Abstract
High levels of homocysteine (Hcy), known as hyperhomocysteinmia (HHcy), are correlated with an increase in extracellular matrix remodelling (ECM) via the matrix metalloproteinases (MMPs) and plasminogen/plasmin system. This results in an increase deposition of collagen that leads to endothelial-myocyte (EM) and myocyte-myocyte (MM) uncoupling; the physiological consequences are a plethora of cardiovascular pathologies. Homocysteine-induced increase in intracellular and mitochondrial Ca2+ plays an important role in increasing reactive oxygen species (ROS) within mitochondria and instigating mitophagy within the cell. This occurs via several Hcy-mitigated processes: agonizing N-methyl-d-aspartate receptor-1 (NMDA-R1), decreasing expression of peroxisome proliferator activator receptor (PPAR) [thereby increasing oxidation], impairing Ca2+ handling via Na+/Ca2+ exchanger (NCX1) and Sarco endoplasmic reticulum Ca2+ ATPase (SERCA-2a). The end result is an increase in ROS that directly or indirectly lead to MMP activation within mitochondria or the cytoplasm. Hcy induces a mitochondrial permeability transition that allows MMPs to be released from mitochondria thereby metabolizing matrix and impairing cardiac function. Further work remains to be elucidated concerning the specific mitochondrial mitophagic mechanisms under which matrix metabolism and remodelling occurs. Moreover, the therapeutic implications of NMDA and PPAR ligands are some promise to patient.
Keywords: Homocysteine, NMDA receptor, matrix metalloproteinases, oxidative stress, Ca2+
Introduction
A search for other risk factors for heart disease instigated the discovery of homocysteine (Hcy). Hcy has been shown to be an independent risk factor in several cardiovascular pathologies (Wilcken & Wilcken, 1976). Moreover, elevated levels of Hcy have been found in diabetics, which could explain the association between diabetes and heart disease (Hofmann et al., 1997; Robillon et al., 1994). Vitamin treatment has been shown to be successful in significantly lowering Hcy levels; however, it still remains a puzzle for why clinical trials have yielded mixed results in lowering the risk for cardiovascular-related death.
The heart’s conduction system is of utmost importance for proper cardiac function. The mechanism of transmission of membrane depolarization is via gap junctions; among proteins that make up these gap junctions are the following: connexin 40 (Cx40), connexin 43 (Cx43), connexin 45 (Cx45) (Gros & Jongsma, 1996; Miquerol et al., 2004). Deposition of collagen in extracellular matrix (ECM) can impair cardiovascular function via endothelial-myocyte uncoupling (EM uncoupling) and myocyte-myocyte (MM uncoupling) whereby the signal is not properly transmitted (Givvimani et al., 2011; Moshal et al., 2008b). Matrix metalloproteinase’s (MMPs) and the plasminogen/plasmin system are responsible for modifying the ECM; activation of MMPs is considered pathological, whereas activation of plasmin is thought to be beneficial in most cases (Iimoto et al., 1988; Jalil et al., 1989; Mukherjee & Sen, 1990; Norton et al., 1997; Takeshita et al., 2004). Most MMPs are generally considered to be either secretory or membrane-anchored (Hadler-Olsen et al., 2011; Pei et al., 2000). However, MMPs have also been found to have roles within the nucleus and mitochondria (McCawley & Matrisian, 2001).
Activation of NMDA-R1 will increase intracellular Ca2+ levels, and mitochondrial Ca2+ levels, resulting in oxidative stress (Gao et al., 2007). Mitochondria are involved in translocating and activating several proteins (Hansson Petersen et al., 2008). Importing proteins involves translocation through outer (OMM) and inner mitochondrial membranes (IMM) via complex protein machinery (Ow et al., 2008; Rassow et al., 1994). Another function of mitochondria aside from the role of ATP generation is sequestration of Ca2+, and the generation and detoxification of cellular ROS; the electron transport chain is a key connector for these roles (Brand et al., 2004; Turrens, 2003; Xi et al., 2005). It is concluded that an increase in Ca2+ will disrupt the membrane potential of mitochondria, decrease oxygen utilization (since oxygen is the final electron acceptor to generate water) and produce greater amounts of superoxide (Archer, 2010; Dalton et al., 1999). One other mechanism for MMP-9 activation involving mitochondria is via the calpain system. It was shown that Hcy activates and translocates calpain-1 from the cytosol to mitochondria; this results in intra-mitochondrial oxidative stress, resulting in MMP-9 activation within mitochondria (Tyagi et al., 2010).
The activation of the PPAR receptor by Hcy has been shown to promote a reducing environment (antioxidant) (Brude et al. 1999; Hunt & Tyagi, 2002; Inoue et al., 1998). However, an increased concentration of Hcy correlates with a decreased expression of the PPAR receptor and its antioxidant effects (Brude et al., 1999; Gillespie et al., 2011; Inoue et al., 1998).
Homocysteine as a marker for cardiovascular pathology
Homocysteine (Hcy) is a sulphur-containing amino acid that is derived from the essential amino acid, methionine (HofMann et al., 2001; Zhou et al., 2001). Moreover, Hcy is shown to be metabolized from two pathways: trans-sulphuration by cystathionine-β-synthase (CBS) in hepatic cells or via re-methylation to methionine in non-hepatic cells (Loscalzo 2006). Hyperhomocysteinemia (HHcy) is considered an independent risk factor for cardiovascular disease (CVD) (Zhou and Austin 2009). Elevated Hcy levels are caused by two factors: genetic defects in enzymes involved in Hcy metabolism or nutritional deficiencies in vitamin cofactors (folate, vitamin B12, vitamin B6 (Milani and Lavie 2008). Other factors involved are the following: chronic kidney disease, hypothyroidism, psoriasis, cancers, and several drugs (Milani & Lavie, 2008).
Folic acid, Vitamin B12, and Vitamin B6 combinations are able to reduce Hcy concentrations without lowering the risk of further cardiovascular events; it has been suggested that some of these vitamin treatments may counteract the beneficial effects of lowering Hcy, and cause damage themselves (Bonaa et al. 2006). For instance, treatment with B vitamins did not lower the risk of recurrent CV disease after MI. Pathogenesis of coronary artery disease show reduced ability to metabolize Hcy in premature coronary artery disease (Nasir et al., 2007; Wilcken & Wilcken 1976). One study indicates that the greater the increase in Hcy induced by fenofibrate, the smaller the increase in HDL-c and apoA-I, two proteins that are helpful in cholesterol uptake; hence cholesterol levels would remain higher (Taskinen et al., 2009). Similar results were noted in a study of ischemic stroke patients: a higher level of Hcy was independently associated with ischemic stroke (Dhamija et al., 2009).
