Abstract
Aim
To prospectively examine the association of retinal microvascular signs with incident diabetes and impaired fasting glucose (IFG) in a multi-ethnic population-based cohort.
Methods
The multi-ethnic study of atherosclerosis comprised Caucasians, African-Americans, Hispanics and Chinese aged 45–84 years. Retinal vascular calibre and retinopathy were quantified from baseline retinal photographs. Incident diabetes and IFG were ascertained prospectively.
Results
After a median follow-up of 3 years, 243 (4.9%) people developed diabetes and 565 (15.0%) developed IFG. After adjusting for known risk factors, participants with wider retinal arteriolar calibre had a higher risk of developing diabetes [HR: 1.60; 95% CI: 1.12–2.29, p = 0.011 comparing highest with lowest arteriolar calibre tertile]. In ethnic subgroup analysis, the association between wider retinal arteriolar calibre and incident diabetes was stronger and statistically significant only in Caucasians [HR: 2.78; 95% CI: 1.37–5.62, p = 0.005]. Retinal venular calibre and retinopathy signs were not related to risk of diabetes or IFG.
Conclusion
Wider retinal arteriolar calibre is independently associated with an increased risk of diabetes, supporting a possible role for early arteriolar changes in diabetes development. This effect was largely seen in Caucasians, and not in other ethnic groups, and may reflect ethnic differences in susceptibility to diabetes from microvascular pathways.
Keywords: Retinal microvascular calibre, Retinopathy, Diabetes, Impaired fasting glucose
1. Introduction
Microvascular disease has been hypothesized to play a key role in the pathogenesis of diabetes [1] and pre-diabetic states such as impaired fasting glucose (IFG) [2]. However, the precise temporal sequence of microvascular disease to diabetes development remains uncertain [3].
An assessment of the retinal microvasculature may offer the opportunity to examine early microvascular changes that may precede diabetes development [4]. Cross-sectional studies suggest that persons with diabetes have wider retinal arterioles [5–8]. However, narrower retinal arterioles have been reported to predict an increased risk of developing diabetes in some prospective studies [9–11] but not in others, which found that diabetes risk was related to venular rather than arteriolar calibre changes [12]. A few studies have also reported that retinal vascular calibre was related to incident IFG [12,13].
Furthermore, isolated retinopathy signs in persons without diabetes have also been suggested to be markers of future diabetes risk [14], although most studies show that these retinopathy signs do not appear to be related to an increased risk of diabetes [15–17] except perhaps in younger individuals [16] and those with a family history of diabetes [15].
The majority of previous studies have been conducted in white populations, and no data are currently available to inform how these relationships may vary in different racial/ethnic groups. We therefore examined prospectively the relationship of retinal vascular calibre and retinopathy signs with incident diabetes and IFG in a multi-ethnic population-based cohort.
2. Methods
2.1. Study population
The multi-ethnic study of atherosclerosis (MESA) is a prospective cohort study of 6814 men and women aged 45–84 years without clinical cardiovascular disease living in six United States communities. The main objective of this study was to identify risk factors for subclinical and clinical cardiovascular disease progression. Sampling and recruitment procedures have been described in detail elsewhere [18]. In brief, 6814 participants comprising 4 ethnic groups (Caucasians, African-Americans, Hispanics and Chinese) were recruited between July 2000 and July 2002 from Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York City, New York; and St. Paul, Minnesota. Each field centre recruited approximately 1100 participants, with an approximately equal number of men and women from two or more of the ethnic groups to minimize confounding of ethnicity by site. The Tenets of the Declaration of Helsinki were followed and institutional review board approval was granted at all MESA sites. Written informed consent was obtained from each participant.
Retinal photography was performed at the second examination (August 2002–February 2004), which occurred immediately after the baseline examination. 6231 (91.6%) participants returned for this second examination, and 5946 (87.3%) and 5818 (85.4%) participants returned for the third (March 2004–September 2005) and fourth examinations (September 2005–May 2007), respectively.
