Abstract
Salvage chemotherapy followed by high dose autologous stem cell transplantation (HD-ASCT) is the standard of care for patients who have relapsed or refractory Hodgkin Lymphoma (HL). Few trials have had long-term follow-up post HD-ASCT in the ABVD era of treatment. We reviewed 95 consecutive patients who received HD-ASCT for relapsed or refractory HL following ABVD failure between 1990 and 2006 at the University of Rochester. Median follow-up for survivors was 8.2 years. All patients received HD-ASCT following up-front ABVD (or equivalent) failure. At 5 years, overall survival (OS) and event-free survival (EFS) were 54% and 37%, respectively. In total, 54 patients have died; 37 of these patients died directly of HL. Notably, there were 19 deaths > 3 years post HD-ASCT and 13 of these late deaths are directly attributable to HL. Furthermore, there were 51 documented relapses, 9 of which occurred >3 years post HD-ASCT. In contrast to other studies, we did not observe a plateau in EFS following transplantation. Patients appear to be at continuous risk of recurrence beyond 3 years after HD-ASCT. Our results emphasize the importance of long-term follow-up for both toxicity and recurrence, and have important implications in defining success of post-transplant maintenance strategies.
Keywords: Hodgkin lymphoma, hematopoietic stem cells, late effects of therapy, relapsed and refractory disease, HD-ASCT
Introduction
Approximately 80% of patients with advanced stage Hodgkin Lymphoma (HL) achieve a complete remission (CR) when treated with ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine), the current gold-standard up-front HL therapy in the United States, or ABVD/MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) hybrid therapy [1]. Thus, 20% of patients fail primary induction and an additional 20% will relapse within one year of initial therapy [1]. High-dose chemotherapy followed by autologous stem cell transplantation (HD-ASCT) post salvage therapy is the standard of care for patients with relapsed or primary refractory HL [2–12].
Early relapses following HD-ASCT in the pre-ABVD era are well-defined in the literature. Indeed, the majority of HL relapses occur within the first two years following HD-ASCT [13–15]. Poor prognostic factors have been identified, including: systemic symptoms, extra-nodal disease at relapse, increased age, increased cycles of pre-transplant regimens, bulky disease, and poor performance status at transplant [7, 15–20]. Several studies, however, have called for systematic examination of long-term effects and, specifically, relapse following HD-ASCT [10, 17]. Long term complications have been identified, including mortality associated with infections, cardiac problems, including myocardial infarction and cardiomyopathy, and secondary malignancies [6, 14, 21–23]. However, very few studies have exclusively examined late relapses following autologous transplant. While relapse itself does not necessarily pose an immediate threat to survival, only a small number of patients who relapse after HD-ASCT will achieve long-term disease-free survival [4, 12]. The median time to progression (TTP) following HD-ASCT is approximately 6 months [24]. Several studies conducted at large transplant centers have clearly identified a plateau in OS beginning at 2 years post HD-ASCT in the pre-ABVD therapeutic era, implying that HL mortality after this point is negligible. The Vancouver group’s landmark 2005 study on the first 100 patients to undergo HD-ASCT at their center, universally viewed as the paradigm of long-term surveillance post-transplant, defines a plateau in OS beginning at 2 years post HD-ASCT [14].
There is therefore a lack of studies which examine the long-term outcomes and late relapses of HD-ASCT following first-line therapy in the modern ABVD era; additional studies that characterize the late effects associated with ABVD failure rescued by HD-ASCT are valuable as ABVD is the current standard of care for advanced stage HL in the United States [25]. We analyzed all consecutive patients who underwent HD-ASCT following ABVD failure at the University of Rochester from 1990–2006 to characterize the long-term outcomes and late effects of relapsed and refractory HL treated with HD-ASCT in the ABVD therapeutic era.
Materials and Methods
Patients
This study was reviewed and approved by the Institutional Review Board (IRB) at URMC. All consecutive patients over the age of 17 with documented HL who received HD-ASCT at the University of Rochester Medical Center James P. Wilmot Cancer Center (URMC) between the years 1990 and 2006 following up-front ABVD therapy or equivalent and who were registered in the URMC Bone Marrow Transplant (BMT) Registry were examined in this study. All patients who underwent HD-ASCT after either a failure to achieve complete remission or disease relapse following initial ABVD chemotherapy or equivalent were eligible for inclusion. All HL diagnoses were confirmed by hematopathology review at URMC. Disease status was established prior to HD-ASCT by the treating physician and was not re-evaluated at the time of transplant. Relapsed and primary refractory disease prior to HD-ASCT was confirmed by biopsy or imaging. In the event that both CT +/− PET and biopsy confirmed relapsed disease, the date of biopsy was taken as date of relapse. Patients were categorized as: primary refractory or induction failure if they had relapsed less than one year after initial HL diagnosis; first relapse if they had one relapse ≥ 1 year after initial diagnosis; and advanced disease if they had experienced 2 or more HL relapses at the time of HD-ASCT.
