Abstract
Understanding the requirements for protection against pneumococcal carriage and pneumonia will greatly benefit efforts in controlling these diseases. Several antigens, in addition to the polysaccharide capsule, have been implicated in both the virulence and protective immunity against Streptococcus pneumoniae; one of the best-studied S. pneumoniae antigens is pneumococcal surface protein A (PspA). Recently, it was shown that genetic polymorphisms could diminish CCL5 expression, which results in increased susceptibility to and progression of infectious diseases. We previously showed CCL5 blockade reduced PspA-specific humoral and cellular pneumococcal immunity, during S. pneumoniae strain EF3030-induced carriage, by diminishing IFN-γ and enhancing IL-10 secretion by effector T cells. We also identified immuno-dominant helper T lymphocyte (HTL) epitopes in PspA peptide 19-23 (PspA199-246), which caused comparatively more cytokine secretion and proliferation responses by splenic and cervical lymph node (CLN) CD4+ T cells from mice previously challenged with S. pneumoniae strain EF3030. In this study, we sought to determine if PspA199-246–specific CD4+ T cells responses were resistant to the effect of CCL5 deficiency. In short, T cell responses against these HTL epitopes were resistant to CCL5 inhibition, than compared to cells from control or naïve mice, and unaffected by reduced co-stimulatory molecule expression caused by CCL5 blockade. CCL5 deficiency also corresponded with a higher number of IL-10+ CD11b+ CD11cLo and CD11b+ CD11cHi cells and lower IFN-γ expression by similar cells, than compared to controls. These data confirm CCL5 is an essential factor for optimal pneumococcal adaptive immunity and show CD4+ T cell responses to PspA199-246 are largely resistant to CCL5 deficiency.
Keywords: T helper cytokine, Streptococcus pneumoniae, HTL epitope
Introduction
Pneumonia caused by S. pneumoniae, is the most common cause of childhood deaths in the developing world and among the top ten causes of death in aged populations. Recently, antibiotic-resistant S. pneumoniae strains have emerged worldwide [1-3]. Pneumococci in nasopharyngeal carriage are thought to be the main human reservoir for these potentially lethal bacteria. Moreover, nasopharyngeal carriage is thought to be an intermediate stage that precedes invasive disease [4]. Vaccination against pneumococcal infections is greatly needed. However, the host factors that determine pneumococcal immunity are imprecisely known. This study addresses the contribution of an essential host factor and dominant HTL epitopes in pneumococcal immunity.
Chemokines have emerged as important factors and possible mucosal adjuvants that function in lymphocyte activation and recruitment [5-7]. Indeed, a qualitative relationship exists between the class of chemokines secreted following infection, the type of immune response (cellular or humoral immunity) elicited, and the fate of the host following infection [8-11]. The profile of chemokine expression serves as an indicator of immune response type (i.e., Th1 vs. Th2). In this respect, the CCL5-CCR5 axis has been demonstrated to be involved in the activation and function of Thl cells [6, 12, 13]. CCL5 is secreted by epithelial cells, macrophages, fibroblasts, platelets, and activated T cells [14]. This CC chemokine is known to regulate T cell differentiation and polarize Th1 ⪢ Th2 subtypes as well as numerous physiological functions of leukocytes including migration [6, 9, 14, 15]. Genetic variations in ccl5 contribute to differences in infectious disease progression. Indeed, polymorphisms in ccr5 and ccl5 genes play critical roles in susceptibility to and progression of infectious diseases, namely HIV/AIDS and Chlamydia [16-18]. CCL5 acts as an adjuvant for antigen-specific humoral and cellular immune responses in both mucosal and systemic compartments [6]. However, it is not certain what effect these variations have on S. pneumoniae disease susceptibility, progression, and/or protective T cell immunity.
Recently, we showed that S. pneumoniae strain EF3030 induced bronchial epithelium to express CCL5, which was required for optimal pneumococcal humoral and cellular immunity [19]. In fact, CCL5 inhibition resulted in fewer local and systemic antigen-specific CD4+ T cells that produced IL-4 and IFN-γ, while increasing T helper cells that secreted IL-10. Recently, we revealed a region in PspA, spanning residues 199 to 246 (PspA 199-246), with dominant HTL epitopes that theoretically bind a broad range of HLA-DR, -DQ, and –DP alleles as well as I-A and I-E. Overlapping peptides in this region, i.e., PspA peptides 19, 20, 21, 22, and 23, induced significant IFN-γ and IL-10 secretion and proliferative responses after ex vivo stimulation of T helper cells from previously pneumococcal-challenged mice [20].
Our study specifically addresses an important question “are dominant HTL epitopes resistant to CCL5 deficiency?” This is an important question to design better vaccines against S. pneumoniae, especially when one considers the health disparities associated with CCL5 expression caused by the In 1.1 T/C mutation [17]. We used a novel human isolate of capsular group 19 pneumococci that was passed in mice to yield S. pneumoniae strain EF3030, which has a greater propensity to cause nasal or pulmonary infections than to cause sepsis and death when given intranasally [21]. Through antibody-mediated inhibition, we show that dominant PspA HTL epitopes are largely resistant to CCL5 deficiency, despite the significant contribution this chemokine has on pneumococcal immunity.
Materials and Methods
Mice
Female F1 (C57BL/6 × BALB/c) mice, aged 8 to 12 weeks, were procured from Jackson Laboratories (Bar Harbor, MA). All mice were housed in horizontal laminar flow cabinets free of microbial pathogens. Routine Ab screening for a large panel of pathogens and histological analysis of organs and tissues were performed to insure that mice were pathogen - free. The Morehouse School of Medicine Institutional Review Board approved all procedures using mice.
Anti-CCL5 antibody generation and treatment
CCL5 and Freund’s or incomplete Freund’s adjuvants (Sigma, St. Louis, MO) were used to generate anti-CCL5 antibody titers of ~ 1:106 such that 10 μl of rabbit anti-CCL5 antiserum neutralized 20 ng of CCL5. This antiserum was titrated by direct ELISA and no cross-reactivity was detected, when tested against other CCR5 ligands, chemokines (CXCL8, CXCL9, CXCL10, CXCL11, CXCL12, CXCL13, XCL1, CCL1, CCL2, CCL4, CCL7, CCL8, and CCL11), and cytokines (IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and TNF-α). Subsequently, normal or anti-CCL5 sera were heat-inactivated and purified using an IgG isotype-specific protein A column (Pierce Biotechnology, Rockford, IL). Anti-CCL5 antibody titers or non-immunized sera were adjusted to 1:4×105 (i.e., 50 × dilution) in PBS (CCL5 antibody solution), which were administered to mice two days before bacterial challenge and every third day thereafter.
S. pneumoniae strain EF3030 growth and challenge
S. pneumoniae capsular strain EF3030 was among the human isolates of capsular group 19 that were previously examined and found to be relatively non-invasive in mice [22]. Pneumococci were grown in Todd Hewitt broth and stored frozen in aliquots at −70 °C, in 20% glycerol, in sterile lactated Ringer’s solution (Ringer’s) (Abbott Labs) [23, 24]. To establish nasal carriage, groups of F1 (C57BL/6 × BALB/c) mice were nasally administered 107 colony-forming units (CFU) of EF3030 in 15 μL of Ringer’s solution [25]. Experimental groups consisted of 10 mice and studies were repeated 3 times. The guidelines proposed by the committee for the Care of Laboratory Animal Resources Commission of Life Sciences - National Research Council were followed to minimize animal pain and distress. The Morehouse School of Medicine review board approved all procedures involving mice.