Vitamin treatment did lower Hcy levels by 27% among patients given folic acid plus Vitamin B12 (Bonaa et al.,2006). One study found that a Hcy level of ≥20 μmol/L is associated with a high mortality risk (odd ratio 2.57) (Maurer et al., 2010). Another study found that increased Hcy levels cause abnormalities in Na+ currents in human atrial cells via the following mechanism: slowing inactivation and promoting recovery of Na+ channels (Cai et al.,2009). Another study found that folic acid supplementation resulted in a significant intima-media thickness reduction after 18 months in patients with at least one CV risk (Ntaios et al., 2010). Finally, Hcy was shown to act as an independent risk factor for an increase in arterial stiffness (Ruan et al., 2009).
Homocysteine and diabetes act in synergy
Elevated levels of Hcy have been found in diabetics, which could explain the association between diabetes and heart disease (Hofmann et al., 1997; Robillon et al. 1994; Wijekoon et al. 2007). Another study showed an association between Hcy and silent myocardial infarction (SMI) in diabetic patients (Tarkun et al., 2004). However, HHcy was not detected in adolescent patients with type 1 diabetes (Pavia et al., 2000). In cases where there were no renal complications in both Type 1 and Type 2 diabetes, Hcy levels were even lower than controls (Wijekoon et al., 2007). It was concluded that in Type 1 diabetes, increased activity of the trans-sulphuration enzymes were the major cause of reduction in plasma Hcy. In Type 2 diabetes, BHMT(betaine:homocysteinemethyltransfer ase) was considered to be responsible in increased Hcy catabolism (Wijekoon et al., 2007). Plasma levels of Hcy are usually normal in diabetes; however, both high and low values have been reported. They have been modulated by hyperfiltration and renal dysfunction, and low folate status, while insulin resistance does not seem to play a role in HHcy (Huijberts et al., 2005). It appears that the presence of diabetes contributes to worsening HHcy determined cardiovascular risk, and may even act in synergy in evoking their vascular effects (Becker et al., 2003; Soinio et al., 2004). One review indicates that, in patients with Type 2 diabetes, elevated Hcy levels have an independent risk associated with higher rates of CHD events and CHD mortality (Soinio et al., 2004).
Contraction and conduction – gap junctions
The integrity of the heart’s conduction system is paramount to maintaining proper cardiac function. This specialized pacemaker system consists of modified cardiomyocytes and includes the following: sinoatrial node (SA), atrioventricular node (AV), His Bundle with two branches, and Purkinje fibres (PFs). The molecular mechanism of transmission of membrane depolarization is via gap junctions; among proteins that make up these gap junctions are the following: Connexin 40 (Cx40), Connexin 45 (Cx45), and Connexin 43 (Cx43) (Gros & Jongsma, 1996; Miquerol et al., 2004). A single gap junction, for instance, is composed of 12 Cx43 units (Schulz and Heusch, 2006). Cx40 is highly expressed in active atrial myocytes, the central part of the AV node, the His bundle, as well as the Purkinje fibres. One study found that a null mutation in Cx40 results in impaired conduction and conduction block, suggesting the pivotal role that this connexin plays in transmitting signal from atria to ventricles (Gros et al., 2004; Tamaddon et al., 2000; van Rijen et al., 2001). Another connexin involved in gap junction machinery is Connexin 30.2 (Cx30.2); this protein was found in both SA and AV nodes; (Kreuzberg et al. 2006). Cx30.2 was actually shown to dampen the rate of impulse propagation in AV node in control mice versus mutant, determined by the PQ interval (Kreuzberg et al., 2006). This provides further evidence of the importance of different types of connexins as structural components of gap junctions in the cardiac pacemaker.
Furthermore, Cx43 is the primary connexin component of active myocytes of the atria and ventricles; ablation causes reduced conduction velocity, increased dispersion of conduction, and enhanced electrical sensitivity on the ventricle (Beyer et al., 1989; Reaume et al., 1995; van Rijen et al., 2004). Connexin 45 is located in SA and AV nodes (Coppen et al., 1998; Kruger et al., 2000; Verheijck et al., 2001), and its ablation is lethal in mice due largely to defects in vascularization (Kruger et al., 2000). Connexins are expressed in several tissues including heart, blood vessels, and neural tissue (Rackauskas et al., 2007). In fact, basilar artery SMCs are coupled in vivo with Cx43, Cx40, and other conductance channels; many of the channels involve non-homotypic components [not entirely comprised of the same connexin protein] (Li and Simard, 1999).
Endothelial-myocyte uncoupling and myocytemyocyte uncoupling
Remodelling is a process whereby there is synthesis and degradation of the ECM involving a very precise balance of proteinase/antiproteinase activity; an increase in this ratio has been shown to result in systolic and diastolic heart failure with uncoupling cardiomyocytes (Hunt et al., 2002). This would result in impaired depolarization of the signal between endothelial cells and cardiomyocytes [Endothelial-Myocyte uncoupling, E-M uncoupling], or between cardiomyocytes [Myocyte-Myocyte uncoupling, M-M uncoupling]. In fact, one study suggests a direct cause-and-effect relationship between MMP-9 activation and EM uncoupling in LV myocardium after chronic volume overload (Moshal et al., 2008b).
During heart disease, including hypertension, tissue inhibitors of matrix metalloproteinase’s (TIMPs) are oxidized and inactivated, thereby allowing matrix metalloproteinase’s (MMPs) to be activated (Rucklidge et al. 1992). A normal heart expresses four TIMP species: TIMP1, TIMP2, TIMP3, TIMP4; these TIMPs are altered during progression of human heart failure (Mann & Taegtmeyer, 2001). A reduction in TIMP3, thereby allowing MMP activation, results in adverse remodelling affects (Fedak et al. 2003). MMPs act as collagenases and elastases; however, their primary function is as collagenases. Hence, collagen deposition is greater than elastin deposition when MMPs are active. The accumulation of collagen disrupts the aforementioned connexin proteins, interfering with depolarization of cardiomyocytes, and impairing heart function. One study found that congenic transfer of TIMP ameliorated LV hypertrophy and cardiac dysfunction by inactivating MMP-9 involved in remodelling (Rodriguez et al., 2008). It was found that there are chamber-specific alterations in myocardial collagen content and MMP and TIMP levels that may provide for diagnostic and other insight into pathogensis of atrial fibrillation and chronic heart failure (CHF) (Mukherjee et al., 2006).