Data Collection
The first day of follow-up was defined as the day after HD-ASCT; follow-up continued until date of most recent medical contact or date of confirmed death. All data were abstracted through extensive review of URMC medical records (including clinical, histological, laboratory and diagnostic imaging records), URMC BMT records, outside clinic charts, physician correspondence, death certificates, and autopsy reports when available. Data collected included: disease characteristics and staging; pre-HD-ASCT treatment history; salvage, conditioning, and HD-ASCT regimens; progression of disease; treatments following post HD-ASCT relapse; secondary malignancies; vital status; and cause of death.
Information regarding cause of death (COD) and vital status of patients with a prolonged follow-up time was confirmed via the National Death Index (NDI). Records were matched according to NDI’s published recommendations [26–27]. At the time of data collection, NDI records extended through the year 2006; thus date and cause of death for patients with a date of last contact beyond 2006 was determined solely by our URMC chart review process. In cases where there was no URMC cause of death, the NDI search results were used exclusively. Cause of death was classified according to a modified version of the three-tiered system described by Lavoie et al in 2005: progressive HL (including treatment related death from recurrent HL after HD-ASCT), treatment-related mortality (TRM) (including organ failure or second malignancy), or other (death unrelated to HL disease progression or treatment) [14].
Statistical Analysis
Demographic characteristics of the study cohort were summarized and reported as median values (and range) for continuous variables, and proportions for categorical variables. Event Free Survival (EFS) was defined as time from HD-ASCT date to relapse or death from any cause, and patients without an event were censored at their most recent medical contact. Overall Survival (OS) was defined as time from HD-ASCT date to death from any cause; patients who were still alive at the end of follow-up were censored as either December 31, 2006 (end of NDI record availability) or their most recent medical contact if after January 1, 2007. Both EFS and OS were estimated by standard Kaplan-Meier survival techniques. We compared OS from time of first documented relapse post HD-ASCT by timing of relapse (<6 months post HD-ASCT, excluding TRM; 6–12 months post HD-ASCT, and >12 months post HD-ASCT) using the log-rank test. Expecting that those patients that relapsed within the first year post HD-ASCT were going to have an inferior prognosis, we further evaluated whether or not there was a difference in OS post HD-ASCT relapse for those relapsing 1–3 years post HD-ASCT and those that relapsed late (defined arbitrarily as >3 years post HD-ASCT), though we acknowledge that this comparison is exploratory due to limited power to formally test this comparison. Median follow-up was calculated both based on median time from HD-ASCT to censoring date among the living patients, and using the reverse Kaplan-Meier estimator [28]. Statistical analysis was conducted using SAS statistical software (SAS, Cary, NC).
Results
Patient Characteristics
Ninety-five patients were eligible and are included in this analysis. Demographic attributes of patients and therapy details are included in Table 1. The majority of patients were white (88%) and female (51%). Median age was 34 years (19–66). Patients predominantly presented with stage III-IV (53%), nodular sclerosing HL (70%), and B symptoms (62%) at the time of diagnosis. All patients underwent ABVD or ABVD equivalent therapy. The majority of patients in this study had chemosensitive disease (78%). Median time to HD-ASCT from diagnosis was 1.9 years (0.7 – 19.6 years). A greater proportion of patients (60%) was transplanted in the first relapse.
Table 1.
Patient, Disease and Transplant Characteristics
| Characteristic | N=95 | % |
|---|---|---|
| Age (median, range) | 34 | (19–66) |
| Female | 48 | 50.5% |
| Race | ||
| White | 84 | 88.4% |
| Black | 7 | 7.4% |
| Other | 4 | 4.2% |
| Hodgkin Lymphoma Histology | ||
| Nodular Sclerosis | 66 | 69.5% |
| Mixed cellularity | 19 | 20.0% |
| Classical NOS | 1 | 1.1% |
| Lymphocyte Predominant | 4 | 4.2% |
| Lymphocyte Depleted | 3 | 3.2% |
| Unknown | 2 | 2.1% |
| Disease Stage at Diagnosis | ||
| Early Stage (I/II) | 43 | 45.3% |
| Advanced Stage (III/IV) | 50 | 52.6% |
| B symptoms at Diagnosis | 59 | 62.1% |
| Disease Status at Transplant | ||
| First Relapse | 57 | 60.0% |
| Primary Refractory or Induction Failure | 33 | 34.7% |
| Advanced Disease | 5 | 5.3% |
| Chemosensitivity | ||
| Chemosensitive | 74 | 77.9% |
| Conditioning Regimen | ||
| BEAC | 50 | 52.6% |
| BEAM | 36 | 37.9% |
| TBI+cyclophosphamide | 9 | 9.5% |
| Consolidative Radiation Therapy | 47 | 49.5% |
| Graft Type | ||
| Bone Marrow | 17 | 17.9% |
| Peripheral Blood | 66 | 69.5% |
| Bone Marrow + Peripheral Blood | 12 | 12.6% |
HL, Hodgkin Lymphoma; HD-ASCT, high-dose autologous stem cell transplant
Treatment and Conditioning Regimens
Patients received one of several salvage therapies, including: DHAP (dexamethasone, cytarabine, and cisplatin), ESHAP (etoposide, methylprednisone, cytarabine, and cisplatin), ICE (ifosfamide, carboplatin, and etoposide), ABVD, EVA (etoposide, vinblastine, and doxorubicin), bortezomib and gemcitabine on a clinical trial [29], cyclophosphamide, local radiotherapy, and surgical debulking. A minimum of 2 × 108 mononuclear cells/kg body weight from the bone marrow, or 2 × 106 CD34+ cells/kg body weight from the peripheral blood were required in order to proceed to autologous transplantation. Conditioning regimens were: BEAM (carmustine, etoposide, cytarabine, and melphalan) (38%), BEAC (carmustine, etoposide, cytarabine, and cyclophosphamide) (53%), or TBI + cyclophosphamide (9%). Forty-seven patients (49%) also received consolidative radiotherapy following HD-ASCT.