Pneumococcal antigens
Previously, we revealed immuno-dominant PspA helper T lymphocyte (HTL) epitopes recognized by S. pneumonia strain EF3030-challenged CD4+ T cells [20]. These epitopes were identified by “peptide walking” using PspA peptides 15 amino acids in size, which overlapped by four amino acids. This region spanned residues 199-246 of the PspA protein sequence (NCBI Acession no. NP_359087). For this study, we synthesized peptides 19-23, DAEEVAPQAKIAELE, AELENQVHRLEQELK, QELKEIDESESEDYA, EDYAKEGFRAPLQSK, and LQSKLDAKKAKLSKL, respectively, by the multipin synthesis method by Chiron Mimotopes Peptide Systems [26] (Table 1). All peptides were acetylated at the N-terminus and ended with a COOH-terminal. Purity of these peptides was approximately 95% and free of endotoxin contamination. The peptides were dissolved in a mixture (v/v) of 75 % dimethyl sulfoxide and 25 % water, to a concentration of 70 mM, divided into small aliquots and stored frozen at −80 °C.
Table 1. Overlapping PspA peptides.
| 01 | MNKKKMILTSLASVA |
| 02 | ASVAILGAGFVASQP |
| 03 | ASQPTVVRAEESPVA |
| 04 | SPVASQSKAEKDYDA |
| 05 | DYDAAKKDAKNAKKA |
| 06 | AKKAVEDAQKALDDA |
| 07 | LDDAKAAQKKYDEDQ |
| 08 | DEDQKKTEEKAALEK |
| 09 | ALEKAASEEMDKAVA |
| 10 | KAVAAVQQAYLAYQQ |
| 11 | AYQQATDKAAKDAAD |
| 12 | DAADKMIDEAKKREE |
| 13 | KREEEAKTKFNTVRA |
| 14 | TVRAMVVPEPEQLAE |
| 15 | QLAETKKKSEEAKQK |
| 16 | AKQKAPELTKKLEEA |
| 17 | LEEAKAKLEEAEKKA |
| 18 | EKKATEAKQKVDAEE |
| 19 | DAEEVAPQAKIAELE |
| 20 | AELENQVHRLEQELK |
| 21 | QELKEIDESESEDYA |
| 22 | EDYAKEGFRAPLQSK |
| 23 | LQSKLDAKKAKLSKL |
| 24 | LSKLEELSDKIDELD |
| 25 | DELDAEIAKLEDQLK |
| 26 | DQLKAAEENNNVEDY |
| 27 | VEDYFKEGLEKTIAA |
| 28 | TIAAKKAELEKTEAD |
| 29 | TEADLKKAVNEPEKP |
| 30 | PEKPAPAPETPAPEA |
| 31 | APEAPAEQPKPAPAP |
| 32 | APAPQPAPAPKPEKP |
| 33 | PEKPAEQPKPEKTDD |
| 34 | KTDDQQAEEDYARRS |
| 35 | ARRSEEEYNRLTQQQ |
| 36 | TQQQPPKAEKPAPAP |
| 37 | APAPKTGWKQENGMW |
| 38 | NGMWYFYNTDGSMAT |
| 39 | SMATGWLQNNGSWYY |
| 40 | SWYYLNSNGAMATGW |
| 41 | ATGWLQYNGSWYYLN |
| 42 | YYLNANGAMATGWAK |
| 43 | GWAKVNGSWYYLNAN |
| 44 | LNANGAMATGWLQYN |
| 45 | LQYNGSWYYLNANGA |
| 46 | ANGAMATGWAKVNGS |
| 47 | VNGSWYYLNANGAMA |
| 48 | GAMATGWLQYNGSWY |
| 49 | GSWYYLNANGAMATG |
| 50 | MATGWAKVNGSWYYL |
| 51 | WYYLNANGAMATGWV |
| 52 | TGWVKDGDTWYYLEA |
| 53 | YLEASGAMKASQWFK |
| 54 | QWFKVSDKWYYVNGL |
Individual, yet overlapping, Streptococcus pneumoniae strain R6 PspA peptides, 15 amino acids in length were used in ex vivo and in silico assays.
Tissue collection and cell isolation
Mice were sacrificed by CO2 inhalation to collect the spleen and CLNs for single cell isolation of lymphocytes. Single cell suspensions of spleen and CLNs were collected 0, 7, 14 and 28 days following S. pneumoniae strain EF3030 challenge, prepared by aseptically removing tissues and passage through a sterile wire screen. CD4+ T cells were further separated by OctoMACS™ (Miltenyi Biotec) using negative selection. Remaining (non-CD4+ T cells), were used as accessory feeder cells for peptide-specific stimulation assays after mitomycin C (Sigma) treatment.
Cytokine quantitation by Luminex™ analysis
Purified CD4+ T cells and mitomycin C-treated feeder cells were cultured at a density of 5 × 106 and 106 cells per ml, respectively, in complete medium containing 1 μM of each PspA peptide at 37°C in 5% CO2. For the assessment of cytokine production, 100 μL of culture supernatants from 96-well flat bottom plates (Corning Glass Works) were harvested 3 days after ex vivo PspA peptide stimulation to determine the levels of IL-10 and IFN-γ secreted by CD4+ T cells. Phorbol-12-myristate-13-acetate (PMA) 1 μg/mL was used as a positive control, ovalbumin (1 μg/mL) and medium only were used as negative controls. Supernatant cytokine levels were determined by the Beadlyte™ mouse multi-cytokine detection (Bio-Rad). Briefly, filter bottom ELISA plates were rinsed with 100 μL of Bio-plex assay buffer and liquid was removed using a Millipore™ Multiscreen Separation Vacuum Manifold System set at 5 mm Hg. Analyte beads in assay buffer were added to the wells followed by 50 μL of serum or standard solution. The plates were incubated for 30 minutes at room temperature with continuous shaking (at setting #3) using a Lab-Line™ Instrument Titer Plate Shaker. The filter bottom plates were washed, as before, and centrifuged at 300 × g for 30 seconds. Subsequently, 50 μL of anti-mouse IL-10 or IFN-γ antibody-biotin reporter solution was added in each well, after which the plates were incubated with continuous shaking for 30 min followed by centrifugation and washing. Next, 50 μL streptavidin-phycoerythrin (PE) solution was added, and the plates were incubated with continuous shaking for 10 min at room temperature (25°C). 125 μL of Bio-plex assay buffer was added, and Beadlyte™ readings were measured using a Luminex™ System and calculated using Bio-plex™ software (Bio-Rad). The cytokine Beadlyte™ assays were capable of detecting > 5 pg/mL for each analyte.
Cell proliferation
Lymphocyte proliferation was measured by a 5-bromo-2′-deoxy uridine (BrdU) absorption and detection (Roche Diagnostics). In brief, purified CD4+ T cells were cultured at a density of 5 × 106 cells/mL, with 106 mitomycin C-treated feeder cells/mL in complete medium containing 1 μM of PspA peptide at 37°C in 5% CO2. After 2 days of ex vivo antigen stimulation, cells were transferred to polystyrene 96 well plates (Corning Glass Work). 10 μL of BrdU labeling solution (10 μM final concentration per well) were added and incubated for 18 hours at 37°C with 5% CO2. The cells were then fixed and incubated with 100 μL of nuclease in each well for 30 minute at 37°C. The cells were washed with complete media and incubated with BrdU-POD solution for 30 minute at 37°C. BrdU incorporation was developed with a 2,2′–azino-bis 3-ethylbenzthia-zoline-6-sulfonic acid (ABTS) solution and optical density (OD) was read at 450 nm. The proliferation index (PI) was calculated as follows. Antigen-specific CD4+ T cell proliferation was obtained by measuring 5-Bromo-2′-deoxy uridine (BrdU) incorporation, according to manufacturer’s instructions (Roche Diagnostics). BrdU absorption or optical density at 450nm (OD450) was detected using a scanning multi-well SpectraMax 250 spectrophotometer (Molecular Devices). PI = BrdU OD450 of peptide -stimulated cells / BrdU OD450 in un-stimulated cells × 100 %. The results were expressed as mean ± the standard error mean (SEM) of the response of three replicate determinations from three independent experiments. Statistical significance was assessed by student’s t test.