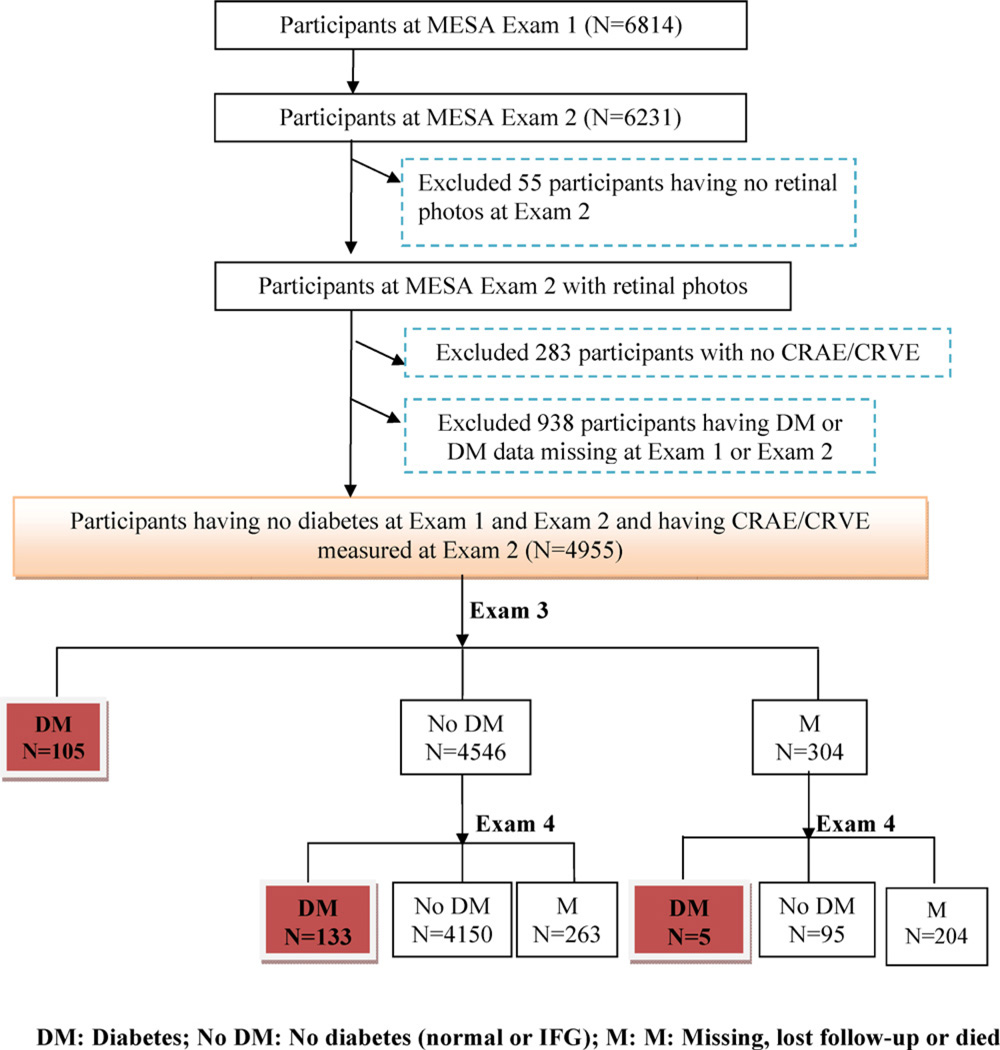
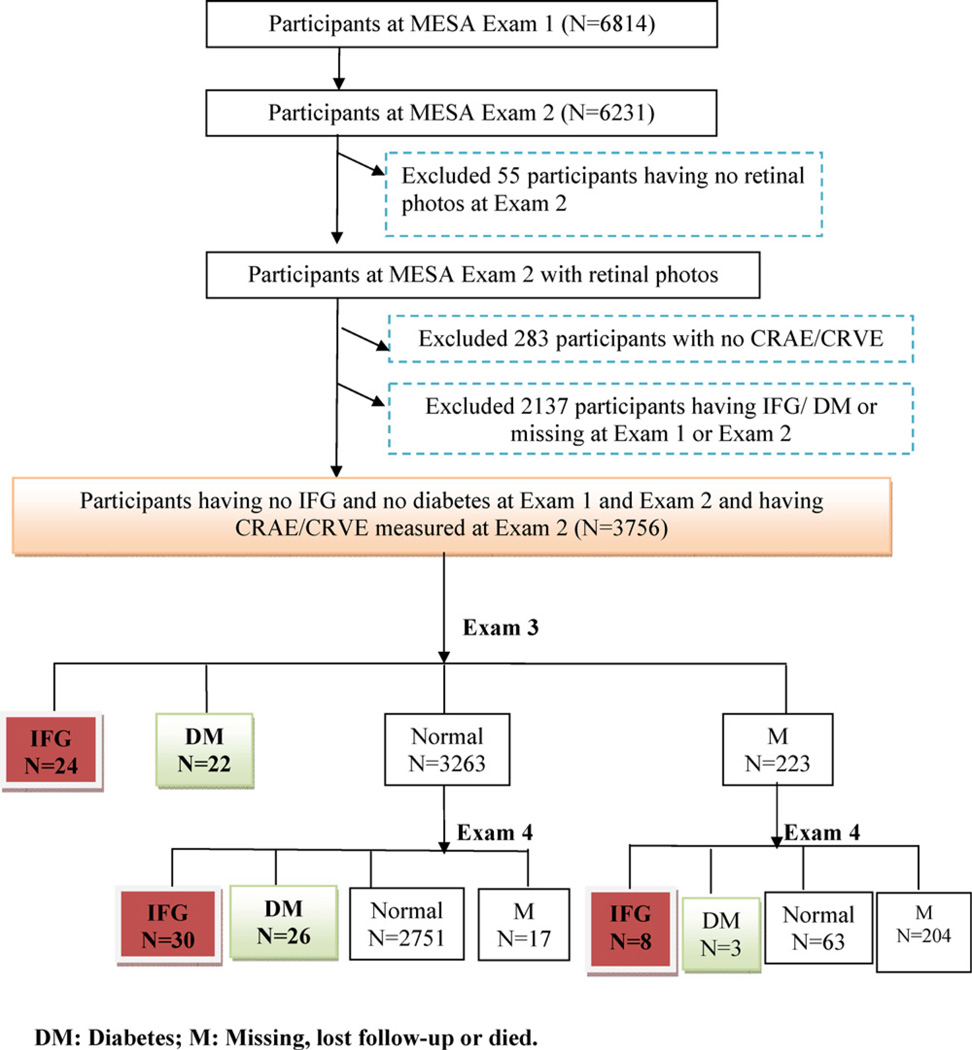
For the purpose of this study, eligible participants were identified from the second examination, which was considered the baseline examination for this report. Incident diabetes and IFG were prospectively identified at the third and fourth examinations. Of the 6231 participants that returned for the second examination, 6176 (99.0%) had retinal photographs taken. Participants in the following categories, which are not mutually exclusive, were excluded in separate models: those with prevalent diabetes or missing diabetes status, n = 938 (in incident diabetes prediction model); those with prevalent IFG, diabetes or missing diabetes status, n = 2137 (in incident IFG prediction model); and those with ungradable retinal photographs or missing data for retinal vessel calibre or retinopathy, n = 283 (Figs. 1 and 2).