Post HD-ASCT
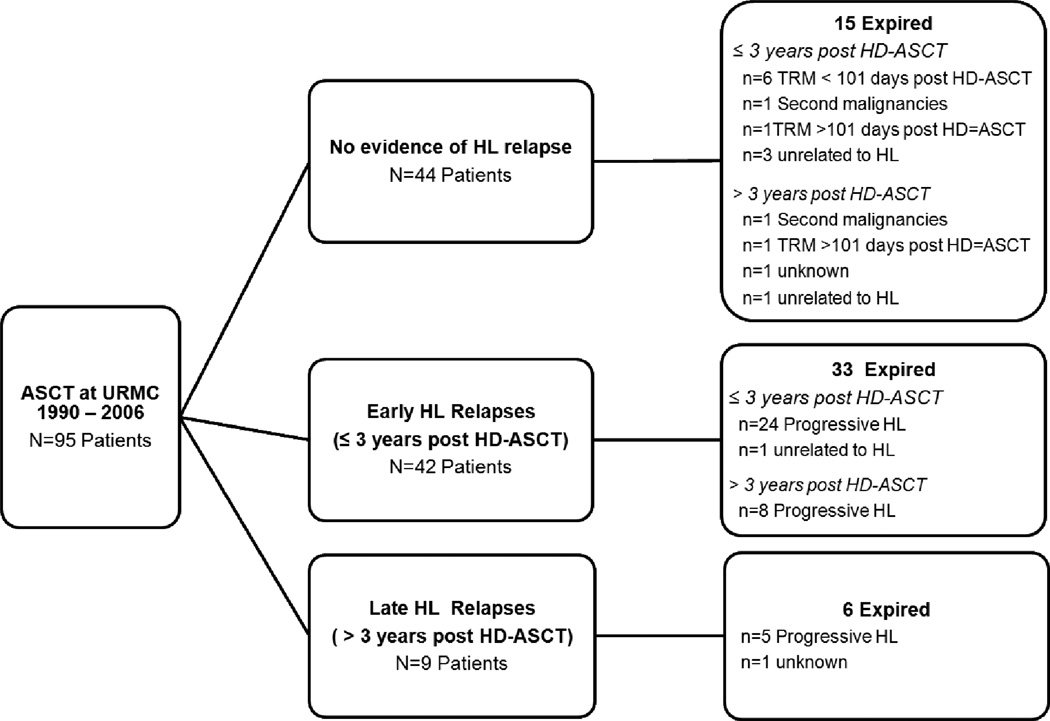
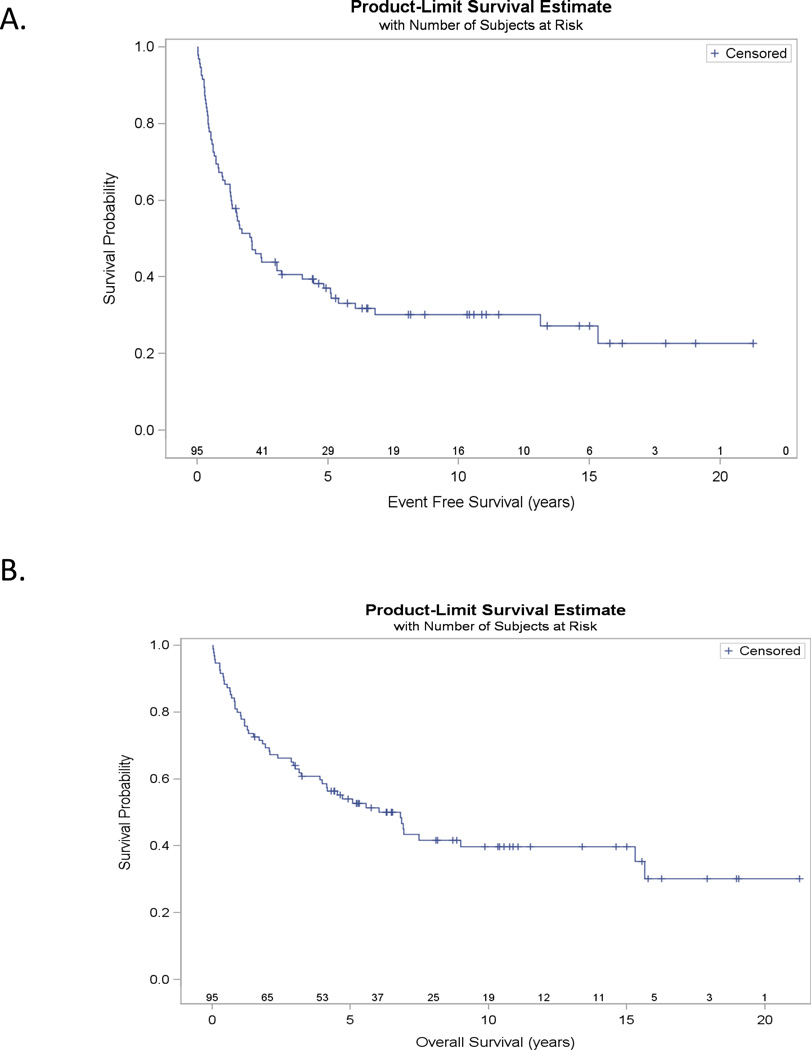
Twenty-nine patients were alive without relapse as of August 5, 2011. The median follow-up time for survivors was 8.2 years (1.5–21.2.1 years), and the KM estimated median follow-up was 10.3 years (95% CI: 6.5–11.1 years). Patient outcomes are summarized in Figure 1, and causes of death for the 54 observed deaths to date are summarized in Table 2. Kaplan-Meier estimated EFS and OS at 5 years was 37% and 54%, respectively (Figure 2). Our central findings is that 19 of the 54 deaths (35%) occurred more than 3 years after HD-ASCT, and 13 (68% of all late deaths) are directly attributable to HL.
Figure 1.
Summary of patient outcomes following HD-ASCT
Table 2.
Causes of death for N= 54 patients (Median follow-up = years)
| Characteristic | N=54 | % |
|---|---|---|
| Progressive HL (including TRM for recurrent HL) | 37 | 68.5% |
| Treatment Related Mortality | 10 | 18.5% |
| Early deaths (within 101 days of HD-ASCT) | 6 | |
| Second malignancies | 2 | |
| Deaths secondary to treatment (>101 days post HD-ASCT)* | 2 | |
| Deaths unrelated to HL | 5 | 9.3% |
| Acute MI | 2 | |
| Acute cholecystitis | 1 | |
| Non-infective gastroenteritis/colitis | 1 | |
| COPD | 1 | |
| Unknown | 2 |
TRM, Treatment related mortality; HL, Hodgkin Lymphoma; HD-ASCT, high-dose autologous stem cell transplant; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease;
pneumothorax (1), infection pneumonitis (1)
Figure 2.