Flow cytometry
After single cell isolation of lymphocytes, fluorescently tagged monoclonal antibodies (BD-Pharmingen) were added to characterize CD4+ CD11b+ and CD11c+ lymphocytes. Cells were washed 3 times in PBS (supplemented with 0.5% BSA) and treated with 1 μg of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, allophycocyanin (APC)-, or Cy5-conjugated IgG control isotype or rat anti-mouse CD4, CD11b, CD11c, IL-10 and/or IFN-γ antibodies per 105 cells at 4°C for 30 minutes. Subsequently, wells were washed with PBS to remove unbound antibodies. Labeled cells were fixed in 500 μL of 2% paraformaldehyde and 104 CD4− or CD11b-gated cells were analyzed using a FACS Caliber™ flow cytometer (BD) and Flowjo software (Tree Star).
Statistics
Data were expressed as mean ± SEM and compared using a two-tailed student’s t-test or an unpaired Mann Whitney U test. The results were analyzed using Microsoft Excel and considered statistically significant if p < 0.01. When expression levels were below the detection limit (BD), then values were recorded as one-half the lower detection limit for statistical analysis. Kolmogorov-Smirnov (K-S) two sample tests were used to compute the statistical significance between histograms; results were considered statistically significant if p < 0.01.
Results
Proliferation and cytokine responses of PspA199-246-specific systemic and local CD4+ T cells following pneumococcal carriage
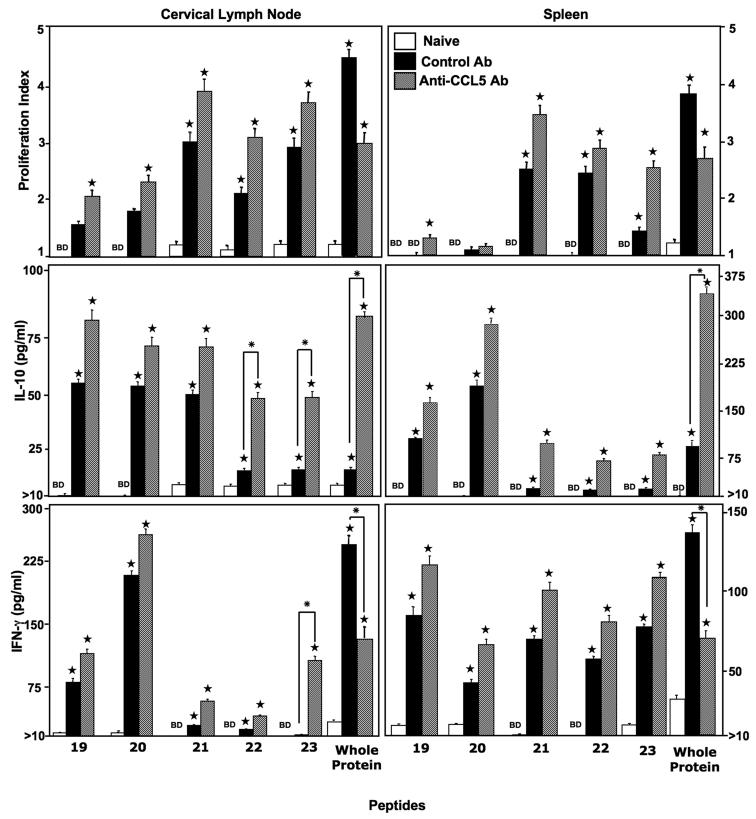
PspA199-246-specific CD4+ T cell responses were characterized 28 days after S. pneumoniae strain EF3030 challenge along with anti-CCL5 or control antibody treatment. In general, pneumococcal carriage led to substantial increases in PspA-specific proliferative responses (Figure 1). In confirmation with our previous finding, CCL5 inhibition significantly lowered PspA (i.e., whole protein) proliferative ex vivo antigen restimulation responses [19, 27]. Surprisingly, CCL5 blockade during pneumococcal carriage resulted in slightly increased splenic as well as CLN CD4+ T cell proliferative recall responses to PspA peptides 19-23, than compared to naïve mice or control antibody-treated mice. Of the peptides tested, PspA peptide #21 induced the highest recall proliferation response by both systemic as well as local CD4+ T cells, which were not significantly effected by CCL5 deficiency.
Figure 1. Proliferation and cytokine response of PspA peptide-specific systemic and local CD4+ T cells that express CCL5 during pneumococcal carriage.
Cervical lymph node (CLN) and spleen lymphocytes were isolated from female F1 (C57BL/6 × BALB/c) naïve mice (open box) and 28 days after intranasal challenge with Streptococcus pneumoniae strain EF3030 and treated with control (solid box) or anti-CCL5 (hashed box) antibody (Ab) solutions. CD4+ T cells were incubated with 1μM of PspA peptides plus mitomycin C-treated naïve syngeneic feeder cells, for 3 days, at a ratio of 5:1 × 106 cells. Proliferation was measured by BrdU incorporation, which was measured by ELISA. The data presented are the mean OD450. Stars (★) indicate statistically significant (p < 0.01) increases between naïve versus control and anti-CCL5 antibody-treated groups. Whereas asterisks (*) indicate statistically significant (p < 0.01) increases between control antibody- and anti-CCL5 antibody-treated groups. Experimental groups consisted of 10 mice and experiments were repeated three times. The data presented are the mean ± SEM optical densities of quadruplicate cultures from each group. IL-10 and IFN-γ production of cultured supernatants was determined by luminex capable of detecting > 2 pg/ml.
In confirmation with our prior studies [19, 27], splenic and CLN derived CD4+ T cells, from pneumococcal -challenged mice that received anti-CCL5 antibody, secreted significantly more IL-10 in response to ex vivo restimulation with whole PspA protein, than compared to cells from naïve mice or similar cells from infected mice treated with control antibody. In general, CLN CD4+ T cells from S. pneumoniae strain EF3030-challenged mice secreted significantly higher levels of IL-10 after PspA peptide 19-23 ex vivo restimulation, than compared to similar splenic T helper cells from control mice. However, PspA peptides 22 and 23 induced significantly more IL-10 secretion by ex vivo restimulated CLN CD4+ T cells from pneumococcal-challenged, anti-CCL5 antibody-treated mice than compared to naïve T helper cells or similar cells from mice treated with control antibody that were ex vivo restimulated with PspA peptides 19, 20, or 21. Splenic CD4+ T cells from S. pneumoniae strain EF3030-challenged mice secreted similar levels of IL-10 after ex vivo restimulation with PspA peptides 19-23. However, splenic T helper cells from infected mice that were ex vivo restimulated with PspA peptides 19 and 20 secreted comparatively more IL-10 than similar cells ex vivo restimulated with PspA peptides 21-23. Taken together, local and systemic CD4+ T cells from pneumococcal-challenged mice, treated with either control antibody or anti-CCL5 antibody during carriage, secreted similar levels of IL-10 in response to PspA peptides 19 and 20, but not PspA peptides 21-23.