Fig. 1.
Participants included/excluded at each MESA follow-up examination in the incident diabetes prediction model.
Fig. 2.
Participants included/excluded at each MESA follow-up examination in the incident IFG prediction model.
2.2. Retinal photography and measurement of baseline retinal vascular calibre
Fundus photography was performed at each site using a standardized protocol described previously [19]. In brief, participants were seated in a darkened room and both eyes were photographed with a 45 degree 6.3 megapixel digital nonmydriatic camera. Two photographic fields were taken of each eye, the first centred on the optic disc and the second on the fovea. Images were sent from the six field centres to the University of Wisconsin, Madison, for measurement of retinal vascular calibre and quantification of retinopathy.
Retinal vascular calibre was measured using a computer-based program (IVAN, University of Wisconsin, Madison), based on a detailed protocol [20]. Trained graders, masked to participant characteristics performed these measurements. Optic-disc centred right eye photographs were selected for measurement; the left eye photograph was used if retinal vascular calibre could not be measured in the right eye. For each photograph, all arterioles and venules coursing through an area 0.5–1 disc diameter from the optic disc margin were measured, and using formulas developed by Hubbard et al. [21] and later modified by Knudtson et al. [22], these measurements were combined and summarized into a single central retinal artery equivalent (CRAE) and central retinal venular equivalent (CRVE). Reproducibility of these measurements has been reported, with intra- and intergrader intraclass correlation coefficients ranging from 0.78 to 0.99 [20].
2.3. Assessment of retinopathy signs
Assessment of retinopathy signs has been previously published [19]. In brief, retinopathy was considered to be present if any characteristic lesions as defined by the early treatment diabetic retinopathy study (ETDRS) [23] severity scale was present: microaneurysms, haemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading, and new vessels. These lesions were defined as present if graded as either definite or probable.
2.4. Ascertainment of incident diabetes mellitus and IFG
Methods of ascertainment and diagnosis of diabetes mellitus have been previously described [18]. Participants underwent a 12-h overnight fast and had morning blood collection. Diabetes mellitus was defined as fasting plasma glucose value of ≥126 mg/dl (7.0 mmol/L) or use of insulin or oral hypoglycaemic medication. IFG was defined as fasting plasma glucose level of 110–125 mg/dL (6.0–6.9 mmol/L). All other participants were defined as having normal glucose metabolism.
Incident diabetes was defined in persons free of diabetes at the first and second examinations who subsequently developed diabetes by the third or fourth examination. Incident IFG was defined in persons with normal fasting glucose at the first and second examinations who subsequently developed IFG by the third or fourth examination.
2.5. Assessment of other risk factors
All participants underwent an extensive interview, physical examination and laboratory investigation at each follow-up examination. Information on past medical history, medication use, cigarette smoking status, alcohol consumption, physical activity and family history were self-reported [18]. A positive family history of diabetes was defined by participant report of diabetes in either biological parent. Physical activity was surveyed in detail and for this report the MESA-computed value for total intentional exercise was analysed. Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or current use of antihypertensive medications. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters. Fasting (>12 h) blood samples were drawn from participants and analysed for serum glucose and insulin levels, HbA1C, plasma total and HDL-cholesterol and triglycerides. LDL-cholesterol was calculated using the Friedewald equation [24]. A urine sample was collected and analysed for albumin and creatinine.
2.6. Power calculation
When the sample size in each group is 1621, with a total number of events required, E, of 3139, a 0.050 level two-sided log-rank test for equality of survival curves will have 80% power to detect the difference between groups by CRVE. The proportion of incident diabetes in people with higher CRVE is 0.025 and the proportion of incident diabetes in people with lower is 0.035 (a constant hazard ratio of 1.05); this assumes no dropouts before time t.
2.7. Statistical analysis
Descriptive statistics were computed for all variables. Retinal arteriolar and venular calibre values (CRAE/CRVE) were categorized into tertiles, with the first tertile representing the narrowest calibre and the third tertile the widest. Retinopathy signs, categorized as presence of microaneurysms, haemorrhage, hard exudates, or any retinopathy, were analysed as binary categorical variables (present vs. absent).