Kaplan-Meier estimates of: A) Event Free survival, EFS (years; N=84); and B) Overall Survival, OS (years; N=84)
Post HD-ASCT Treatment Related Mortality
In total, 10 deaths of 54 total deaths were attributed to complications of therapy (TRM defined previously). Included in this category are 6 deaths within 101 days of transplant, 2 deaths beyond 101 days due to post-transplant complications, and 2 deaths due to secondary malignancy without evidence of HL progression.
Progression of HL post HD-ASCT
We observed documented relapses in 51 patients. Forty-two relapses occurred within 3 years post-transplant. However, we observed 9 relapses (18% of all relapses) beyond 3 years; the disease characteristics of these late relapsed patients are provided in Table 3. Out of all 51 patients who relapsed, 39 patients have expired: 37 deaths are due to HL (including TRM for recurrent HL), 1 death is unrelated to HL, and 1 death is due to unknown causes. Twelve of the 51 relapsed patients remain alive and many have received multiple treatment regimens since relapse post HD-ASCT: 7 patients were treated with localized radiotherapy; 5 received additional chemotherapy; 4 received allogeneic transplants (3 have relapsed; 1 is in remission); 3 were treated with monoclonal antibodies, 2 with Brentuximab vedotin (SGN 35) [30], and 1 with SGN 30; and 1 patient had a second autologous transplant. Four of these 12 patients achieved complete remission: 1 following a second autologous stem cell transplant; 1 following an allogeneic transplant; 1 following radiation therapy; and 1 following SGN 30.
Table 3.