We previously showed splenic and CLN CD4+ T cells from pneumococcal-challenged mice receiving anti-CCL5 antibody produced significantly less IFN-γ in response to whole PspA protein ex vivo restimulation, than compared to similar cells from infected mice treated with control antibody during infection [19, 27]. In contrast, T helper cells from S. pneumoniae strain EF3030-challenged mice secreted similar amounts of IFN-γ when ex vivo restimulated with PspA peptides 19-23, regardless of whether these mice received control antibody or anti-CCL5 antibody during infection. As seen with CLN T helper cell IL-10 recall responses, PspA peptide 23 induced CLN-derived CD4+ T cells, from anti-CCL5 antibody-treated, pneumococcal-challenged mice to secrete significantly more IFN-γ than compared to similar cells from infected mice treated with control antibody during infection. Hence, CLN- and spleen-derived T helper cells from mice receiving either control antibody or anti-CCL5 antibody during pneucoccal carriage secrete comparable amounts of IFN-γ when ex vivo restimulated with PspA peptides 19-22, but not PspA peptide 23.
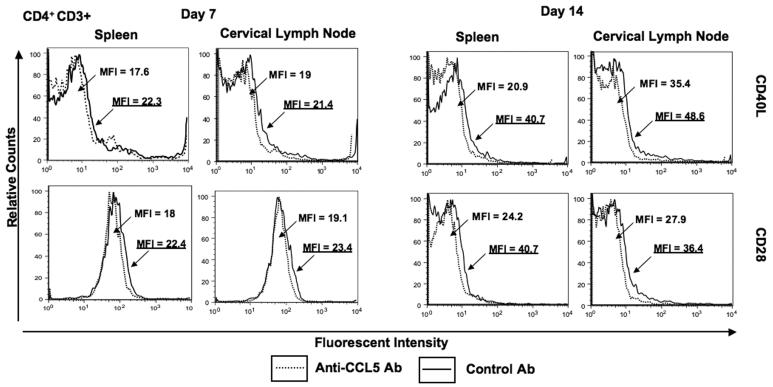
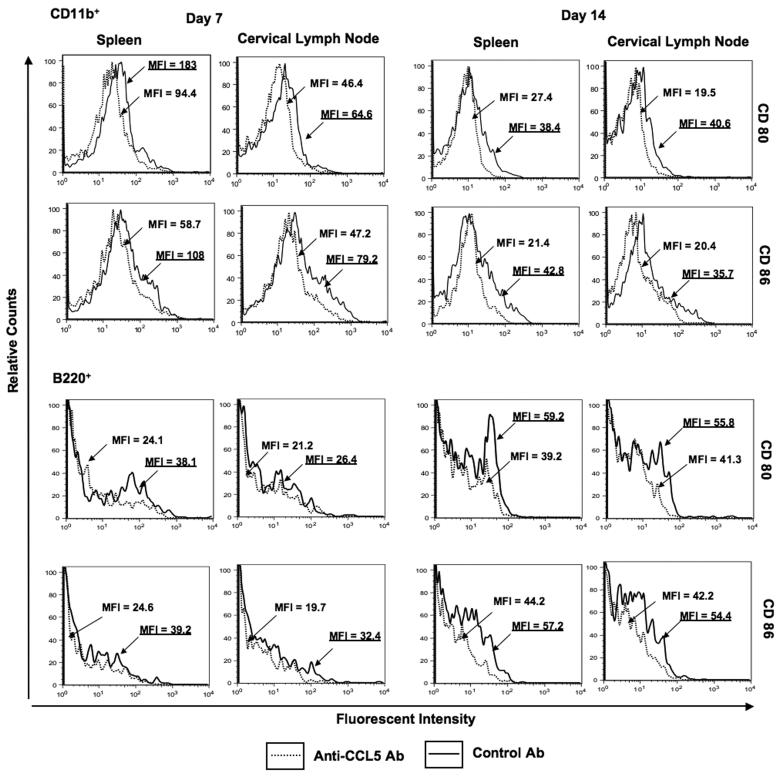
Effect of CCL5 blockade on co-stimulatory molecule expression
Co-stimulatory molecule expression by leukocytes from pneumococcal-infected mice was evaluated to determine whether CCL5 blockade modulated CD40L, CD28, CD80 and CD86 expression during carriage. Anti-CCL5 antibody treatment during carriage lead to an decrease in CD40L and CD28 expression by splenic as well CLN-derived CD4+ T cells 7 and 14 days after pneumococcal challenge (Figure 2). There were also significant decreases in CD80 and CD86 expression by spleen and CLN-derived CD11b+ and B220+ cells from anti-CCL5 antibody-treated mice, than compared to control mice 7 and 14 days after pneumococcal challenge (Figure 3). Our findings show anti-CCL5 antibody treatment during carriage lead to a reduction in CD28, CD40L, CD80 and CD86 expression by both systemic and local leukocytes up to 2 weeks after pneumococcal challenge.
Figure 2. Flow cytometry analysis of CD28 and CD40L expression by CD4+ T cells following pneumococcal challenge.
Representative plots from three separate experiments are shown where spleen- and cervical lymph node (CLN)-derived CD4+ T cells from female F1 (C57BL/6 × BALB/c) mice, treated with control antibody (Ab, solid line) or anti-CCL5 Ab (dotted line) solutions, were isolated 7 and 14 days after intranasal challenge with Streptococcus pneumoniae strain EF3030. Mean fluorescence intensity (MFI) and fluorescence intensity histograms of CD28 and CD40L expression by CD4+ cells are illustrated and were analyzed using Flow Jo version 8.3 software. Underlined MFI values represent bacterial-challenged, anti-CCL5 antibody-treated groups.
Figure 3. Flow cytometry analysis of CD80 and CD86 expression by CD11b+ and B220+ cells following pneumococcal challenge.
Representative plots from three separate experiments are shown where spleen- and cervical lymph node-derived CD4+ T cells from Female F1 (C57BL/6 × BALB/c) mice, treated with control or anti-CCL5 antibody (Ab) solutions, were isolated 7 and 14 days after intranasal challenge with Streptococcus pneumoniae strain EF3030. Mean fluorescence intensity (MFI) and fluoroscence intensity histograms of CD80 or CD86 expression by CD11b+ and B220+ cells are illustrated and were analyzed using Flow Jo version 8.3 software. Underlined values represent MFI recorded bacterial-challenged, anti-CCL5 antibody-treated groups.