The relationship between baseline retinal vascular calibre/retinopathy and incident diabetes/IFG was examined using Kaplan–Meier survival estimates and Cox’s proportional hazards regression model after adjusting for potential confounders. We constructed 3 models: Model 1 initially adjusted for age, gender, race, study centre and CRVE in models of CRAE and vice versa [25]. Models for arteriolar diameter were adjusted for venular diameter (and vice versa) because this allows for controlling the potential confounding effects of fellow vessels on the outcomes of interest. This is detailed in a study by Liew et al. [25] and is an approach used in previous publications such as in MESA [26,27]. Stepwise Cox regression was used to find the best-fitting survival model from all available covariates. Model 2 included additional adjustments for SBP, family history of diabetes, BMI, HbA1C, fasting insulin concentration, antihypertensive treatment and urinary albumin excretion (UAE), which were significant in the stepwise Cox regression model; and Model 3 was adjusted for all the variables in Model 2 plus presence of any retinopathy. The latter additional covariate was selected to demonstrate that the relationship between CRAE/CRVE and incident diabetes is independent of baseline retinopathy. All pertinent variables were examined for correlations and multicollinearity using Pearson product-moment correlation. We used the Schoenfeld residual tests to evaluate the proportional hazard assumption and checked interactions between variables. Tests for interaction between identified risk factors were carried out. No interaction between such variables was found. A p-value of 0.05 was used for significance testing. We also performed supplementary analysis of retinal vascular calibre with incident diabetes by ethnic subgroups. All statistical analyses were performed using Stata software, version 11.0 (Stata Corp., College Station, TX).
3. Results
Among participants with baseline retinal vascular calibre, retinopathy data and known diabetes status who attended at least one follow-up examination, 4955 participants were free of diabetes and 3756 of both diabetes and IFG at baseline. The proportion of participants with ungradable photographs did not differ between the incident diabetes and incident IFG groups. Table 1 compares the baseline characteristics of participants that developed incident diabetes with those that did not. In general, participants that developed incident diabetes were more likely to be overweight and hypertensive; have a positive family history of diabetes; have baseline IFG and higher fasting serum glucose and insulin levels; and have poorer lipid profiles and physical activity scores (Table 1.1).
Table 1.
Participant characteristics at baseline (Exam 2), comparing those who did and did not develop diabetes at the end of Exam 4 (N = 4955).
| Participants who did not develop diabetes (N = 4712) |
Participants who developed diabetes (N = 243) |
p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender, Men | 2221 | 47.1 | 112 | 46.1 | 0.75 |
| Race | |||||
| White Caucasian | 2059 | 43.7 | 67 | 27.6 | <0.001 |
| African American | 1167 | 24.7 | 81 | 33.3 | |
| Hispanics | 936 | 19.9 | 67 | 27.6 | |
| Chinese American | 550 | 11.7 | 28 | 11.5 | |
| Hypertension, present | 1891 | 40.5 | 128 | 54.2 | <0.001 |
| Antihypertensive treatment | 1616 | 35.9 | 121 | 52.2 | <0.001 |
| Lipid-lowering medication | 881 | 19.6 | 54 | 23.3 | 0.16 |
| Fasting glucose status at Exam 2 | |||||
| Normal | 3907 | 82.9 | 82 | 33.7 | <0.001 |
| IFG | 805 | 17.1 | 161 | 66.3 | |
| Family history of diabetes | 606 | 12.9 | 57 | 23.6 | <0.001 |
| Smoking status | |||||
| Never smoked | 2331 | 49.7 | 119 | 49.2 | 0.96 |
| Ex smoker | 1804 | 38.5 | 93 | 38.4 | |
| Current | 554 | 11.8 | 30 | 12.4 | |
| Median | p25, p75 | Median | p25, p75 | ||
| Age, years | 62 | 54, 70 | 61 | 54, 69 | 0.27 |
| Systolic blood pressure, mmHg | 119 | 108, 136 | 124 | 114, 138 | 0.0002 |
| Diastolic blood pressure, mmHg | 71 | 64, 77 | 72 | 66, 78 | 0.023 |
| Waist circumference (cm) | 95.4 | 86.5, 104.2 | 103.0 | 94.3, 115.2 | <0.001 |
| Waist hip ratio (unit) | 0.93 | 0.87, 0.98 | 0.96 | 0.91, 0.99 | <0.001 |
| Fasting plasma glucose, mg/dL | 90 | 85, 97 | 107 | 95, 115 | <0.001 |
| Serum insulin (µmol/L) | 4.9 | 3.4, 7.6 | 8.0 | 5.6, 12.3 | <0.001 |
| Haemoglobin A1c (%) | 5.4 | 5.2, 5.7 | 5.9 | 5.5, 6.3 | <0.001 |
| Total cholesterol, mg/dL | 191 | 169, 215 | 187 | 166, 212 | 0.14 |
| HDL cholesterol, mg/dL | 50 | 42, 61 | 45 | 40, 53 | <0.001 |
| Triglycerides, mg/dL | 108 | 77, 155 | 123 | 90, 174 | <0.001 |
| Physical activity (total intentional exercise (Q9–15) MET-min/wk) | 825 | 105, 1920 | 630 | 0, 1575 | 0.04 |
| HOMA_IR | 1.05 | 0.70, 1.69 | 1.98 | 1.34, 3.05 | <0.001 |
| Urinary creatinine (mg/dl) | 113 | 67, 164 | 124 | 73, 181 | 0.014 |
| Urinary albumin excretion | 0.60 | 0.30, 1.10 | 0.80 | 0.40, 1.80 | <0.001 |
| Urinary albumin creatinine ratio (mg/g) | 113 | 67, 164 | 124 | 73, 181 | 0.014 |
| Retinal arteriolar calibre (CRAE) | 144.1 | 134.9, 152.8 | 146.3 | 137.4, 155.2 | 0.009 |
| Retinal venular calibre (CRVE) | 212.7 | 198.6, 227.1 | 219.0 | 207.2, 232.5 | <0.001 |
Data shown as medians or proportions at 25th and 75th percentiles (p25 and p75).
