objects when memory was tested one day later. Objects for which the initial encounter was followed by brief BLA stimulation were remembered well on a test given one day after the study phase, but objects for which the initial encounter was not followed by BLA stimulation appeared to be forgotten by that point. On a test given immediately after the study phase (for which the study-test delay for each object averaged 72 minutes), memory was unaffected by BLA stimulation. The observation that the benefit of BLA stimulation emerged only after a protracted period of time suggests that the stimulation exerted its influence on memory for individual objects by modulating memory processes related to cellular consolidation, a suggestion consistent with a large body of work on the role of the amygdala in emotional memory in both humans and experimental animals (LaBar & Cabeza, 2006; McGaugh, 2004; 2006) and with studies specifically examining the role of the BLA in enhancing object recognition memory in rats (Roozendaal, Okuda, Van der Zee, & McGaugh, 2006; Roozendaal et al., 2008).
The present study adds to that body of work by indicating that direct activation of the BLA can target memory for individual events. Previous studies in experimental animals established that sustained activation of the BLA following a learning session could enhance subsequent memory, but this enhancement was thought to have encompassed all trials during the learning session. In some scenarios, a blanket enhancement in memory that extends to all information acquired in the past hour or so would be advantageous (e.g., training on a procedural task). However, in the case of episodic memory, modulation by the BLA would be most effective if the activation could selectively target only the important memories (i.e., those of events that immediately trigger amygdala activation in real-world encounters). The present results obtained with interleaved Stimulation and No Stimulation object encounters indicate that BLA activation, when brief and temporally-targeted, can indeed prioritize some memories over others. These results correspond well with studies in humans in which indirect activation of the amygdala using emotional images led to item-specific memory enhancement (Anderson, Wais, & Gabrieli, 2006) and trial-specific modulation of amygdala activity (Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000).
The observation that BLA stimulation did not affect performance on the immediate test highlights a role for protracted processes related to memory consolidation but also indicates that stimulation did not induce positive or negative dispositions towards objects. Rats explored repeated objects similarly on the immediate test irrespective of whether or not the initial encounter with the object was followed by stimulation of the BLA (mean sec ± SEM; Stimulation = 0.89 ± 0.32; No Stimulation = 0.86 ± 0.27). In a previous study that used a similar task but did not include BLA stimulation (Manns & Eichenbaum, 2009), objects encountered for the sixth time were explored for a much shorter time (their Figure 2; mean sec ± SEM = 0.33 ± 0.16). When median exploration times were used to calculate exploration times for each rat in the previous study (in the same manner used in the present study), the exploration time for the sixth encounter averaged across rats was even lower (0.03 ± 0.03 sec), which corresponded to a discrimination score of 0.99 when calculated using median exploration times for novel objects from the previous study (mean of medians = 1.78 ± 0.01). This comparison indicates that the similar exploration of the Stimulation and No Stimulation objects in the present study was not due to a floor effect on exploration times (or to a corresponding ceiling effect on discrimination scores).
It remains unclear exactly what the neuronal effects of BLA stimulation were, and thus the mechanisms of event-specific memory enhancement in the present study are unknown. However, structures in the hippocampal memory system are important for object recognition memory (Clark, Zola-Morgan, & Squire, 2000; Ennacuer, Neave, & Aggleton, 1996), and BLA activation can alter synaptic plasticity in the hippocampus (Akirav & Richter-Levin, 1999; Frey et al., 2001; Ikegaya et al., 1995; McIntyre et al., 2005) as well as other cortical regions (Chavez, McGaugh, & Weinberger, 2009). Thus, one possibility is that BLA stimulation engaged projections from the BLA to the entorhinal cortex, perirhinal cortex, and/or hippocampus (Petrovich, Canteras, & Swanson, 2001; Pitkanen, Pikkarainen, Nurminen, & Ylinen, 2000) and that triggering of these projections selectively benefited plasticity in at least one of these targets.
The slow emergence of the memory enhancement (> 1 hour) in the present study would suggest that the BLA stimulation influenced molecular cascades related to late-phase LTP at these synapses, as these cascades have been observed to take hours to unfold (Alberini, 2009). In particular, it has been suggested that BLA activation may not directly impact early-phase LTP in target structures but instead may encourage the transition of synapses in early-phase LTP to late-phase LTP (Akirav & Richter-Levin, 1999; Ikegaya et al., 1995). For example, a previous study in anesthetized rats found that BLA stimulation following LTP induction had minimal impact on LTP at 1 hour but reinforced maintenance of LTP beyond 1 hour (Frey et al., 2001), findings that parallel the current behavioral results. Thus, BLA stimulation may be able to facilitate late-phase LTP at specific synapses by impacting only synapses at which early-phase LTP was most recently initiated by an object encounter event. Regardless of the mechanism, the finding in the present study of event-specific enhancement of object recognition memory via brief electrical stimulation of the BLA is an important finding in that it demonstrates the ability of the BLA to target individual events within the stream of incoming information, a necessary ability for the BLA to modulate episodic memory effectively.
Acknowledgements
We thank Arick Wang and Josephine Duan for their assistance. This research was supported by NIH Grant MH079564 and NSF Grant 0824199.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Conflict of Interest: none
References
- Alberini C. Transcription Factors in Long-Term Memory and Synaptic Plasticity. Physiology Reviews. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. Journal of Neuroscience. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences. 2006;103:1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrielli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez CM, McGaugh JL, Weinberger NM. The basolateral amygdala modulates specific sensory memory representations in the cerebral cortex. Neurobiology of Learning and Memory. 2009;91:382–392. doi: 10.1016/j.nlm.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola-Morgan S, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. Journal of Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennacuer A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behavioral Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennacuer A, Neave N, Aggleton J. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transaction in the rat. Behavioral Brain Research. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. Journal of Neuroscience. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Research. 1975;86:509–513. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- Gold PE, Hankins LL, Rose RP. Time-dependent post-trial changes in the localization of amnestic electrical stimulation sites within the amygdala in rats. Behavioral Biology. 1977;20:32–40. doi: 10.1016/s0091-6773(77)90454-0. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. High-frequency stimulation of the basolateral amygdala facilitates induction of long-term potentiation in the dentate gyrus in vivo. Neuroscience Research. 1995;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Brain Stimulation: Effects on Memory. Behavioral and Neural Biology. 1982;36:315–367. doi: 10.1016/s0163-1047(82)90762-2. [DOI] [PubMed] [Google Scholar]
- Labar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. A cognitive map for object memory in the hippocampus. Learning & Memory. 2009;16:616–624. doi: 10.1101/lm.1484509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology. 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex and postrhinal cortex in rat. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning and Memory. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. Post-tetanic time window for reinforcement of long-term potentiation by appetitive and aversive stimuli. Proceedings of the National Academy of Sciences. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaei AA, Jezek K, Bures J, Fenton AA, Rashidy-Pour A. Post-training reversible inactivation of the rat’s basolateral amygdala interferes with hippocampus-dependent place avoidance in memory in a time-dependent manner. Neurobiology of Learning and Memory. 2007;88:87–93. doi: 10.1016/j.nlm.2007.02.004. [DOI] [PubMed] [Google Scholar]