Moreover, nitric oxide (NO) generation from endocardial endothelium has a role in myocyte contraction, relaxation and heart rate (Brady et al., 1993; Pinsky et al., 1997). Hence, an increase of collagen between the endothelium and myocyte will lead to a longer contracting period since endothelial-mediated relaxation via NO is impaired (Moshal et al., 2005). In accordance with other results, another study found that an increased MMP activation contributes to the LV dilation and increased wall stress with pacing CHF (McElmurray III et al., 1999).
Remodelling via plasmin and MMPs
The dynamic nature of the cardiovascular architecture requires such remodelling systems to be in place and work in harmony to accommodate various pressures and stresses. However, when these systems are not in proper balance, or when the stresses exceed the accommodating capacity, physiological problems ensue (Heistad et al., 1991; Wilson et al., 1993). It was found that myocardial fibrosis and ECM remodelling are characteristics of the failing heart (Weber et al. 1992).
The purpose of collagen fibres is to provide an elastic force in the myocardium that allows Starling Forces to operate properly (Weber et al., 1989). The following are characteristic of ECM remodelling that can contribute to stiffness and systolic/diastolic failure: total collagen content, collagen subtypes, collagen protein stability, collagen cross-linking (Iimoto et al., 1988; Jalil et al., 1989; Mukherjee & Sen 1990; Norton et al., 1997). The activation of MMPs involves collagen degradation with replacement of fibrotic tissue (Dollery et al., 1995; Li et al., 2000b; Maquart et al., 1988).
Remodelling of the vessel wall will determine lumen diameter after vascular injury or hemodynamic forces (de Smet et al., 2000; Mintz et al., 1996; Mondy et al., 1997; Tyagi 1999). The smooth muscle cells (SMCs) remodel existing extracellular matrix, as well as contributing to further matrix deposition/removal, thereby altering the phenotype (Tummalapalli & Tyagi 1999). One aspect of atherosclerosis involves the migration of SMCs from media to intima of vasculature (Ross, 1993; Schwartz, 1997). In order for this deleterious migration to occur, a degradation of matrix is necessary via MMPs and fibrinolytic plasminogen/plasmin (Cho and Reidy, 2002; Clowes et al., 1990; Kenagy et al., 1996).
This destructive cascade is first initated from the conversion of plasminogen to plasmin by two plasminogen activators: tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) (Carmeliet et al., 1995; Lijnen, 2001). Since MMPs are secreted as inactive zymogens (pro-MMPs), they require proteolytic activation by t-PA (Malemud, 2006). Plasminogen activator inhibitor-1 (PAI-1) is responsible for inhibition of uPA and tPA, thereby mediating the destructive process whereby matrix is metabolized and migration of SMCs occurs (Carmeliet et al., 1997; Hasenstab et al., 2000). Further evidence for higher levels of PAI-1 becoming detrimental was cited in a review of cancer and plasmin activators (Andreasen et al., 1997). One study showed the effects of mutations in the following: u-PA, t-PA, MMP-9 (Heymans et al., 2005). In t-PA deficient mice, cardiomyocyte hypertrophy was discovered in conjunction with myocardial fibrosis, LV dilation, dysfunction after 7 weeks (Heymans et al., 2005); this is logical since plasmin levels would decrease, thereby reducing fibrinolysis. One study showed evidence that reduced inactivation (moderate activation) will result in a decrease in plasmin levels, thereby decreasing remodelling after myocardial infarction (Askari et al., 2003). In conjunction with this study, another study showed that an increase of PAI will serve the opposite role: decrease plasmin, and increase in myocardial fibrosis after infarction (Takeshita et al., 2004). One study indicated that the use of MMP-inhibitors would preserve cardiac pump function in LV overloading (Heymans et al., 2005).
Injury of a vessel can lead to leakage of proteins into the interstitial space, which activates the coagulation cascade with deposits of fibrin, the major substrate for plasmin (Loskutoff & Quigley, 2000). However, it is generally accepted that fibrinolysis is a good thing since fibrotic disease is detrimental in all major tissues. Hence, activation of PAI-1 results in greater fibrosis, whereas the inactivation of PAI-1 results in increased fibrinolysis. This was demonstrated in PAI-1 deficient mice compared with control mice: fibrinolysis was enhanced, collagen build-up was reduced, and survival was dramatically prolonged in bleomycin-treated mice (Hattori et al., 2000). One in vivo study found that in SMCs PAI-1 plays a role in limiting flow-induced SMC migration, thereby playing a pivotal role in controlling vascular remodelling (Cullen et al., 2004).
MMPs: intracellular, extracellular, intranuclear, intramitochondrial
A second system of remodelling includes MMPs; MMPs are a class of zinc endopeptidases that initially exist in a pro-form that is further activated upon cleavage. Like most biological mediators, the two systems [MMPs, plasminogen/plasmin] that play a role in remodelling are not mutually exclusive. Plasmin, for instance, can also cleave the inactive zymogen MMP to the active form (Dollery et al., 1995; Lijnen 2001). Both of these systems are known to be active in plaque formation as part of the atherosclerotic process; all of the following are increased in expression/activity: MMPs (MMP-2, MMP-9), tPA, uPA (Dollery et al., 1995; Lijnen 2001). This role was further confirmed by using MMP inhibitors and in vivo models of mice lacking uPA or uPA and tPA; in such cases, SMC migration and intimal thickening were reduced (Bendeck et al., 1996; Dollery et al., 1995).
Most MMPs have two methods for completing their role of digesting substrate: secretory and membrane-anchored roles [via a type 1 transmembrane domain or glycosylphosphatidylinositol linkage] (Pei et al., 2000). However, MMPs have also been found to have roles within the nucleus and mitochondria. There has been a recent understanding that MMPs are not only located in the matrix (McCawley & Matrisian 2001), but also act intracellularly. In fact, it has been shown that MMP-2 is expressed by fibroblasts and cardiomyocytes, and can be found with contractile proteins such as troponin-I and sarcomeres (Schulz, 2007; Wang et al., 2002). Moreover, MMP-2 activation has been shown to reduce performance of contraction after ischemia-reperfusion injury (Singh et al., 2000). The mitochondria have also been shown to contain MMPs: mtMMPs. One study has shown that ROS, possibly generated from mitochondria, can increase MMP-2 expression as well as activation (Nelson and Melendez, 2004).
Another study has found that MMP-1 was not only found in interstitial space, but also intracellularly, intranuclear, and within mitochondria (Limb et al., 2005). Inhibition of the enzyme with interference RNA (RNAi) or broad MMP inhibitor resulted in faster degradation of lamin A, activation of caspases, and fragmentation of DNA compared to controls. This suggests that MMP-1 expression allows the cell to resist apoptosis, thereby explaining a known mechanism whereby tumour cells may survive for a longer period of time (Limb et al., 2005).