Table 1.1.
Participant characteristics at baseline (Exam 2), comparing those who did and did not develop IFG at the end of Exam 4 (N = 3756).a
| Participants who did not develop IFG (N = 3140) |
Participants who developed IFG (N = 565) |
p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender, Men | 1374 | 43.8 | 284 | 50.3 | 0.004 |
| Race | |||||
| White Caucasian | 1459 | 46.5 | 223 | 39.5 | <0.001 |
| African American | 751 | 23.9 | 137 | 24.3 | |
| Hispanics | 566 | 18.0 | 152 | 26.9 | |
| Chinese American | 364 | 11.6 | 53 | 9.4 | |
| Hypertension, present | 1115 | 35.8 | 255 | 45.6 | <0.001 |
| Antihypertensive treatment | 937 | 31.2 | 219 | 40.3 | <0.001 |
| Lipid-lowering medication | 514 | 17.1 | 129 | 2378 | <0.001 |
| Family history of diabetes | 402 | 13.1 | 81 | 14.3 | 0.31 |
| Smoking status | |||||
| Never smoked | 1605 | 51.3 | 272 | 48.2 | 0.29 |
| Ex smoker | 1151 | 36.8 | 227 | 40.3 | |
| Current | 371 | 11.9 | 65 | 11.5 | |
| Median | p25, p75 | Median | p25, p75 | ||
| Age, years | 61.0 | 53.0, 70.0 | 62.0 | 55.0, 70.0 | 0.16 |
| Systolic blood pressure, mmHg | 117.5 | 107.0, 134.5 | 122.0 | 111.5, 136.5 | <0.001 |
| Diastolic blood pressure, mmHg | 69.5 | 63.0, 76.0 | 71.2 | 64.7, 78.0 | 0.003 |
| BMI (cm) | 26.4 | 23.7, 29.7 | 28.2 | 25.6, 31.6 | <0.001 |
| Fasting plasma glucose, mg/dL | 87.0 | 84.0, 92.0 | 93.0 | 89.0, 96.0 | <0.001 |
| Serum insulin (µmol/L) | 4.6 | 3.2, 7.0 | 4.4 | 3.2, 6.9 | <0.001 |
| Haemoglobin A1c (%) | 5.3 | 5.1, 5.6 | 5.5 | 5.3, 5.7 | <0.001 |
| Total cholesterol, mg/dL | 192.0 | 170.0, 216.0 | 190.0 | 168.0, 214.0 | 0.09 |
| HDL cholesterol, mg/dL | 52.0 | 43.0, 63.0 | 49.0 | 42.0, 59.0 | 0.001 |
| Triglycerides, mg/dL | 104.0 | 75.0, 147.0 | 111.0 | 80.5, 159.0 | 0.006 |
| Physical activity (total intentional exercise (Q9–15) MET-min/wk) | 840.0 | 157.5, 1927.5 | 735.0 | 67.5, 1972.5 | 0.16 |
| HOMA_IR | 0.90 | 0.62, 1.39 | 1.24 | 0.84, 1.85 | <0.001 |
| Urinary albumin | 0.50 | 0.30, 1.0 | 0.6 | 0.3, 1.2 | 0.002 |
| Urinary albumin creatinine ratio (mg/g) | 4.6 | 3.1, 8.2 | 4.7 | 3.1, 8.3 | 0.15 |
| Retinal arteriolar calibre (CRAE) | 144.4 | 135.2, 153.1 | 143.7 | 134.0, 152.8 | 0.53 |
| Retinal venular calibre (CRVE) | 212.1 | 198.3, 225.5 | 212.2 | 199.7, 227.3 | 0.14 |
Data shown as medians or proportions at 25th and 75th percentiles (p25 and p75).
51 participants with unknown IFG status.
Over a median follow-up of 3 years (range: 2 months–4.5 years), 243 people developed diabetes and 565 developed IFG. The incidence rates of diabetes and IFG were 1.60 and 5.25 per 100 person years respectively. Table 2 shows that incident diabetes was associated with CRAE but not CRVE. The incidence rate of diabetes increased from 1.26% to 1.41% and 2.19% with each increasing tertile of CRAE. After adjusting for age, gender, race, study centre, SBP, family history of diabetes, BMI, HbA1C and other risk factors, persons with the highest compared to the lowest CRAE tertile were 60% more likely to develop incident diabetes [HR 1.60; 95% CI: 1.12–2.29]. This association remained similar with additional adjustment for retinopathy.
Table 2.
Cox proportional hazard model of the association between retinal vascular calibre and incident diabetes.
