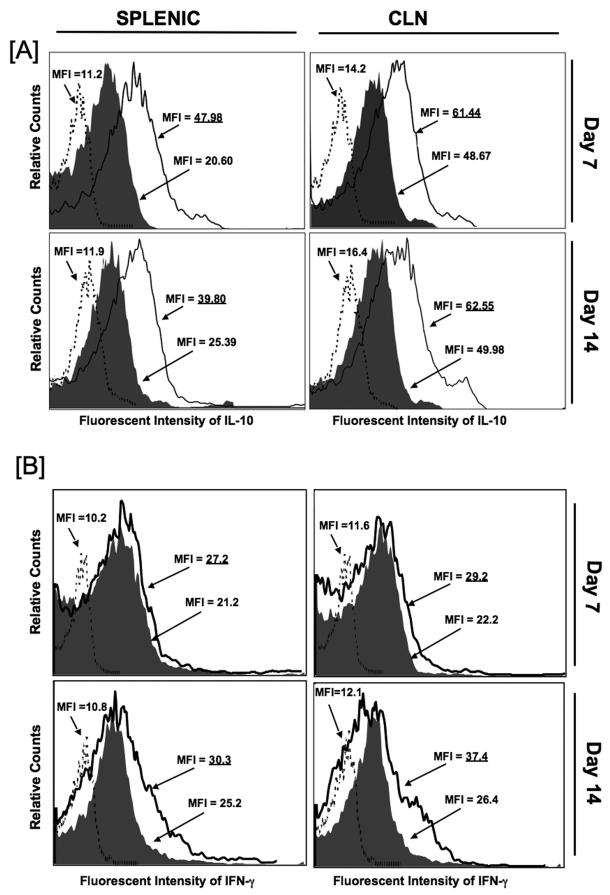
Figure 4. Change in IL-10- and IFN-γ-expressing splenic and cervical lymph node CD4+ T cells following pneumococcal challenge.
Female F1 (C57BL/6 × BALB/c) mice were intranasally challenged with 107 CFUs of Streptococcus pneumoniae strain EF3030 in a 15μl volume of Ringer’s solution. Anti-CCL5 antibody (open histogram) or control antibody (solid histogram) antibody were administered by intraperitoneal route every 3 days, starting 2 days before challenge. Anti-CCL5 antibody- and control antibody-treated groups consisted of 10 mice each and studies were repeated 3 times. Panel A shows the mean fluorescence intensity (MFI) and fluorescence intensity histograms of IL-10 expression by cervical lymph node (CLN)- and spleen-derived CD4+ T cells from anti-CCL5 and control antibody-treated groups as well as isotype control antibody staining (dotted histogram) of pooled lymphocytes from these groups, which were analyzed using Flow Jo version 8.3 software. Underlined values represent MFI recorded bacterial-challenged, anti-CCL5 antibody-treated groups. Panel B shows the mean fluorescence intensity (MFI) and fluorescence intensity histograms of IFN-γ expression by CLN- and spleen-derived CD4+ T cells from anti-CCL5 and control antibody-treated groups as well as isotype control antibody staining (dotted histogram) of pooled lymphocytes from these groups, which were analyzed using Flow Jo version 8.3 software. Underlined values represent MFI recorded bacterial-challenged, anti-CCL5 antibody-treated groups