One study has found that MMP-3 is located in the nucleus and is involved in apoptosis (Si-Tayeb et al., 2006). A mutation of MMP-3 resulted in decreased apoptosis; hence, MMP-3 activation is involved in apoptosis (Si-Tayeb et al., 2006). This is in stark contrast to MMP-1 expression in cells that allow cells to resist apoptosis (Limb et al., 2005). Another study found that MMP-2 is present in the nucleus of cardiac myocytes with the role of cleaving poly (ADP-ribose) polymerase (PARP) in vitro (Kwan et al., 2004).
Hcy and NMDA-R1 activation leading to an increase in Ca2+
Chronic heart failure (CHF) includes propensity of arrhythmias, systolic failure and diastolic failure; moreover, an overactive sympathetic system can contribute to the abnormality, and decrease in normal function (Colucci et al., 1981; Singh et al., 2000; Sood et al., 2002). CHF has been shown to correlate with an increase in glutamatergic activity that mediates sympathetic regulation. An upregulation of N-methyl-D-aspartate receptor-1 subunits (NMDA-R1) in the hypothalamus during CHF has been demonstrated (Li et al., 2003). Moreover, ischemia and reperfusion-induced arrhythmias are sensitive to NMDA-R1 blockade (D’Amico et al., 1999). One study has shown that the antagonist to NMDA-R1, MK-801, protects against Hcy-induced oxidative damage in neurons (Folbergrova, 1994), and an increase in heart rate by an analogue of NMDA (D’Amico et al., 1999). Another study found that the activation of NMDA-R by Hcy increases oxidative stress and Ca2+ load in mitochondria, leading to cardiomyocyte death in neonatal rats (Gao et al., 2007).
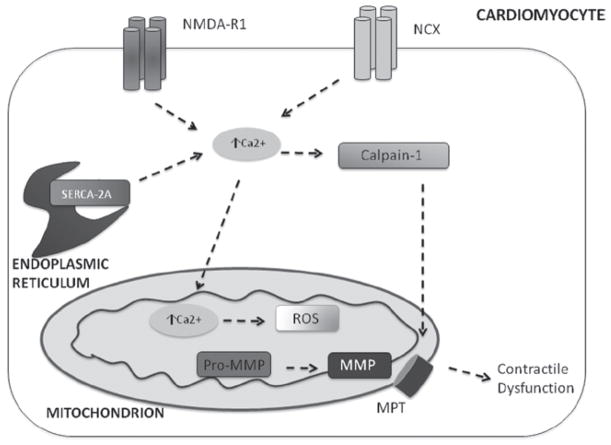
NMDA-R1 is well-known as being expressed in neural tissue; however, NMDA-R1 is now known to be expressed in cardiomyocytes and endothelial cells (Huang & Su, 1999; Krainc et al., 1998; Qureshi et al., 2005). Activation of NMDA-R1 will increase intracellular Ca2+ levels, and mitochondrial Ca2+ levels, resulting in oxidative stress (Gao et al., 2007). HHcy was shown to decrease myocyte contractile performance by agonizing the NMDA-R1 receptor. An increase in Hcy decreased the contraction amplitude with an increase in Ca2+ concentration; recent studies suggest that HHcy condition increased mitochondrial NO levels and mitochondrial permeability transition (MPT), leading to the poor cardiac performance (Moshal et al., 2009). This cascade of events is illustrated in Figure 1.
Figure 1.
Hcy increases intracellular Ca2+ by: agonizing NMDA-R1 receptor, impairing ability of NCX-1 protein to extrude Ca2+ from the cell in exchange for Na+, impairs SERCA-2a uptake of ER Ca2+. This increases Ca2+ in mitochondria, disrupting electron transport chain, and increasing presence of ROS. An increase in ROS will activate MMPs; Calpain-1 will activate mitochondrial pore transition, resulting MMPs exiting mitochondria and causing contractile dysfunction.
Mitochondrial mechanism of ECM metabolism
Hcy-induced increase of Ca2+ leads to ROS production. The mitochondria are involved in several cellular processes aside from its well-known role of providing energy through oxidative phosphorylation. In fact, mitochondria have a well-known role in cellular death that includes the release of many pro-apoptotic intermembrane space proteins: cytochrome c, apoptosis inducing factor, endonuclease G, and DIABLO/Smac (Du et al., 2000; Kroemer & Reed 2000; Li et al., 2001; Liu et al., 1996; Spiess et al., 1999; Susin et al., 1999; Van et al., 2001; Verhagen et al., 2000). Another protein found to be released is Omi, a homologue to the bacterial HtrA gene product: a chaperone and active protease (Van et al., 2002a). HtrA2/Omi has a role in degrading improperly folded proteins in times of cellular and endoplasmic reticulum stress, heat-shock, and even ischemia/reperfusion (Faccio et al., 2000; Gray et al., 2000). In fact, many studies demonstrate a role for serine proteases in apoptotic cell death (Kagaya et al., 1997; Wright et al., 1997). One study also found that the alteration of mitochondrial membrane potential contributes to apoptosis. A decrease in mitochondrial membrane potential leads to matrix condensation with an exposure of cytochrome c into IMM space; this facilitates cytochrome c release and cell death (Gottlieb et al., 2003).
Mitochondria are involved in translocating and activating several proteins. For example, amyloid β-peptide is imported into mitochondria via translocase of outer membrane (TOM) import machinery, and is localized into the mitochondrial cristae (Hansson Petersen et al., 2008). Import of proteins involves translocation through the outer (OMM) and inner mitochondrial membranes (IMM) involving complex protein machinery (Rassow et al., 1994). One such example of this is the MIM44 and mt-hsp70 cooperation in translocation of pro-proteins (Rassow et al., 1994). For instance, it was found that the translocation of the protein, Bax, and its activation to mitochondria will alter the mitochondrial transmembrane potential; the consequences could be cell death (Tikhomirov and Carpenter, 2005).
Another function of mitochondria aside from the role of ATP generation is sequestration of Ca2+, and the generation and detoxification of cellular ROS; the electron transport chain is a key connector for these roles (Brand et al., 2005). One study found that endothelin-1 (ET-1) promoted oxidative stress through mitochondrial ROS in vascular smooth muscle cells (Touyz et al., 2004). Rate of superoxide formation within mitochondria is greatly affected by the coupling state of mitochondria; Ca2+ plays a great role in this state (Dalton et al., 1999). Furthermore, the redox state can determine the oxidation state of thiols and pyrimidine nucleotides (Dalton et al., 1999). An increase in intracellular Ca2+ will also increase mitochondrial Ca2+ via a simple mechanism linked to the proton gradient that is established via the electron transport chain (Dalton et al., 1999). Hence, it is concluded that an increase in Ca2+ will disrupt the membrane potential of mitochondria, decrease oxygen utilization (since oxygen is the final electron acceptor to generate water) and produce greater amounts of superoxide (Dalton et al., 1999).