| Person time (years) |
Incident DM cases |
Incident DM rate (%) |
Model 1a: Adjusted HR |
Model 2b: Adjusted HR |
Model 3c: Adjusted HR |
||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| Retinal arteriolar calibre | |||||||||
| Tertile 1, ≤139 µm | 5165 | 65 | 1.26 | 1 | 1 | 1 | |||
| Tertile 2, 139–150 µm | 5104 | 72 | 1.41 | 0.97 (0.69, 1.37) | 0.88 | 1.03 (0.72, 1.46) | 0.84 | 1.03 (0.72, 1.46) | 0.85 |
| Tertile 3, ≥150 µm | 4845 | 106 | 2.19 | 1.34 (0.96, 1.87) | 0.08 | 1.60 (1.12, 2.29) | 0.010 | 1.60 (1.12, 2.29) | 0.011 |
| Retinal venular calibre | |||||||||
| Tertile 1, ≤205 µm | 5259 | 56 | 1.06 | 1 | 1 | 1 | |||
| Tertile 2, 205–223 µm | 4054 | 79 | 1.55 | 1.22 (0.85, 1.76) | 0.27 | 1.02 (0.71, 1.48) | 0.90 | 1.02 (0.71, 1.48) | 0.91 |
| Tertile 3, ≥223 µm | 4771 | 108 | 2.26 | 1.57 (1.09, 2.27) | 0.016 | 1.0 (0.68, 1.46) | 0.97 | 1.00 (0.68, 1.48) | 0.91 |
Model 1: Adjusted for age, gender, race, study centre and venular calibre (in models of arteriolar calibre) or arteriolar calibre (in models of venular calibre).
Model 2: Adjusted for variables in Model 1 plus SBP, family history of diabetes, BMI, HbA1C, fasting insulin concentration, antihypertensive treatment, and urinary albumin excretion.
Model 3: Adjusted for variables in Model 2 plus presence of any retinopathy.
Subgroup analysis by ethnic/racial subgroups showed higher odds of incident diabetes with wider CRAE in all 4 groups; however, the association was statistically significant only among the Caucasians (Table 3). Caucasians with the highest compared to the lowest tertile of CRAE were almost three times [HR: 2.78; 95% CI: 1.37–5.62] more likely to develop incident diabetes. Retinal vascular calibre was not associated with risk of incident IFG (data not shown).
Table 3.
Cox proportional hazard model of the association between retinal vascular calibre and incident diabetes by ethnicity.a
| White Caucasians (n = 2126, incident DM: 1.02%) |
Chinese Americans (n = 578, incident DM: 1.57%) |
African-Americans (n = 1248, incident DM: 2.15%) |
Hispanics (n = 1003, incident DM: 2.21%) |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Retinal arteriolar calibre | ||||||||
| Tertile 1, ≤139 µm | 1 | 1 | 1 | 1 | ||||
| Tertile 2, 139–150 µm | 1.22 (0.60, 2.46) | 0.59 | 1.06 (0.38, 3.00) | 0.91 | 0.88 (0.48, 1.62) | 0.70 | 0.87 (0.46, 1.66) | 0.71 |
| Tertile 3, ≥150 µm | 2.78 (1.37, 5.62) | 0.005 | 1.71 (0.49, 5.93) | 0.40 | 1.26 (0.70, 2.28) | 0.45 | 1.20 (0.64,2.24) | 0.57 |
| Retinal venular calibre | ||||||||
| Tertile 1, ≤205 µm | 1 | 1 | 1 | 1 | ||||
| Tertile 2, 205–223 µm | 0.98 (0.52, 1.83) | 0.91 | 0.65 (0.16, 2.65) | 0.55 | 1.03 (0.48 2.23) | 0.94 | 1.10 (0.59, 2.04) | 0.75 |
| Tertile 3, ≥223 µm | 1.10 (0.55, 2.21) | 0.78 | 1.83 (0.53, 6.29) | 0.34 | 1.14 (0.54 2.44) | 0.75 | 0.67 (0.34, 1.33) | 0.26 |
Adjusted for age, gender, venular calibre (in models of arteriolar calibre) or arteriolar calibre (in models of venular calibre), SBP, family history of diabetes, BMI, HbA1C, fasting insulin concentration, antihypertensive treatment, urinary albumin excretion and retinopathy.
Retinopathy signs such as retinal haemorrhage [HR: 0.61; 95% CI: 0.27–1.37], microaneurysms [HR: 0.97; 95% CI: 0.53–1.79], hard exudates [HR: 0.77; 95% CI: 0.20–2.95] and “any retinopathy signs” [HR: 0.82; 95% CI: 0.56–1.20] were not related to incident diabetes (data not shown). There were also no associations between focal retinopathy signs and incident IFG (data not shown).
4. Discussion
In this large prospective multi-ethnic cohort study of persons free of diabetes, we found that persons with wider retinal arterioles were more likely to develop incident diabetes than those with narrower arterioles, independent of age, gender, race, study centre, SBP, HbA1C, fasting insulin levels, family history of diabetes, and other risk factors. We found no association between retinal venular calibre and retinopathy with incident diabetes or IFG.
Our finding that wider retinal arteriolar calibre is associated with subsequent development of diabetes has not been previously reported (Table 4). Cross-sectional data in different population-based studies support our findings and suggest that persons with diabetes have wider retinal arterioles [5–8]. However, prospective data have been more conflicting. In the Atherosclerosis Risk In Communities (ARIC) and Beaver Dam Eye Study (BDES) [9,10], after adjusting for baseline risk factors, persons with narrower, and not wider, retinal arterioles at baseline were more likely to subsequently develop diabetes over a median of 3.5 years (OR: 1.71; 95% CI: 1.13–2.57) and 10 years (OR: 1.53; 95% CI: 1.03–2.27), respectively. Recent data from the Australian Diabetes, Obesity and Lifestyle (AusDiab) study also found a similar relationship between narrower retinal arterioles and 5-year incidence of diabetes (OR: 2.21; 95% CI: 1.02–4.80) [11]. In comparison, the Rotterdam Study and Blue Mountains Eye Study (BMES) found no association between retinal arteriolar calibre and incident diabetes; however, reported that wider venules were related to incident IFG [12,13].