Characteristics of Late Relapses (relapse >3 years postHD-ASCT, n=9)
| Age^ | Stage* | Histology | Conditioning Regimen |
Disease Characteristics† |
Post-transplant Radiation Consolidation |
Event Free Survival (years) |
Survival | COD |
|---|---|---|---|---|---|---|---|---|
| 40 | IV B | Lymphocyte Predominant |
BEAC | Advanced disease; Chemosensitive |
No | 3.1 | Expired | TRM |
| 20 | II B | Nodular Sclerosis | BEAC | PIF; Chemosensitive | Yes | 3.1 | Expired | Unknown |
| 40 | II | Indeterminate | BEAC | Chemosensitive | No | 4.0 | Alive | |
| 33 | IV | Mixed cellularity | BEAM | Chemosensitive | No | 4.4 | Alive | |
| 40 | III | Nodular Sclerosis | BEAC | Chemosensitive | No | 4.8 | Expired | HL |
| 46 | IV B | Nodular Sclerosis | TBI/Cy | Chemosensitive | Yes | 5.1 | Alive | |
| 39 | IV B | Nodular Sclerosis | BEAM | Chemoresistant | No | 5.1 | Alive | |
| 44 | III | Nodular Sclerosis | BEAM | PIF; Chemosensitive | No | 5.4 | Expired | HL |
| 64 | II | Nodular Sclerosis | BEAC | Unknown Chemosensitivity |
Yes | 13.1 | Expired | HL |
age at high-dose-autologous stem cell transplant;
stage at diagnosis;
all patients were transplanted after salvage following first relapse unless noted to be primary induction failures or have advanced disease (2 or more relapses before transplant); HD-ASCT, high-dose autologous stem cell transplant; PIF, primary induction failure; COD, cause of death; TRM, treatment related mortality; TBI, total body irradiation; Cy, cyclophosphamide
Death from Progression of HL post HD-ASCT
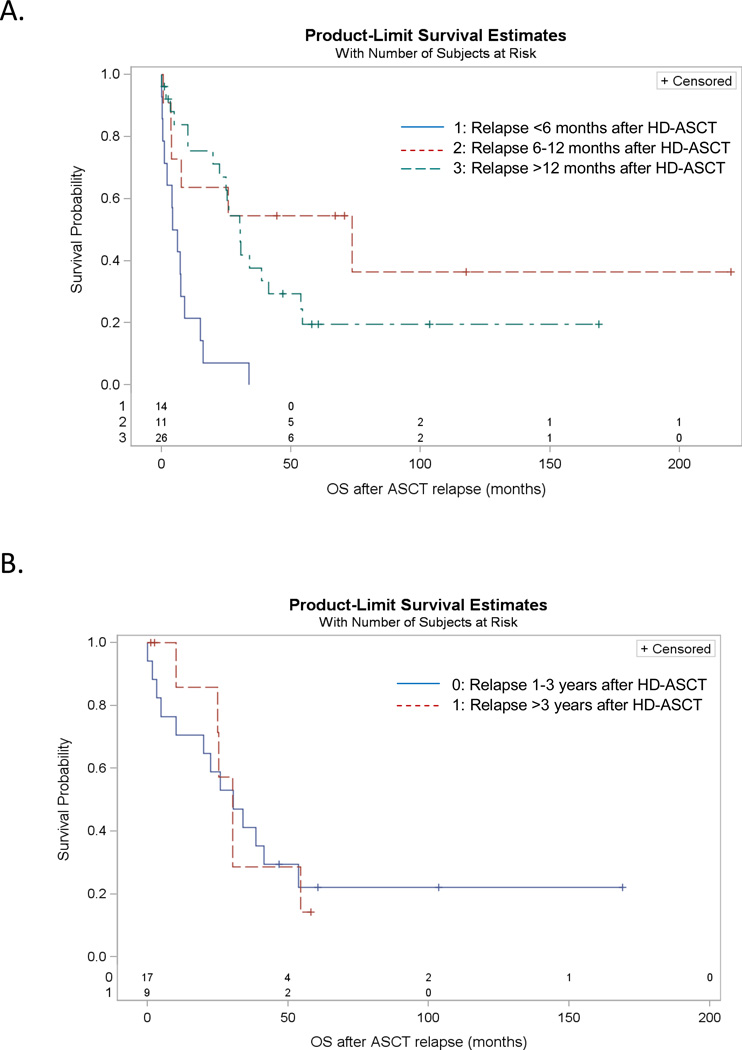
Thirty-seven patients (39%) have died of progressive HL. Included in progressive HL deaths are patients who died of treatment related complications for recurrent HL. We do observe inferior OS for those that relapse early in the first year post HD-ASCT, as expected (Figure 3, Panel A). However, among those who relapse more than 1 year post HD-ASCT, the survival experience past relapse appears similar for those relapsing later (defined as >3 years post HD-ASCT for this study) as compared to those patients that relapse earlier (1–3 years post HD-ASCT) (Figure 3, Panel B).