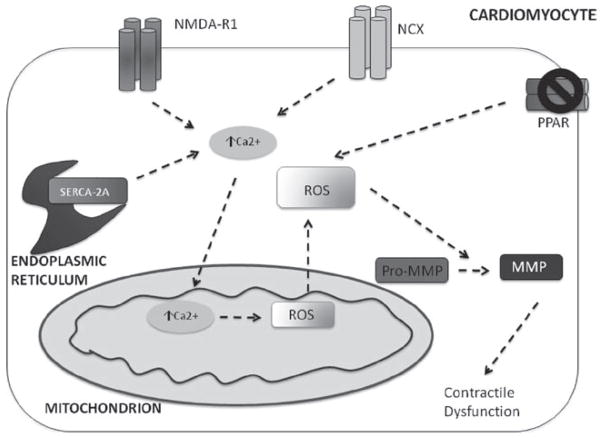
A mitochondrial manganese-containing superoxide dismutase (Sod2) is one of several enzymatic defences to reduce injury from oxidation (Van et al., 2002b). Sod2, for instance, is responsible for catalysing the reaction of superoxide to hydrogen peroxide. One study also indicates that a Sod2-dependent production of hydrogen peroxide leads to MMP-1 expression. Mice that have this gene removed will develop fibrosis and collagen deposition. Under basal conditions, mitochondria have a buffering capacity that is largely determined by glutathionine redox system (Ranganathan et al., 2001). Sod2 expression results when such systems are overwhelmed (Ranganathan et al., 2001). Another study showed that over expression of Sod stimulated activation of MMP-2 with an increase of ROS (Zhang et al., 2002). One possible mechanism for MMP activation in mitochondria is generation of hypochlorous acid (HOCl) from H2O2 by the enzyme, myeloperoxidase (Fu et al., 2001). One study indicated that HOCl regulates the activity of MMP-7 in vitro. In addition, HOCl activated pro-MMP-7 to MMP-7 in vivo via converting thiol residue of cysteine switch to sulphinic acid (Fu et al., 2001). Figure 2 illustrates how ROS, produced from mitochondria, can cleave pro-MMP to active MMPs; this results in contractile dysfunction. This is a distinctly different mechanism from proteolytic cleavage of MMP (Mukherjee et al., 2006).
Figure 2.
Hcy increases intracellular Ca2+ by: agonizing NMDA-R1 receptor, impairing ability of NCX-1 protein to extrude Ca2+ from the cell in exchange for Na+ impairs SERCA-2a uptake of ER Ca2+. This increases Ca2+ in mitochondria, disrupting electron transport chain, and increasing presence of ROS. An increase in ROS will activate MMPs. ROS is also generated via decreased expression of PPAR receptor, allowing greater presence of ROS that will activate MMPs within the cell, and result in contractile dysfunction.
One other mechanism for MMP-9 activation involving mitochondria is via the calpain system. It was shown that Hcy activates and translocates calpain-1 from the cytosol to mitochondria; this results in intra-mitochondrial oxidative stress, resulting in MMP-9 activation within mitochondria. There has also been a link to ERK ½ pathway in activating calpain-1 (Moshal et al., 2006). Moreover, this is a Ca2+-dependent mechanism whereby calpain-1 is activated via induction of dissociation of calpain subunits (Moshal et al., 2006). The requirement for Ca2+ activated calpain-1 in MMP-2 and MMP-9 expression was also demonstrated via the calpain inhibitor, CP1B which reduces expression of MMP-2 and MMP-9 (Popp et al., 2003). Use of ERK ½ blocker also resulted in a decrease in MMP-9 expression (Moshal et al. 2006). It has also been suggested that there is a negative feedback mechanism involved whereby an increase in ROS would impair mitochondrial membrane potential, thereby disrupting function (Zhou et al., 2007).
A mechanism whereby Hcy controls this process begins with calpain protease activation; upon activation, there is an induction of mitochondrial permeability transition (MPT) (Moshal et al., 2008a). Treatment with MK-801, a blocker of NMDA-R1 will attenuate the induction of MPT in the presence of Hcy (Moshal et al., 2008a). The mechanism by which this is proposed to occur is Hcy-induced ROS and Ca2+ load in the mitochondria. One study reports that HHcy increases MMP-9 expression by agonizing the NMDA-R1 receptor, the consequences of which are an increase in ROS and Ca2+ load in mitochondria (Moshal et al., 2008c). One study found that in conditions of HHcy, the Ca2+ clearance rate declined from a decrease in the expression of SERCA-2a and NCX – Ca2+ handling proteins. An increase in Ca2+ may have induced MPT, thereby reducing the ability of the mitochondria to generate ATP resulting in a decline of myocyte contractility (Moshal et al., 2008c). The mechanism of Hcy-induced activation of calpain and MPT is emphasized in Figure 1, whereby activated MMPs translocate and can cause contractile dysfunction.
Another study analysed the effects of reactive oxygen species on neutrophil and fibroblast collegnases; it was found that pro-MMP-8 was preferentially activated by ROS such as hydrogen peroxide and hypochlorous acid versus the traditional serine proteinases: trypsin, chymotrypsin (Saari et al., 1992). Again, figure 2 illustrates this kind of interaction whereby ROS of mitochondrial origin cleaves pro-MMP to MMP. Studies have shown that the plasminogen/plasmin system can be activated by oxidative stress (Tanaka et al., 1997), and also by xanthine/xanthineoxidase generation (Liu & Gaston Pravia, 2010). Another study indicated that reactive oxygen species are able to activate NF-κB. NF-κB is involved in many cytokine genes, such as TNF that stimulates plasminogen activators (Mariappan et al., 2010).