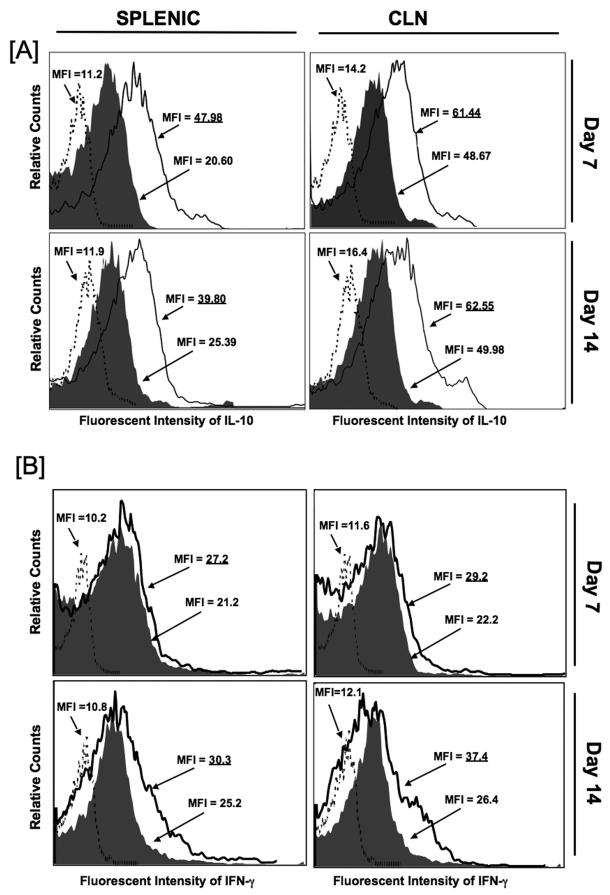
Effect of CCL5 blockade on IL-10 and IFN-γ expression by T helper cells and monocytes
Previously, we showed CCL5 inhibition increased IL-10 secretion by PspA-specific splenic CD4+ T cells, but decreased IFN-γ production by similar cells isolated from CLNs, 28 days post pneumococcal challenge [19]. To better elucidate changes in the expression of these cytokines caused by CCL5 deficiency during carriage, the effect of anti-CCL5 antibody treatment on the frequency of IL-10- and IFN-γ-expressing spleen- and CLN-derived CD4+ T cells were examined 7 and 14 days post pneumococcal challenge. CCL5 blockade during pneumococcal carriage resulted in dramatically higher IL-10 expression by both spleen- and CLN-derived T helper cells isolated 7 and 14 days after challenge (Figure 4A), than compared to similar cells from the control antibody-treated mice or isotype control antibody staining of pooled lymphocytes from both groups. In contrast to our previous findings observed 28 days post bacterial challenge, anti-CCL5 antibody treatment during carriage lead to a modest, yet measurable, increase in IFN-γ expression, than compared to similar cells from infected mice receiving control antibody (Figure 4B).
Figure 4. Change in IL-10- and IFN-γ-expressing splenic and cervical lymph node CD4+ T cells following pneumococcal challenge.
Female F1 (C57BL/6 × BALB/c) mice were intranasally challenged with 107 CFUs of Streptococcus pneumoniae strain EF3030 in a 15μl volume of Ringer’s solution. Anti-CCL5 antibody (open histogram) or control antibody (solid histogram) antibody were administered by intraperitoneal route every 3 days, starting 2 days before challenge. Anti-CCL5 antibody- and control antibody-treated groups consisted of 10 mice each and studies were repeated 3 times. Panel A shows the mean fluorescence intensity (MFI) and fluorescence intensity histograms of IL-10 expression by cervical lymph node (CLN)- and spleen-derived CD4+ T cells from anti-CCL5 and control antibody-treated groups as well as isotype control antibody staining (dotted histogram) of pooled lymphocytes from these groups, which were analyzed using Flow Jo version 8.3 software. Underlined values represent MFI recorded bacterial-challenged, anti-CCL5 antibody-treated groups. Panel B shows the mean fluorescence intensity (MFI) and fluorescence intensity histograms of IFN-γ expression by CLN- and spleen-derived CD4+ T cells from anti-CCL5 and control antibody-treated groups as well as isotype control antibody staining (dotted histogram) of pooled lymphocytes from these groups, which were analyzed using Flow Jo version 8.3 software. Underlined values represent MFI recorded bacterial-challenged, anti-CCL5 antibody-treated groups
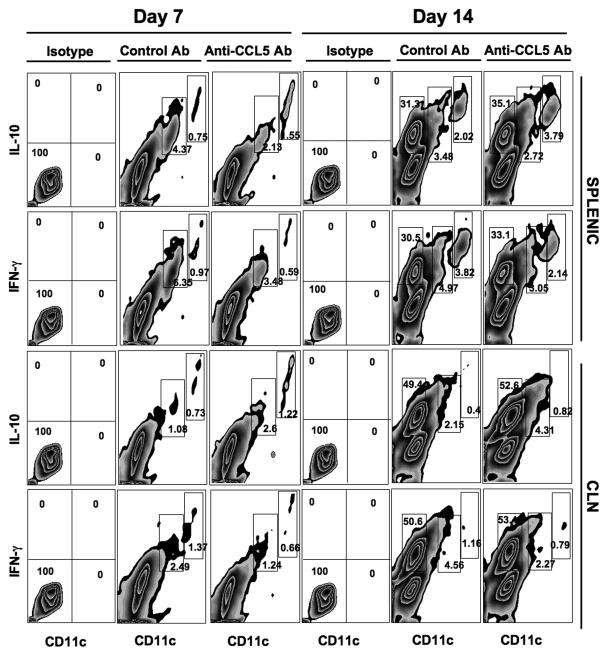
Seven days after challenge, 0.75% of splenic CD11b+ CD11cHi cells from control antibody-treated mice were IL10+, compared to 1.55% of similar cells isolated from anti-CCL5 antibody-treated mice (Figure 5). This two-fold increase was also observed 14 days post challenge. Interestingly, CCL5 inhibition led to a two fold decrease in the percentage of IL-10+ CD11b+ CD11cLo spleen cells 7 days (i.e. 4.37% to 2.13%) and more modest decreases (3.48% to 2.72%) 14 days after pneumococcal challenge. A similar trend was observed in the percentage of IL-10+ CD11b+ CD11cHi cells isolated from the CLN. Unlike splenic IL-10+CD11b+CD11cLo cells, the percentage of these cells in the CLN were significantly higher and 14 days post pneumococcal carriage following CCL5 blockade, than cells from mice treated with control antibody (Tables 1 and 2, respectively). Remarkably, the establishment of pneumococcal carriage lead to a significant increase in the percentage of IFN-γ+ CD11b+ CD11c− and IL-10+ CD11b+ CD11c− cells 14 days post challenge, than compared to cells 7 days post challenge. However, these B220+ cells (data not shown) did not appear to be significantly affected by CCL5 deficiency, because there were no major changes in the percentage of IFN-γ+ CD11b+ CD11c− or IL-10+ CD11b+ CD11c− cells from mice treated with either control antibody or anti-CCL5 antibody.
Figure 5. Change in IL-10+ and IFN-γ+ splenic and cervical lymph node CD11c+ leukocytes following pneumococcal challenge.
Female F1 (C57BL/6 × BALB/c) mice were intra-nasally challenged with 107 CFUs of S. pneumoniae strain EF3030 in a 15μl volume of Ringer’s solution. Anti-CCL5 antibody or control antibodies were administered by intra peritoneal route every 3 days, starting 2 days before challenge with Pneumococci. Splenic and cervical lymph node (CLN) lymphocytes from anti-CCL5 and control antibody-treated groups as well as isotype control antibody groups were stained and analyzed by flow cytometry. Experimental and control groups consisted of 10 mice each and studies were repeated 3 times. Representative density plots along with percentages of IL-10+, IFN-γ+ and CD11b+ CD11cHi or CD11b+ CD11cLo populations are shown of CD11b-gated cells isolated 7 or 14 days after bacterial challenge.