Figure 3.
Kaplan-Meier estimates of: A) Overall Survival from time of relapse, stratified by length of time between HD-ASCT and relapse (n=51 relapses, n=6 TRM excluded), log-rank p<0.001; and B) Overall Survival from time of relapse for those that relapsed 1–3 years post HD-ASCT versus those that relapsed >3 years post HD-ASCT (n=26), log-rank p=0.93, Hazard Ratio for OS with relapse >3 years post HD-ASCT vs. OS with relapse 1–3 years post HD-ASCT = 1.05 (95% CI: 0.39–2.77).
Discussion
This study examined the long-term outcomes of patients with relapsed or refractory HL who underwent HD-ASCT in the ABVD era. We reported a median follow-up time of 8.2 years. Similar to comparable studies [13–15, 31–34] the majority of our patients remain alive at 5 years and over one-third is disease free, emphasizing the continued utility of HD-ASCT. In contrast to these studies, however, we observed a noticeably higher number of late relapses. In particular, we observed 9 late relapses ≥3 years post HD-ASCT (18% of total relapses) without a plateau in either OS or EFS. We also observed 19 late deaths occurring 3 years post HD-ASCT; 13 of these late deaths are directly attributed to HL. While the 3 year post HD-ASCT threshold for defining late relapses and late deaths is somewhat arbitrary, our experience demonstrates a clear, continual risk of late relapse and HL-related mortality following HD-ASCT. Most notably our threshold is even later than the 2-year OS plateau identified in large cohort series, such as the Vancouver study and the CCTCI/MSKCC study, underscoring the finding that there is no plateau in OS. We did observe, however, a similar survival experience following HD-ASCT relapse when comparing those who we designated as late relapse (>3 years post HD-ASCT) to those who relapsed earlier (1–3 years post HD-ASCT).
There are several potential explanations for the late relapses observed in this study. We have examined long-term HD-ASCT in the post-ABVD therapeutic era, and suggest that the use of ABVD has important ramifications for our observed results. The majority of studies that have examined HD-ASCT outcomes in relapsed HL (summarized in Table 4) did so drawing heavily on data from the early 1990s before up-front ABVD therapy or equivalent was established unequivocally as standard of care treatment for advanced HL in the United States [1, 15, 25, 35–38]. Our data differs substantially from previous experiences in the pre-ABVD therapeutic era and potentially suggests that the natural history of HL after HD-ASCT following upfront ABVD therapy may differ from the historical experience of MOPP. This finding may have further implications when even more aggressive induction therapies, such as BEACOPP escalated, are utilized [39–40]. Additionally, our findings might be explained by the rigorous methods used to track patients following HD-ASCT. Larger clinical investigations might not employ such aggressive follow-up to patients more than three years removed from autologous transplant. This explanation would suggest that late relapses occur, but are not captured by the majority of studies; thus the current body of literature might be under-reporting the true incidence of late relapses following HD-ASCT. Lastly, it is possible that we observed an increased number of late relapses because our “real world” patient population was not selected for favorable prognostic features. The majority of the clinical trial literature examines HD-ASCT outcomes of a selected group of relatively young patients with favorable prognostic features. Many of the patients in our series had prognostically unfavorable characteristics at diagnosis, including B symptoms (62%), and advanced stage (III/IV) HL (53%). In addition, 33 patients in our series (35%) were primary induction failures, and 5 patients (5%) had advanced disease. Interestingly, the majority of our patients had chemosensitive disease (78%), and only one of our late relapses had documented chemoresistance. Comparable studies often include a highly selected patient population, with additional cardiac, pulmonary, renal, and hepatic function inclusion criteria. We submit that late events and relapses detailed in our series might be more applicable to the average HL patient with relapsed or refractory disease requiring aggressive treatment encountered in a clinic setting.
Table 4.