Other mechanisms of ROS generation and extracellular metabolism
There are several nuclear transcription factors (NF) receptors that control oxidation/reduction balance of the cell. NF-κB has been shown to be induced by Hcy (Ferlazzo et al., 2008; Wang et al., 2002). Activation of the PPAR receptor by Hcy has been shown to promote a reducing environment (antioxidant) (Brude et al., 1999). Moreover, an increased concentration of Hcy correlates with a decreased expression of the PPAR receptor and its antioxidant effects (Inoue et al., 1998). An activated PPAR receptor will increase the expression of superoxide dismutase (SOD) and catalase while decreasing NAD/NADPH oxidase (Inoue et al., 2000; Inoue et al., 2001; Poynter and Daynes, 1998; Takenouchi et al., 2010). This can serve as one potential mechanism by which high levels of Hcy result in the increased production of reactive oxygen species (ROS) and cell injury (Berman & Martin, 1993; Jiang et al., 2011; Mujumdar et al., 2001; Zhang et al., 2000). Moreover, it was found that agonists of PPAR will decrease oxidative stress and MMP activity in macrophages (Lee et al., 2011; Marx et al., 1998; McGregor et al., 2000). Another method whereby matrix remodelling is decreased by PPAR activity is via a decrease in mRNA expression of the plasminogen activators and increase of plasminogen activator inhibitors (Xin et al., 1999). One study suggests Hcy may enhance vascular constrictive remodelling by inactivating PPARα and PPARγ in ECs and PPARγ in SMCs (Mujumdar et al., 2002). Some data indicates members of the plasminogen activator system, in addition to MMP-2/9, increase with growing potential of ovarian tumours; hence there has been some interest in using MMP-inhibitors to treat certain types of cancer (Schmalfeldt et al., 2001).
The transcription factor, TNFα, was also found to increase MMP expression; over expression of TNFα, and subsequent MMP expression, can cause heart failure phenotype (Kubota et al., 1997; Li et al., 2002). One study found that ECM remodelling in transgenic mice that over express TNFα can be modulated using an anti-TNFα treatment (Li et al., 2000a).
Conclusions
The role of mitochondria in myocardial matrix metabolism and remodelling is still not clear. This review briefly mentions some mechanisms that could activate the MMP system and modulate plasminogen/plasmin that involves Hcy-induced production of oxidative stress during cardiovascular remodelling.
Acknowledgments
This work was supported by NIH grants: HL-71010; HL-74185; HL-88012; HL108621 and NS-51568.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72 (1):1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Archer SL. The mitochondrion as a Swiss army knife: implications for cardiovascular disease. J Mol Med (Berl) 2010;88(10):963–5. doi: 10.1007/s00109-010-0665-7. [DOI] [PubMed] [Google Scholar]
- Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197(5):615–24. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Smulders YM, van GC, Stehouwer CD. Epidemiology of homocysteine as a risk factor in diabetes. Metab Syndr Relat Disord. 2003;1(2):105–20. doi: 10.1089/154041903322294434. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Irvin C, Reidy MA. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78(1):38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- Berman RS, Martin W. Arterial endothelial barrier dysfunction: actions of homocysteine and the hypoxanthine-xanthine oxidase free radical generating system. Br J Pharmacol. 1993;108(4):920–26. doi: 10.1111/j.1476-5381.1993.tb13487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- Brady AJ, Warren B, Poole-Wilson PA, Williams TJ, Harding SE. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 1993;265(1 Pt 2):H176–82. doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]
- Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37(6):755–67. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Brude IR, Finstad HS, Seljeflot I, Drevon CA, Solvoll K, Sandstad B, Hjermann I, Arnesen H, Nenseter MS. Plasma homocysteine concentration related to diet, endothelial function and mononuclear cell gene expression among male hyperlipidaemic smokers. Eur J Clin Invest. 1999;29(2):100–8. doi: 10.1046/j.1365-2362.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- Cai B, Shan L, Gong D, Pan Z, Ai J, Xu C, Lu Y, Yang B. Homocysteine modulates sodium channel currents in human atrial myocytes. Toxicology. 2009;256(3):201–6. doi: 10.1016/j.tox.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Bouche A, De CC, Janssen S, Pollefeyt S, Wyns S, Mulligan RC, Collen D. Biological effects of disruption of the tissue-typeplasminogen activator, urokinase-type plasminogen activator, and plasminogen activator inhibitor-1 genes in mice. Ann NY Acad Sci. 1995;748:367–81. doi: 10.1111/j.1749-6632.1994.tb17333.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation: a gene targeting and gene transfer study in mice. Circulation. 1997;96(9):3180–91. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91(9):845–51. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Clowes MM, Au YP, Reidy MA, Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue–type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990;67(1):61–7. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Alexander RW, Williams GH, Rude RE, Holman BL, Konstam MA, Wynne J, Mudge GH, Jr, Braunwald E. Decreased lymphocyte beta-adrenergic-receptor density in patients with heart failure and tolerance to the beta-adrenergic agonist pirbuterol. N Engl J Med. 1981;305(4):185–90. doi: 10.1056/NEJM198107233050402. [DOI] [PubMed] [Google Scholar]
- Coppen SR, Dupont E, Rothery S, Severs NJ. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res. 1998;82(2):232–43. doi: 10.1161/01.res.82.2.232. [DOI] [PubMed] [Google Scholar]
- Cullen JP, Nicholl SM, Sayeed S, Sitzmann JV, Okada SS, Cahill PA, Redmond EM. Plasminogen activator inhibitor-1 deficiency enhances flow-induced smooth muscle cell migration. Thromb Res. 2004;114(1):57–65. doi: 10.1016/j.thromres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- D’Amico M, Di FC, Rossi F, Rossi F. Arrhythmias induced by myocardial ischaemia-reperfusion are sensitive to ionotropic excitatory amino acid receptor antagonists. Eur J Pharmacol. 1999;366(2–3):167–74. doi: 10.1016/s0014-2999(98)00914-5. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- de Smet BJ, de KD, Hanemaaijer R, Verheijen JH, Robertus L, van Der Helm YJ, Borst C, Post MJ. Metalloproteinase inhibition reduces constrictive arterial remodeling after balloon angioplasty: a study in the atherosclerotic Yucatan micropig. Circulation. 2000;101(25):2962–7. doi: 10.1161/01.cir.101.25.2962. [DOI] [PubMed] [Google Scholar]
- Dhamija RK, Gaba P, Arora S, Kaintura A, Kumar M, Bhattacharjee J. Homocysteine and lipoprotein (a) correlation in ischemic stroke patients. J Neurol Sci. 2009;281(1–2):64–8. doi: 10.1016/j.jns.2009.02.341. [DOI] [PubMed] [Google Scholar]
- Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77(5):863–8. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Faccio L, Fusco C, Chen A, Martinotti S, Bonventre JV, Zervos AS. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J Biol Chem. 2000;275(4):2581–8. doi: 10.1074/jbc.275.4.2581. [DOI] [PubMed] [Google Scholar]