Table 2. Number of CD11b+CD11cHi and CD11b+CD11cLo cells 7 days after pneumococcal challenge.
| IL-10 | IFN-γ | CCR5 | ||
|---|---|---|---|---|
| Spleen | CD11b+CD11cHi Control Ab |
4.40 ± 0.3 | 7.93 ± 0.2 | 17.10 ± 0.3 |
| CD11b+CD11c Hi Anti-CCL5 Ab |
8.96 ± 0.1* | 5.12 ± 0.1* | 11.50 ± 0.2* | |
|
Cervical Lymph Node |
CD11b+CD11c Hi Control Ab |
0.10 ± 0.1 | 0.38±0.1 | 1.70 ± 0.2 |
| CD11b+CD11c Hi Anti-CCL5 Ab |
0.35 ± 0.2* | 0.18 ± 0.1* | 1.60 ± 0.2 | |
| Spleen | CD11b+CD11cLo Control Ab |
4.76 ± 0.3 | 4.79 ± 0.1 | 7.40 ± 0.2 |
| CD11b+CD11c Lo Anti-CCL5 Ab |
5.90 ± 0.2 | 7.09 ± 0.3* | 5.43 ± 0.2* | |
|
Cervical Lymph Node |
CD11b+CD11c Lo Control Ab |
0.10 ± 0.1 | 0.17 ± 0.1 | 0.84 ± 0.1 |
| CD11b+CD11c Lo Anti-CCL5 Ab |
0.15 ± 0.1 | 0.14±0.2 | 0.40 ± 0.1* |
C57BL/6 × BALB/c F1 mice were intranasally challenged with PBS (uninfected) or 107 CFUs of S. pneumoniae strain EF3030 in a 15 μL volume of Ringer’s solution and treated with either control or anti-CCL5 antibodies. Spleen and cervical lymph node lymphocytes were purified and prepared for cell surface and intracellular flow cytometry analysis 7 days after bacterial challenge. The fold increases ± SEM in the number of (×106) of CD3−CD11b+ CD11cHi or CD3− CD11b+ CD11cLo lymphocytes that were CCL5, IL-10, IFN-γ or CCR5 positive are shown. Asterisks (*) indicate statistically significant (p < 0.01) increases between infected over infected local cell subpopulations from three separate experiments with two groups containing 10 mice each.
In contrast to IL-10+ monocytes and dendritic cells in the spleen, CCL5 deficiency resulted in nearly two fold decreases in the percentage of IFN-γ+ CD11b+ CD11cHi splenocytes 7 and 14 days post pneumococcal challenge, than compared to similar cells from experimental control mice. Similar decreases occurred in CLN –derived IFN-γ+ CD11b+ CD11cHi cells 7 and 14 days after S. pneumoniae strain EF3030-induced carriage from anti-CCL5 antibody -treated mice compared to control antibody-treated mice. The percentage of CLN and splenic IFN-γ+ CD11b+ CD11cLo cells from pneumococcal-challenged mice receiving anti-CCL5 antibody was approximately two-fold lower than similar cells from mice administered control antibody. These changes largely corresponded with trends in CLN- and spleen-derived IFN-γ+ CD11b+ CD11cHi and IFN-γ+ CD11b+ CD11cLo cells. Taken together, the data show that increases in splenic IL-10+ DCs following CCL5 blockade coincided with increases in the number of IL-10+ CD4+ T cells and decreases in splenic CCR5+ DCs, 7 and 14 days following challenge (Tables 1 and 2).
Discussion
PspA is a highly conserved, cell wall-associated surface protein that plays a major role in pneumococcal virulence by inhibiting both bactericidal effect of human apolactoferrin and complement deposition on the bacterial surface [28]. Our previous studies showed CCL3, CCL4, and CCL5 enhance adaptive immunity through cytokine and co-stimulatory molecule modulation [6, 7]. We also demonstrated that pneumoccal carriage induces PspA-specific cellular response [27], which were regulated in part by CCL5 [19]. Indeed, lack of CCL5 resulted in potential lethal effects approximately 5 days after pneumococcal challenge, during the end of the innate response and beginning of the recognition phase of the adaptive immune response. CCL5 blockade resulted in a dramatic (~104 fold) increase in S. pneumonia strain EF3030 colony forming units (CFUs) from nasal tract wash and lung lavage samples. These heightened bacterial loads, relative to infected controls, continued 28 days after challenge. For these reasons and to better determine the effects of CCL5 deficiency on immuno-dominant pneumococcal T cell epitopes after the recognition phase, the current study used F1 (C57BL/6 × BALB/c) mice that were less susceptible to the lethality of CCL5 blockade. I-Ad and I-Ed peptide binding and associated responses have been used to identify sequence motifs for immunogenic peptide regions and correlate with DR-binding [29, 30, Alexander, 1994 #19402, Sidney, 1994 #19403]; hence, studying PspA199-246-specific CD4+ T cell responses in mice might give insights into similar responses in man. Using these approaches, this study sought to ascertained whether PspA199-246-specific CD4+ T cells responses were resistant to the previously described effects of CCL5 deficiency.
IL-10 leads to macrophage/monocyte deactivation as well as suppresses the release of reactive -oxygen species and -nitrogen intermediates, which are involved in the pathophysiology of pneumococcal meningitis [31]. This Th2 cytokine reduces pulmonary vascular leakage and the appearance of red blood cells in the alveoli during pneumococcal pneumoniae [32]. IL-10 has also been shown to enhance susceptibility to pneumococcal infections [33]. Interestingly, this selective increase in IL-10 secretion was representative of unique Th2 cell subsets that are often prevalent at early stages of Th2 differentiation that diminish over time [34]. IL-10+ CD4+ T cells also exist as T regulatory (Tr1) cells. While additional studies will be necessary to characterize the potential of CCL5 deficiency on CD4+ CD25+ Tr1 cells or merely the lack of appropriate T helper cell development, our data suggest that the development of antigen-specific IL-10-secreting CD4+ T cells, in the presence of diminished Th1 (e.g. IFN-γ and TNF-α), correlated with a higher number of IL-10+ CD11b+ CD11c+ cells, 7 days post infection.
Marginal zone (MZ) B cells interact with B1 B cells to generate a massive wave of IgM antibodies in the initial 3 days of a primary response to pneumococcal infection [35]. B1 B cell anti-phosphorylcholine (PC) responses confer protection against S. pneumoniae [36], although homing to lung and/or nasal tract is not required. While the precise role of MZ, B1, B2, and follicular B cells in thymus-dependent or -independent pneumococcal responses remains uncertain, B cell apoptosis controls the level of humoral S. pneumoniae responses [37]. Interestingly, high CCL5 expression during an immune response is associated with expanded conventional B2 cells, but not MZ or B1 cells proliferation [38]. In contrast, innate-like B1 B cells play housekeeping roles, including spontaneous production of IgM, anti-PC antibody, and IL-10 [39]; the latter has been shown to regulate macrophage polarization during inflammation and infection [40]. Our data show that CD11b+ CD11c− leukocytes were a major source of IL-10 (Figure 5), at least 14 days after pneumococcal carriage was established in mice receiving control or anti-CCL5 antibody. The precise function of these B220+ B cells (data not shown) in the context of CCL5 and S. pneumoniae carriage remains to be determined. Additional studies will be required to determine the role of CCL5 in MZ, follicular, and B1 B cell IL-10 responsiveness and their collective role in pneumococcal immunity.
IFN-γ is required for protective host immunity against pneumococcal disease(s) [41]. PspA peptide-specific IFN-γ CD4+ T cell secretion were dramatically reduced in the absence of CCL5. In contrast to Th2 cells, Th1 cells preferentially express CCR5 [12]. Hence, expression of CCR5 ligands (e.g. CCL5, CCL4 and CCL3) often precedes recruitment of CCR5+ cells for Th1 responses. To this end, we show the number of CCR5+ T cells and antigen-presenting cells significantly increased following pneumococcal challenge. As expected, leukocyte subpopulations in the spleen and CLNs of in anti-CCL5 antibody-treated mice contained less IFN-γ+ CD4+ T cells relative to control antibody-treated mice. PspA199-246-specific CD4+ T cells were able to mount both IL-10 and IFN-γ responses in either control or anti-CCL5 antibody treated mice. IL-10 and IFN-γ double-producers are found in a subpopulation of Tr1 and Th1 cells, which suppress DCs ability to activate CD4+ T cells [42]. It is tempting to speculate that the previously reported IFN-γ+ IL-10+ CD4+ T cells might play some function in the T cell responsiveness attributed by CCL5. However, additional studies will be required to elucidate the precise role of IL-10 and IFN-γ double-producer T cell populations in pneumococcal immunity.