Comparison of Studies Examining Long-Term Outcomes after HD-ASCT for Relapsed/Refractory HL
| Author | Date | Study Duration | N | % ABVD |
R/P | Median f/u time (yrs) |
% EFS | %OS | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Bierman | 2002 | 1984–1999 | 379 | NS^ | R | 5.1 | 24 (10y) | 32 (10y) | |
| Stiff | 2003 | 1990–1995 | 74 | 14 | P | 10 | 41 (5y) | 54 (5y) | No late events in sensitive* pts; some late events in resistant† pts |
| Moskowitz | 2004 | 1985–1998 | 75 | 81.3 | P | 10 | 45 (10y) | 48 (10y) | OS, EFS plateau |
| Lavoie | 2005 | 1985–1992 | 100 | NS^ | P | 11.4 | 51(15y) | 54 (15y) | OS plateau |
| Majhail | 2006 | 1985–2003 | 141 | 63.8 | R | 6.3 | 48 (5y) | 53 (5y) | OS plateau, some late events |
| Sirohi | 2008 | 1985–2005 | 195 | NS^ | P | 10.3 | 44 (5y) | 55 (5y) | |
| Smith | 2011 | 1995–2008 | 214 | 82.7 | R | 6 | 45 | 55 | Study limited to early relapses; EFS plateau |
| URMC | 2011 | 1990–2006 | 95 | 100 | R | 8.2 | 37 (5y) | 54 (5y) | Notable late events |
NS, not specified; R, retrospective study; P, prospective study; sensitive*, chemosensitive disease; resistant,†, chemoresistant disease
With a median follow-up time of 8.2 years, we demonstrated a substantial number of relapses and deaths ≥ 3 years post HD-ASCT. Clinicians need to be aware that HL patients remain at a continued risk of relapse, in addition to the well-documented risk of late therapy-related toxicity. Based on our results, HL patients may benefit from close monitoring post-transplant, perhaps for at least 5–10 years after HD-ASCT. Furthermore, our results emphasize the potential role for novel therapeutic approaches currently under evaluation to improve HD-ASCT outcomes, including maintenance approaches with therapeutic agents such as Brentuximab vedotin, anticipated to receive FDA approval, as well as with intensive, double HD-ASCT [30, 41–44]. Reduced-intensity allogeneic transplant might yield lasting remission in those patients who fail HD-ASCT, while limiting toxic exposure. Additionally, consolidative radiotherapy following autologous transplant was shown in the 2003 SWOG study to improve outcomes following HD-ASCT and should be considered as a potential strategy to maximize response in high-risk patients or those with refractory disease [34]. Moreover, given that late relapse is a reality following HD-ASCT in the ABVD era, the long natural history of HL has significant implications on endpoints for clinical trials of novel conditioning regiments prior to autologous transplantation, as well as maintenance strategies post HD-ASCT.
Acknowledgements
This research was supported by the University of Rochester SPORE in lymphoma CA 130805 (Drs Kelly, Fisher, Bernstein, and Friedberg). Additional research support was received from the University of Rochester Weed Hematology Fellowship (Keller, Sensenig, and Andreozzi). Dr. Friedberg is a Scholar in Clinical Research of the Leukemia & Lymphoma Society. Dr. Kelly is a Leukemia Research Foundation Postdoctoral Fellow. We thank Diane Nichols of the University of Rochester Medical Center Bone Marrow Transplant Registry for assistance in collecting transplant data on patients undergoing HD-ASCT, and Margie Richardson of the New York State Cancer Registry for assistance in determining post HD-ASCT outcomes.
Footnotes
Financial Disclosure Statement: The authors declare no competing financial interests, corporate involvement or patent holdings.
Presented in part at the American Society of Hematology Annual Meeting 2007, Atlanta, GA and at the 8th International Symposium on Hodgkin Lymphoma 2010, Cologne, Germany.
References
- 1.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: report of an intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Reece DE, Connors JM, Spinelli JJ, et al. Intensive therapy with cyclophosphamide, carmustine, etoposide +/− cisplatin, and autologous bone marrow transplantation for Hodgkin's disease in first relapse after combination chemotherapy. Blood. 1994;83:1193–1199. [PubMed] [Google Scholar]
- 4.Vose JM, Bierman PJ, Anderson JR, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80:2142–2148. [PubMed] [Google Scholar]
- 5.Bierman PJ, Vose JM, Armitage JO. Autologous transplantation for Hodgkin's disease: coming of age? Blood. 1994;83:1161–1164. [PubMed] [Google Scholar]
- 6.Sweetenham JW, Carella AM, Taghipour G, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin's disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17:3101–3109. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- 7.Josting A, Katay I, Rueffer U, et al. Favorable outcome of patients with relapsed or refractory Hodgkin's disease treated with high-dose chemotherapy and stem cell rescue at the time of maximal response to conventional salvage therapy (Dex-BEAM) Ann Oncol. 1998;9:289–295. doi: 10.1023/a:1008283909959. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus HM, Rowlings PA, Zhang MJ, et al. Autotransplants for Hodgkin's disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999;17:534–545. doi: 10.1200/JCO.1999.17.2.534. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin's disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 11.David KA, Mauro L, Evens AM. Relapsed and refractory Hodgkin lymphoma: transplantation strategies and novel therapeutic options. Curr Treat Options Oncol. 2007;8:352–374. doi: 10.1007/s11864-007-0046-9. [DOI] [PubMed] [Google Scholar]
- 12.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program. 2008:326–333. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