Table 4.
Summary of prospective studies examining the association between retinal vascular calibre and retinopathy signs with incident diabetes/IFG, and cross-sectional studies examining the association between retinal vascular calibre and diabetes.
| Retinal vascular calibre and incident diabetes/IFG | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Sample size (n) | Ethnicity | Age (yrs) | Follow-up duration (yrs) |
Measure of retinal calibre |
Association | Odds ratio (95% CI) | |
| 1. | ARIC [23] | 2002 | 7993 | Whites Blacks | 49–73 | 0.7–5.5 Median: 3.5 | Lowest AVR quartile | Higher risk of incident diabetes | 1.71(1.13–2.57) |
| 2. | BDES [25] | 2005 | 3251 | Whites | 43–86 | 10 | Lowest AVR quartile | Higher risk of incident diabetes | 1.53 (1.03–2.27) |
| 3. | Rotterdam [7] | 2006 | 2307 | Whites | ≥55 | 5.2–9.5 Mean: 6.4 | Per SD increase in CRVE | Higher risk of incident IFG | 1.15 (0.99–1.34) |
| 4. | Ausdiab [15] | 2008 | 803 | Whites | ≥25 | 5 | Lowest CRAE tertile | Higher risk of incident diabetes | 2.21 (1.02–4.80) |
| 5. | BMES [11] | 2008 | 2123 | Whites | ≥49 | 10 | Per SD increase in CRVE | Higher risk of incident IFG in persons <70 years | 1.53 (1.11–2.12) |
| Retinopathy signs and incident diabetes | |||||||||
| 1. | ARIC [25] | 2006 | 7992 | Whites Blacks | 49–73 | Median: 3.5 | Any retinopathy | No association in total cohort | 1.10 (0.7–1.9) |
| Higher risk in those with diabetes family history | 2.30 (1.0–5.3) | ||||||||
| 2. | BDES [12] | 2006 | 3402 | Whites | 43–86 | 15 | Any retinopathy | No association in total cohort | 1.35 (0.90–2.03) |
| Higher risk of incident diabetes in persons ≤65 years | 1.80 (1.12–2.89) | ||||||||
| 3. | BMES [14] | 2006 | 3653 | Whites | ≥49 | 5 | Any retinopathy | No association with incident diabetes | NR |
| 4. | Ausdiab [19] | 2008 | 1192 | Whites | ≥25 | 5 | Any retinopathy | Higher risk incident diabetes | 2.66 (1.14–6.21) |
| Cross-sectional association of retinal vascular calibre and diabetes | |||||||||
| 1. | Ausdiab [20] | 2007 | 1998 | Whites | ≥25 | – | Per SD increase in CRAE | Higher odds of diabetes compared to persons with NGT | 1.34 (1.07–1.67) |
| 2. | BMES [10] | 2007 | 3654 | Whites | ≥49 | – | Mean CRAE | Wider in diabetics than in nondiabetics | p < 0.01 |
| 3. | MESA [16] | 2008 | 5976 | Multi-ethnica | 45–84 | – | Mean CRAE Mean CRVE | Wider in persons with diabetes compared to persons with NGT and IFG | p = 0.0008 for increasing trend |
| Wider in persons with diabetes compared to persons with NGT and IFG | p = 0.02 for increasing trend | ||||||||
| 4. | SP2 [9] | 2009 | 3404 | Multi-ethnic Asiansb | 24–95 | – | Mean CRAE Mean CRVE | Wider in persons with diabetes compared to persons with NGT and IFG | p = 0.01 for increasing trend |
| Each mmol increase in FPG associated with a 0.51 µm increase in CRVE | p = 0.006 for increasing trend | ||||||||
CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; AVR, arteriole-to-venule ratio; NR, not reported; NGT, normal glucose tolerance; IFG, impaired fasting glucose; FPG, fasting plasma glucose; SP2, Singapore Prospective Study Program and Singapore Cardiovascular Cohort Study 2.
White Caucasians, African-Americans, Chinese-Americans and Hispanics.
Chinese, Malays and Indians.
Several reasons could account for the discrepancies between studies. First, the findings of MESA may not be directly comparable to those from the above predominantly white populations. The effect of ethnic variation and its associated differences in risk factors could have attenuated some associations or strengthened others. In support of this, we found a stronger relationship among the Caucasians than in other racial/ethnic groups. However, these racial/ethnic variations should be interpreted cautiously because after stratifying by ethnic groups, sample sizes in the Chinese, African-Americans and Hispanics were underpowered to detect significant associations. Second, our median follow-up duration of 3 years was shorter than some prospective studies with 10-year observation periods. We speculate that there may be a sequential dynamic evolution in microvasculature change that occurs during the natural history of diabetes development [4]. Arteriolar narrowing could be the earliest microvascular change that predicts long-term diabetes risk, as shown in studies with longer follow-up [9–11]. Wider retinal arterioles could therefore reflect later and more advanced stages of diabetes development as suggested by our study and other cross-sectional analyses [5–8]. Further research is needed to investigate whether it is the absolute value of CRAE/CRVE or the progression of changes in these variables that is important in determining the association with incident diabetes. Finally, reverse causation accounting for these findings cannot be completely excluded due to the short follow-up duration. This is supported somewhat by the fact that at baseline when the retinal vessels were examined, those who developed diabetes already had elevated glucose, insulin, HbA1c, and HOMA_IR, and 66% had impaired fasting glucose.