The biological determinants that influence the probability of S. pneumoniae transmission and progression can include, but are not limited to, the characteristics of the infecting strain (carriage versus invasive), susceptibility of uninfected hosts as well as infected individuals. Polymorphisms in CCL5 that negatively affect its expression are associated with enhanced susceptibility and progression to HIV-1/AIDS [17, 18, 43]. This accounts for an important health disparity; up to 67% of African Americans carry at least one of these polymorphisms. It is tempting to speculate that some of the health disparities associated with pneumococcal immunity may be partially mediated by this ccl5 genetic variance, which would result in diminished expression of CCL5 protein. We show CCL5 deficiency increased IL-10 expression by DCs as well as CD4+ T cells to support the generation of CD4+ T cells that secrete IL-10 in response to ex vivo PspA199-246 restimulation. However, the contribution of IL-10 from these accessory cells did not prevent the generation of PspA199-246-specific, IFN-γ secreting T helper cells.
T cell epitopes used in a future pneumococcal vaccine should mount optimal responses, even in individuals with CCL5 deficiencies. To explain, African Americans have a higher mortality rate associated with pneumococcal infection, compared to their Caucasian counterparts [45]. The rate of pneumococcal pneumonia is higher in blacks than in whites [46]. Indeed, rates of infection are three-times higher in African Americans than in Caucasian Americans. Moreover, African Americans are known to have a higher incidence of invasive pneumococcal infection than Caucasian Americans [47]. Interestingly, 67% of African Americans, compared to only 3% in Caucasians, possess genetic variants in the ccl5 gene that results in lower CCL5 protein expression. This has been shown to be a critical factor in HIV/AIDS incidence and disease progression, respectively. Unfortunately, there are no animal models that precisely mimic these genetic variations, which is why we used antibodies to limit CCL5 availability. Previously, we showed CCL5 differentially supports CD28, CD40L, CD30, and/or 4-1-BB expression during T cell activation [6]. We also showed that CCL5, IFN and IL-10 expression by CD4+ T cells, CD8+ T cells, CD11b+ monocytes, and CD11b+ CD11cHi DCs were significantly increased four days after pneumococcal carriage challenge [19]. Hence, this is the first study to directly show how CCL5 deficiency during pneumococcal infection affects the ability of T cells as well as CD11b+ CD11c−, CD11b+ CD11cLo CD11b+ CD11cHi leukocytes to produce IL-10 and IFN-γ as well as mount optimal responses to pneumococcal HTL epitopes.
In conclusion, PspA is a highly immunogenic surface protein of S. pneumoniae and considered to be a promising vaccine candidate [47-48]. PspA199-246 is the HTL immunodominant region and likely encompasses MHC II binding epitopes to support pneumococcal immunity. While the precise role of CCL5 interactions in adaptive pneumococcal immunity remains uncertain, this study addresses important questions that are relevant to many individuals that display ccl5 polymorphisms, which diminish CCL5 protein expression. The consequences of the genetic variants might affect infectious disease outcomes and optimal responses to vaccines. In short, the PspA199-246 region is largely resistant to CCL5 deficiency and might prove useful to include in peptide-based pneumococcal vaccines.
PspA is a candidate vaccine antigen against pneumococcal disease.
Unfortunately, little is known regarding PspA peptide epitopes that induce the greatest helper T lymphocyte (HTL) responses.
In previous studies we used in silico analysis tools and mouse models to reveal PspA immuno-dominant peptides of Streptococcus pneumoniae (family 1) PspA.
This region optimally binds with a broad range of HLA-DR, -DQ, and -DP allelles.
In the current study, we show that these immuno-dominant epitopes and possible peptide vaccine candidates are partially resistant to CCL5 deficiencies.
Table 3. Number of CD11b+CD11cHi and CD11b+CD11cLo cells 14 days after pneumococcal challenge.
| IL-10 | IFN-γ | CCR5 | ||
|---|---|---|---|---|
| Spleen | CD11b+CD11cHi Control Ab |
2.32 ± 0.2 | 3.73 ± 0.2 | 19.6 ± 0.1 |
| CD11b+CD11c Hi Anti-CCL5 Ab |
4.41 ± 0.2* | 2.57 ± 0.3* | 12.5 ± 0.2* | |
|
Cervical Lymph Node |
CD11b+CD11c Hi Control Ab |
0.10 ± 0.1 | 0.29±0.1 | 1.66 ± 0.1 |
| CD11b+CD11c Hi Anti-CCL5 Ab |
0.21 ± 0.2* | 0.11 ± 0.1* | 1.58 ± 0.2 | |
| Spleen | CD11b+CD11cLo Control Ab |
8.85 ± 0.3 | 7.68 ± 0.3 | 7.56 ± 0.1 |
| CD11b+CD11c Lo Anti-CCL5 Ab |
10.4 ± 0.2 | 8.62 ± 0.3 | 6.09 ± 0.1 | |
|
Cervical Lymph Node |
CD11b+CD11c Lo Control Ab |
0.18 ± 0.1 | 0.40 ± 0.1 | 0.99 ± 0.2 |
| CD11b+CD11c Lo Anti-CCL5 Ab |
0.22 ± 0.1 | 0.73±0.2* | 0.77 ± 0.1 |
C57BL/6 × BALB/c F1 mice were intranasally challenged with PBS (uninfected) or 107 CFUs of S. pneumoniae strain EF3030 in a 15 μL volume of Ringer’s solution and treated with either control or anti-CCL5 antibodies. Spleen and cervical lymph node lymphocytes were purified and prepared for cell surface and intracellular flow cytometry analysis 14 days after bacterial challenge. The fold increases ± SEM in the number of (×106) of CD3−CD11b+ CD11cHi or CD3− CD11b+ CD11cLo lymphocytes that were CCL5, IL-10, IFN-γ or CCR5 positive are shown. Asterisks (*) indicate statistically significant (p < 0.01) increases between infected over infected local cell subpopulations from three separate experiments with two groups containing 10 mice each.
Acknowledgments
The content of this manuscript benefited from many fruitful conversations with members of the Morehouse School of Medicine and the University of Alabama at Birmingham. This study was supported by National Institute of Health grants (AI021548, AI057808, and RR03034) and also supported by the Research Centers in Minority Institutions Program (RCMI) funded Flow Cytometry and Cell Sorting Core at Morehouse School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no financial conflict of interest.