- Fedak PW, Altamentova SM, Weisel RD, Nili N, Ohno N, Verma S, Lee TY, Kiani C, Mickle DA, Strauss BH, Li RK. Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am J Physiol Heart Circ Physiol. 2003;284(2):H626–34. doi: 10.1152/ajpheart.00684.2002. [DOI] [PubMed] [Google Scholar]
- Ferlazzo N, Condello S, Curro M, Parisi G, Ientile R, Caccamo D. NF-kappaB activation is associated with homocysteineinduced injury in Neuro2a cells. BMC Neurosci. 2008;9:62. doi: 10.1186/1471-2202-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrova J. NMDA and not non-NMDA receptor antagonists are protective against seizures induced by homocysteine in neonatal rats. Exp Neurol. 1994;130(2):344–50. doi: 10.1006/exnr.1994.1213. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276(44):41279–87. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- Gao X, Xu X, Pang J, Zhang C, Ding JM, Peng X, Liu Y, Cao JM. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res. 2007;56(5):559–69. doi: 10.33549/physiolres.931053. [DOI] [PubMed] [Google Scholar]
- Gillespie W, Tyagi N, Tyagi SC. Role of PPARgamma, a nuclear hormone receptor in neuroprotection. Indian J Biochem Biophys. 2011;48(2):73–81. [PubMed] [Google Scholar]
- Givvimani S, Qipshidze N, Tyagi N, Mishra PK, Sen U, Tyagi SC. Synergism between arrhythmia and hyperhomo-cysteinemia in structural heart disease. Int J Physiol Pathophysiol Pharmacol. 2011;3(2):107–19. [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10(6):709–17. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- Gray CW, et al. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem. 2000;267(18):5699–710. doi: 10.1046/j.1432-1327.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- Gros D, Dupays L, Alcolea S, Meysen S, Miquerol L, Theveniau-Ruissy M. Genetically modified mice: tools to decode the functions of connexins in the heart – new models for cardiovascular research. Cardiovasc Res. 2004;62(2):299–308. doi: 10.1016/j.cardiores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Gros DB, Jongsma HJ. Connexins in mammalian heart function. Bioessays. 1996;18(9):719–730. doi: 10.1002/bies.950180907. [DOI] [PubMed] [Google Scholar]
- Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011;278(1):28–45. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- Hansson Petersen CA, et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci USA. 2008;105(35):13145–50. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstab D, Lea H, Clowes AW. Local plasminogen activator inhibitor type 1 overexpression in rat carotid artery enhances thrombosis and endothelial regeneration while inhibiting intimal thickening. Arterioscler Thromb Vasc Biol. 2000;20(3):853–9. doi: 10.1161/01.atv.20.3.853. [DOI] [PubMed] [Google Scholar]
- Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106(11):1341–50. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Lopez JA, Baumbach GL. Hemodynamic determinants of vascular changes in hypertension and atherosclerosis. Hypertension. 1991;17(4 Suppl):III7–11. doi: 10.1161/01.hyp.17.4_suppl.iii7. [DOI] [PubMed] [Google Scholar]
- Heymans S, Lupu F, Terclavers S, Vanwetswinkel B, Herbert JM, Baker A, Collen D, Carmeliet P, Moons L. Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. Am J Pathol. 2005;166(1):15–25. doi: 10.1016/S0002-9440(10)62228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, et al. Hyperhomocyst(e)inemia and endothelial dysfunction in IDDM. Diabetes Care. 1997;20(12):1880–86. doi: 10.2337/diacare.20.12.1880. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107(6):675–83. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CF, Su MJ. Positive inotropic action of NMDA receptor antagonist (+)-MK801 in rat heart. J Biomed Sci. 1999;6(6):387–98. doi: 10.1007/BF02253670. [DOI] [PubMed] [Google Scholar]
- Huijberts MS, Becker A, Stehouwer CD. Homocysteine and vascular disease in diabetes: a double hit? Clin Chem Lab Med. 2005;43(10):993–1000. doi: 10.1515/CCLM.2005.174. [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Aru GM, Hayden MR, Moore CK, Hoit BD, Tyagi SC. Induction of oxidative stress and disintegrin metalloproteinase in human heart end-stage failure. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L239–45. doi: 10.1152/ajplung.00001.2002. [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Tyagi SC. Peroxisome proliferators compete and ameliorate Hcy-mediated endocardial endothelial cell activation. Am J Physiol Cell Physiol. 2002;283(4):C1073–9. doi: 10.1152/ajpcell.00152.2002. [DOI] [PubMed] [Google Scholar]
- Iimoto DS, Covell JW, Harper E. Increase in cross-linking of type I and type III collagens associated with volume-overload hypertrophy. Circ Res. 1988;63(2):399–408. doi: 10.1161/01.res.63.2.399. [DOI] [PubMed] [Google Scholar]
- Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+, Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50(1):3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- Inoue I, Goto S, Mizotani K, Awata T, Mastunaga T, Kawai S, Nakajima T, Hokari S, Komoda T, Katayama S. Lipophilic HMG-CoA reductase inhibitor has an anti–inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci. 2000;67(8):863–76. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- Inoue I, Noji S, Awata T, Takahashi K, Nakajima T, Sonoda M, Komoda T, Katayama S. Bezafibrate has an antioxidant effect: peroxisome proliferator–activated receptor alpha is associated with Cu2+, Zn2+–superoxide dismutase in the liver. Life Sci. 1998;63(2):135–144. doi: 10.1016/s0024-3205(98)00249-5. available from: PM:9674948. [DOI] [PubMed] [Google Scholar]
- Jalil JE, Doering CW, Janicki JS, Pick R, Shroff SG, Weber KT. Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res. 1989;64(6):1041–50. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–42. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- Kagaya S, Kitanaka C, Noguchi K, Mochizuki T, Sugiyama A, Asai A, Yasuhara N, Eguchi Y, Tsujimoto Y, Kuchino Y. A functional role for death proteases in s-Myc- and c-Myc-mediated apoptosis. Mol Cell Biol. 1997;17(11):6736–45. doi: 10.1128/mcb.17.11.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenagy RD, Vergel S, Mattsson E, Bendeck M, Reidy MA, Clowes AW. The role of plasminogen, plasminogen activators, and matrix metalloproteinases in primate arterial smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 1996;16(11):1373–82. doi: 10.1161/01.atv.16.11.1373. [DOI] [PubMed] [Google Scholar]
- Krainc D, Bai G, Okamoto S, Carles M, Kusiak JW, Brent RN, Lipton SA. Synergistic activation of the N-methyl-D-aspartate receptor subunit 1 promoter by myocyte enhancer factor 2C and bSp1. J Biol Chem. 1998;273(40):26218–24. doi: 10.1074/jbc.273.40.26218. [DOI] [PubMed] [Google Scholar]