- 13.Moskowitz CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin's disease. Br J Haematol. 2004;124:645–652. doi: 10.1111/j.1365-2141.2003.04828.x. [DOI] [PubMed] [Google Scholar]
- 14.Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–1478. doi: 10.1182/blood-2004-12-4689. [DOI] [PubMed] [Google Scholar]
- 15.Smith SD, Moskowitz CH, Dean R, et al. Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: results from two transplant centres. Br J Haematol. 2011;153:358–363. doi: 10.1111/j.1365-2141.2011.08616.x. [DOI] [PubMed] [Google Scholar]
- 16.Czyz J, Dziadziuszko R, Knopinska-Postuszuy W, et al. Outcome and prognostic factors in advanced Hodgkin's disease treated with high-dose chemotherapy and autologous stem cell transplantation: a study of 341 patients. Ann Oncol. 2004;15:1222–1230. doi: 10.1093/annonc/mdh304. [DOI] [PubMed] [Google Scholar]
- 17.Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89:801–813. [PubMed] [Google Scholar]
- 18.Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96:1280–1286. [PubMed] [Google Scholar]
- 19.Popat U, Hosing C, Saliba RM, et al. Prognostic factors for disease progression after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33:1015–1023. doi: 10.1038/sj.bmt.1704483. [DOI] [PubMed] [Google Scholar]
- 20.Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin's disease: results and prognostic factors in 494 patients from the Grupo Espanol de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 21.Deeg HJ, Socie G. Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood. 1998;91:1833–1844. [PubMed] [Google Scholar]
- 22.Forrest DL, Nevill TJ, Naiman SC, et al. Second malignancy following high-dose therapy and autologous stem cell transplantation: incidence and risk factor analysis. Bone Marrow Transplant. 2003;32:915–923. doi: 10.1038/sj.bmt.1704243. [DOI] [PubMed] [Google Scholar]
- 23.Forrest DL, Hogge DE, Nevill TJ, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation does not increase the risk of second neoplasms for patients with Hodgkin's lymphoma: a comparison of conventional therapy alone versus conventional therapy followed by autologous hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:7994–8002. doi: 10.1200/JCO.2005.01.9083. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146:158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horning SJ. Risk, cure and complications in advanced hodgkin disease. Hematology Am Soc Hematol Educ Program. 2007:197–203. doi: 10.1182/asheducation-2007.1.197. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Health and Human Services PHS, Centers for Disease Control, National Center for Health Statistics. National Death Index User's Manual. Hyattsville, MD: 2010. [Google Scholar]
- 27.Horm J. Assignment Of Probabilistic Scores To National Death Index Record Matches. A-5-A-13. 12-1-1996. Centers for Disease Control and Prevention/National Center for Health Statistics National Death Index Plus: Coded Causes of Death. 1996 [Google Scholar]
- 28.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 29.Mendler JH, Kelly J, Voci S, et al. Bortezomib and gemcitabine in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008;19:1759–1764. doi: 10.1093/annonc/mdn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 31.Bierman PJ, Lynch JC, Bociek RG, et al. The International Prognostic Factors Project score for advanced Hodgkin's disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol. 2002;13:1370–1377. doi: 10.1093/annonc/mdf228. [DOI] [PubMed] [Google Scholar]
- 32.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008;19:1312–1319. doi: 10.1093/annonc/mdn052. [DOI] [PubMed] [Google Scholar]
- 34.Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9:529–539. doi: 10.1016/s1083-8791(03)00205-2. [DOI] [PubMed] [Google Scholar]
- 35.Canellos GP. Is ABVD the standard regimen for Hodgkin's disease based on randomized CALGB comparison of MOPP, ABVD and MOPP alternating with ABVD? Leukemia. 1996;10 Suppl 2:s68. [PubMed] [Google Scholar]
- 36.Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C. Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer. 1975;36:252–259. doi: 10.1002/1097-0142(197507)36:1<252::aid-cncr2820360128>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Viviani S, Di Nicola M, Bonfante V, et al. Long-term results of high-dose chemotherapy with autologous bone marrow or peripheral stem cell transplant as first salvage treatment for relapsed or refractory Hodgkin lymphoma: a single institution experience. Leuk Lymphoma. 2010;51:1251–1259. doi: 10.3109/10428194.2010.486090. [DOI] [PubMed] [Google Scholar]
- 38.Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–6408. doi: 10.1200/JCO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Diehl V, Franklin J, Hasenclever D, et al. BEACOPP, a new dose-escalated and accelerated regimen, is at least as effective as COPP/ABVD in patients with advanced-stage Hodgkin's lymphoma: interim report from a trial of the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 1998;16:3810–3821. doi: 10.1200/JCO.1998.16.12.3810. [DOI] [PubMed] [Google Scholar]
- 40.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 41.Fung HC, Stiff P, Schriber J, et al. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13:594–600. doi: 10.1016/j.bbmt.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 42.Jona A, Younes A. Novel treatment strategies for patients with relapsed classical Hodgkin lymphoma. Blood Rev. 2010;24:233–238. doi: 10.1016/j.blre.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011 doi: 10.1182/blood-2010-09-288373. [DOI] [PubMed] [Google Scholar]
- 44.Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]