References
- [1].Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect. 2009;3:16–20. doi: 10.1111/j.1469-0691.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- [2].Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis. 2009;199:776–85. doi: 10.1086/597044. [DOI] [PubMed] [Google Scholar]
- [3].Reinert RR. The public health ramifications of pneumococcal resistance. Clin Microbiol Infect. 2009;3:1–3. doi: 10.1111/j.1469-0691.2009.02722.x. [DOI] [PubMed] [Google Scholar]
- [4].Katsarolis I, Poulakou G, Analitis A, Matthaiopoulou I, Roilides E, Antachopoulos C, et al. Risk factors for nasopharyngeal carriage of drug-resistant Streptococcus pneumoniae: data from a nation-wide surveillance study in Greece. BMC Infect Dis. 2009;9:120. doi: 10.1186/1471-2334-9-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lillard JW, Jr., Boyaka PN, Hedrick JA, Zlotnik A, McGhee JR. Lymphotactin acts as an innate mucosal adjuvant. J Immunol. 1999;162:1959–65. [PubMed] [Google Scholar]
- [6].Lillard JW, Jr., Boyaka PN, Taub DD, McGhee JR. RANTES potentiates antigen-specific mucosal immune responses. J Immunol. 2001;166:162–9. doi: 10.4049/jimmunol.166.1.162. [DOI] [PubMed] [Google Scholar]
- [7].Lillard JW, Jr., Singh UP, Boyaka PN, Singh S, Taub DD, McGhee JR. MIP-1alpha and MIP-1beta differentially mediate mucosal and systemic adaptive immunity. Blood. 2003;101:807–14. doi: 10.1182/blood-2002-07-2305. [DOI] [PubMed] [Google Scholar]
- [8].Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunology Today. 1998;19(12):568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- [9].Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nature Immunol. 2001;2:102–7. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- [10].Darville T, Andrews CW, Jr., Sikes JD, Fraley PL, Braswell L, Rank RG. Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response. Infect Immun. 2001;69:7419–24. doi: 10.1128/IAI.69.12.7419-7424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–604. [PubMed] [Google Scholar]
- [12].Bonecchi R, Bianchi G, Bordignon PP, D’ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- [14].Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–7. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- [15].Makino Y, Cook DN, Smithies O, Hwang OY, Neilson EG, Turka LA, et al. Impaired T cell function in RANTES-deficient mice. Clin Immunol. 2002;102:302–9. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]
- [16].Ifere GO, He Q, Igietseme JU, Ananaba GA, Lyn D, Lubitz W, et al. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J Microbiol Immunol Infect. 2007;40:188–200. [PubMed] [Google Scholar]
- [17].Liu H, Chao D, Nakayama EE, Taguchi H, Goto M, Xin X, et al. Polymorphism in RANTES chemokine promoter affects HIV-1 disease progression. Proc Natl Acad Sci U S A. 1999;96:4581–5. doi: 10.1073/pnas.96.8.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McDermott DH, Beecroft MJ, Kleeberger CA, Al-Sharif FM, Ollier WE, Zimmerman PA, et al. Chemokine RANTES promoter polymorphism affects risk of both HIV infection and disease progression in the Multicenter AIDS Cohort Study. Aids. 2000;14:2671–8. doi: 10.1097/00002030-200012010-00006. [DOI] [PubMed] [Google Scholar]
- [19].Palaniappan R, Singh S, Singh UP, Singh R, Ades EW, Briles DE, et al. CCL5 modulates pneumococcal immunity and carriage. J Immunol. 2006;176:2346–56. doi: 10.4049/jimmunol.176.4.2346. [DOI] [PubMed] [Google Scholar]
- [20].Singh R, Singh S, Sharma PK, Singh UP, Briles DE, Hollingshead SK, et al. Helper T cell epitope-mapping reveals MHC-peptide binding affinities that correlate with T helper cell responses to pneumococcal surface protein A. PLoS One. 2010;5:e9432. doi: 10.1371/journal.pone.0009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Briles DE, Hollingshead SK, Paton JC, Ades EW, Novak L, Van Ginkel FW, et al. Immunizations with Pneumococcal Surface Protein A and Pneumolysin Are Protective against Pneumonia in a Murine Model of Pulmonary Infection with Streptococcus pneumoniae. Infect Immun. 2003;188:339–48. doi: 10.1086/376571. [DOI] [PubMed] [Google Scholar]
- [22].Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–6. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aaberge IS, Eng J, Lermark G, Lovik M. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995;18:141–52. doi: 10.1016/s0882-4010(95)90125-6. [DOI] [PubMed] [Google Scholar]
- [24].Briles DE, Ades E, Paton JC, Sampson JS, Carlone GM, Huebner RC, et al. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kadioglu A, Gingles NA, Grattan K, Kerr A, Mitchell TJ, Andrew PW. Host cellular immune response to pneumococcal lung infection in mice. Infect Immun. 2000;68:492–501. doi: 10.1128/iai.68.2.492-501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Valerio RM, Benstead M, Bray AM, Campbell RA, Maeji NJ. Synthesis of peptide analogues using the multipin peptide synthesis method. Anal Biochem. 1991;197:168–77. doi: 10.1016/0003-2697(91)90374-3. [DOI] [PubMed] [Google Scholar]
- [27].Palaniappan R, Singh S, Singh UP, Sakthivel SK, Ades EW, Briles DE, et al. Differential PsaA-, PspA-, PspC-, and PdB-specific immune responses in a mouse model of pneumococcal carriage. Infect Immun. 2005;73:1006–13. doi: 10.1128/IAI.73.2.1006-1013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shaper M, Hollingshead SK, Benjamin WH, Jr., Briles DE. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect Immun. 2004;72:5031–40. doi: 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–61. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- [30].Sette A, Buus S, Appella E, Smith JA, Chesnut R, Miles C, et al. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989;86:3296–300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koedel U, Bernatowicz A, Frei K, Fontana A, Pfister HW. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J Immunol. 1996;157:5185–91. [PubMed] [Google Scholar]
- [32].Wang JF, Park IW, Groopman JE. Stromal cell-derived factor-1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–13. [PubMed] [Google Scholar]
- [33].Van der Sluijs KF, van Elden LJR, Nijhuis M, Schuurman R, Pater JM, Florquin S, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–9. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- [34].Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- [35].Martin I, Shastri VP, Padera RF, Yang J, Mackay AJ, Langer R, et al. Selective differentiation of mammalian bone marrow stromal cells cultured on three-dimensional polymer foams. J Biomed Mater Res. 2001;55:229–35. doi: 10.1002/1097-4636(200105)55:2<229::aid-jbm1009>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [36].Nixon JC, Ferrell S, Miner C, Oldham AL, Hochgeschwender U, Webb CF. Transgenic mice expressing dominant-negative bright exhibit defects in B1 B cells. J Immunol. 2008;181:6913–22. doi: 10.4049/jimmunol.181.10.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chattopadhyay G, Khan AQ, Sen G, Colino J, DuBois W, Rubtsov A, et al. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J Immunol. 2007;179:7523–34. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- [38].Zhang S, Cubas R, Li M, Chen C, Yao Q. Virus-like particle vaccine activates conventional B2 cells and promotes B cell differentiation to IgG2a producing plasma cells. Mol Immunol. 2009;46:1988–2001. doi: 10.1016/j.molimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hansell CA, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, et al. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–24. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- [41].Pomeroy C, Rubins JB. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect Immun. 1997;65:2975–7. doi: 10.1128/iai.65.7.2975-2977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yanagawa Y, Iwabuchi K, Onoe K. Co-operative action of interleukin-10 and interferon-gamma to regulate dendritic cell functions. Immunol. 2009;127:345–53. doi: 10.1111/j.1365-2567.2008.02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Anonymous Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report Series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- [44].Hausmann LR, Ibrahim SA, Mehrotra A, Nsa W, Bratzler DW, Mor MK, et al. Racial and ethnic disparities in pneumonia treatment and mortality. Med Care. 2009;47:1009–17. doi: 10.1097/MLR.0b013e3181a80fdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marrie TJ. Pneumococcal pneumonia: epidemiology and clinical features. Semin Respir Infect. 1999;14:227–36. [PubMed] [Google Scholar]
- [46].Harrison LH, Dwyer DM, Billmann L, Kolczak MS, Schuchat A. Invasive pneumococcal infection in Baltimore, Md: implications for immunization policy. Arch Intern Med. 2000;160:89–94. doi: 10.1001/archinte.160.1.89. [DOI] [PubMed] [Google Scholar]
- [47].McDaniel LS, Yother J, Vijayakumar M, McGarry L, Guild WR, Briles DE. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–94. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–42. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]