The specific mechanisms linking wider arterioles and diabetes are not known. Wider arterioles possibly reflect a combination of tissue hypoxia, hyperperfusion and impaired vascular autoregulation [28]. As a response to hypoxia, retinal arterioles dilate to increase retinal tissue perfusion, which leads to hyperperfusion, impaired vasoregulation, and further and persistent widening of the arterioles [4]. Longitudinal studies of retinal vascular measurements over time are needed to clarify these relationships and to improve our understanding of the pre-diabetic microvascular changes that occur.
We report no association between retinopathy signs and incident diabetes, a finding that is consistent with data from the ARIC, BDES and BMES [15–17] (Table 4). The AusDiab study is the only study so far to report a two-fold higher risk of incident diabetes in nondiabetic individuals with retinopathy signs at baseline [14]. In the ARIC study, among persons with a family history of diabetes, the presence of retinopathy signs was associated with a two-fold increase in diabetes risk [15]. Our cohort did not show such an association (data not shown), which may be related to the small sample size with a family history of diabetes (n = 663 vs. 1727).
The strengths of our study include its large sample size, the recruitment of participants from a population-based cohort with comprehensive data on risk factors, the multi-ethnic composition which enabled exploration of possible ethnic differences, and the quantitative and masked assessment of retinal vascular calibre and retinopathy signs. Potential limitations include its relatively short follow-up duration and the absence of a more accurate method such as an oral glucose tolerance test to diagnose diabetes. The imprecision of a single fasting glucose could have led to misclassification of diabetes status; however, this error is likely to be random and would only tend to bias any associations towards the null.
In conclusion, our population-based study demonstrates a prospective association between wider retinal arteriolar calibre and incident diabetes in middle-aged persons, independent of known risk factors. These findings suggest that arteriolar processes occur early in the course of diabetes development and may play a role and contribute to its pathogenesis. Ethnic differences in association may reflect differential susceptibility to diabetes from microvascular pathways, an area of research that should be further explored.
Acknowledgements
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and NIH Intramural Research award Z01EY000403 from the National Eye Institute (MFC). Additional support was provided by National Institutes of Health grants HL69979-03 (Klein R. and Wong T.Y.) and the National Health and Medical Research Council (NHMRC), 52993, Australia (Wong T.Y.).
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–726. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 2.Jaap AJ, Shore AC, Tooke JE. Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia. 1997;40:238–243. doi: 10.1007/s001250050669. [DOI] [PubMed] [Google Scholar]
- 3.Schiekofer S, Balletshofer B, Andrassy M, Bierhaus A, Nawroth PP. Endothelial dysfunction in diabetes mellitus. Semin Thromb Hemost. 2000;26:503–511. doi: 10.1055/s-2000-13206. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TT, Wang JJ, Wong TY. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care. 2007;30:2708–2715. doi: 10.2337/dc07-0732. [DOI] [PubMed] [Google Scholar]
- 5.Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. Retinal vascular caliber, diabetes, and retinopathy. Am J Ophthalmol. 2007;143:1024–1026. doi: 10.1016/j.ajo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TT, Wang JJ, Sharrett AR, Islam FM, Klein R, Klein BE, et al. Relationship of retinal vascular caliber with diabetes and retinopathy: the multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2008;31:544–549. doi: 10.2337/dc07-1528. [DOI] [PubMed] [Google Scholar]
- 7.Jeganathan VS, Sabanayagam C, Tai ES, Lee J, Lamoureux E, Sun C, et al. Retinal vascular caliber and diabetes in a multiethnic Asian population. Microcirculation. 2009;16:534–543. doi: 10.1080/10739680902975222. [DOI] [PubMed] [Google Scholar]
- 8.Tikellis G, Wang JJ, Tapp R, Simpson R, Mitchell P, Zimmet PZ, et al. The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia. 2007;50:2263–2271. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, et al. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–539. doi: 10.2337/db07-1376. [DOI] [PubMed] [Google Scholar]
- 12.Ikram MK, Janssen JA, Roos AM, Rietveld I, Witteman JC, Breteler MM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55:506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- 13.Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: the Blue Mountains Eye Study. Microcirculation. 2008;15:373–377. doi: 10.1080/10739680701812220. [DOI] [PubMed] [Google Scholar]
- 14.Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31:1349–1354. doi: 10.2337/dc07-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TY, Mohamed Q, Klein R, Couper DJ. Do retinopathy signs in non-diabetic individuals predict the subsequent risk of diabetes? Br J Ophthalmol. 2006;90:301–303. doi: 10.1136/bjo.2005.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- 17.Cugati S, Cikamatana L, Wang JJ, Kifley A, Liew G, Mitchell P. Five-year incidence and progression of vascular retinopathy in persons without diabetes: the Blue Mountains Eye Study. Eye (Lond) 2006;20:1239–1245. doi: 10.1038/sj.eye.6702085. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111:1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 22.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 26.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthal Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun C, Liew G, Wang JJ, Mitchell P, Saw SM, Aung T, et al. Retinal vascular caliber, blood pressure, and cardiovascular risk factors in an Asian population: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:1784–1790. doi: 10.1167/iovs.07-1450. [DOI] [PubMed] [Google Scholar]
- 28.Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80:468–477. doi: 10.1034/j.1600-0420.2002.800503.x. [DOI] [PubMed] [Google Scholar]