- Kreuzberg MM, Schrickel JW, Ghanem A, Kim JS, Degen J, Janssen-Bienhold U, Lewalter T, Tiemann K, Willecke K. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc Natl Acad Sci USA. 2006;103(15):5959–64. doi: 10.1073/pnas.0508512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6(5):513–19. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127(19):4179–93. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81(4):627–35. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–92. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- Lee TI, Kao YH, Chen YC, Pan NH, Lin YK, Chen YJ. Cardiac peroxisome-proliferator-activated receptor expression in hypertension co-existing with diabetes. Clin Sci (Lond) 2011;121 (7):305–12. doi: 10.1042/CS20100529. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li X, Simard JM. Multiple connexins form gap junction channels in rat basilar artery smooth muscle cells. Circ Res. 1999;84(11):1277–84. doi: 10.1161/01.res.84.11.1277. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93(10):990–97. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA. 2000a;97(23):12746–51. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YY, Kadokami T, Wang P, McTiernan CF, Feldman AM. MMP inhibition modulates TNF-alpha transgenic mouse phenotype early in the development of heart failure. Am J Physiol Heart Circ Physiol. 2002;282(3):H983–9. doi: 10.1152/ajpheart.00233.2001. [DOI] [PubMed] [Google Scholar]
- Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000b;46(2):214–24. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost. 2001;86(1):324–333. [PubMed] [Google Scholar]
- Limb GA, Matter K, Murphy G, Cambrey AD, Bishop PN, Morris GE, Khaw PT. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am J Pathol. 2005;166(5):1555–63. doi: 10.1016/S0002-9440(10)62371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010;48(1):1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Homocysteine trials––clear outcomes for complex reasons. N Engl J Med. 2006;354(15):1629–32. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106 (12):1441–3. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Mann DL, Taegtmeyer H. Dynamic regulation of the extracellular matrix after mechanical unloading of the failing human heart: recovering the missing link in left ventricular remodeling. Circulation. 2001;104(10):1089–91. [PubMed] [Google Scholar]
- Maquart FX, Pickart L, Laurent M, Gillery P, Monboisse JC, Borel JP. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ FEBS Lett. 1988;238(2):343–6. doi: 10.1016/0014-5793(88)80509-x. [DOI] [PubMed] [Google Scholar]
- Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsen ious O, Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85(3):473–83. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARgamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma(PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153(1):17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Burri S, de MS, Hullin R, Martinelli M, Mohacsi P, Hess OM. Plasma homocysteine and cardiovascular risk in heart failure with and without cardiorenal syndrome. Int J Cardiol. 2010;141(1):32–8. doi: 10.1016/j.ijcard.2008.11.131. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13(5):534–40. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- McElmurray JH, III, et al. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: comparative effects on left ventricular function and geometry. J Pharmacol Exp Ther. 1999;291(2):799–811. [PubMed] [Google Scholar]
- McGregor D, Shand B, Lynn K. A controlled trial of the effect of folate supplements on homocysteine, lipids and hemorheology in end-stage renal disease. Nephron. 2000;85(3):215–20. doi: 10.1159/000045664. [DOI] [PubMed] [Google Scholar]
- Milani RV, Lavie CJ. Homocysteine: the Rubik’s cube of cardiovascular risk factors. Mayo Clin Proc. 2008;83(11):1200–202. doi: 10.4065/83.11.1200. [DOI] [PubMed] [Google Scholar]
- Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, Hong MK, Kovach JA, Leon MB. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94(1):35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- Miquerol L, Meysen S, Mangoni M, Bois P, van Rijen HV, Abran P, Jongsma H, Nargeot J, Gros D. Architectural and functional asymmetry of the His-Purkinje system of the murine heart. Cardiovasc Res. 2004;63(1):77–86. doi: 10.1016/j.cardiores.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Mondy JS, Williams JK, Adams MR, Dean RH, Geary RL. Structural determinants of lumen narrowing after angioplasty in atherosclerotic nonhuman primates. J Vasc Surg. 1997;26(5):875–83. doi: 10.1016/s0741-5214(97)70103-4. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Kumar M, Tyagi N, Mishra PK, Metreveli N, Rodriguez WE, Tyagi SC. Restoration of contractility in hyperhomocysteinemia by cardiac–specific deletion of NMDA-R1. Am J Physiol Heart Circ Physiol. 2009;296(3):H887–92. doi: 10.1152/ajpheart.00750.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshal KS, Metreveli N, Frank I, Tyagi SC. Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia. Curr Vasc Pharmacol. 2008a;6(2):84–92. doi: 10.2174/157016108783955301. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Rodriguez WE, Sen U, Tyagi SC. Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiol Res. 2008b;57(3):379–84. doi: 10.33549/physiolres.931221. [DOI] [PubMed] [Google Scholar]
- Moshal KS, Singh M, Sen U, Rosenberger DS, Henderson B, Tyagi N, Zhang H, Tyagi SC. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol. 2006;291(6):H2825–35. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- Moshal KS, et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008c;295(2):H890–97. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshal KS, Tyagi N, Henderson B, Ovechkin AV, Tyagi SC. Protease-activated receptor and endothelial-myocyte uncoupling in chronic heart failure. Am J Physiol Heart Circ Physiol. 2005;288(6):H2770–77. doi: 10.1152/ajpheart.01146.2004. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem. 2001;82(3):491–500. doi: 10.1002/jcb.1175. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Tummalapalli CM, Aru GM, Tyagi SC. Mechanism of constrictive vascular remodeling by homocysteine: role of PPAR. Am J Physiol Cell Physiol. 2002;282(5):C1009–15. doi: 10.1152/ajpcell.00353.2001. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Sen S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res. 1990;67(6):1474–80. doi: 10.1161/01.res.67.6.1474. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Herron AR, Lowry AS, Stroud RE, Stroud MR, Wharton JM, Ikonomidis JS, Crumbley AJ, III, Spinale FG, Gold MR. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol. 2006;97(4):532–7. doi: 10.1016/j.amjcard.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Nasir K, Tsai M, Rosen BD, Fernandes V, Bluemke DA, Folsom AR, Lima JA. Elevated homocysteine is associated with reduced regional left ventricular function: the multi-ethnic study of atherosclerosis. Circulation. 2007;115 (2):180–87. doi: 10.1161/CIRCULATIONAHA.106.633750. [DOI] [PubMed] [Google Scholar]
- Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37(6):768–84. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997;96(6):1991–8. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- Ntaios G, et al. The effect of folic acid supplementation on carotid intima-media thickness in patients with cardiovascular risk: a randomized, placebo-controlled trial. Int J Cardiol. 2010;143(1):16–19. doi: 10.1016/j.ijcard.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9(7):532–42. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Pavia C, Ferrer I, Valls C, Artuch R, Colome C, Vilaseca MA. Total homocysteine in patients with type 1 diabetes. Diabetes Care. 2000;23(1):84–87. doi: 10.2337/diacare.23.1.84. [DOI] [PubMed] [Google Scholar]
- Pei D, Kang T, Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J Biol Chem. 2000;275(43):33988–97. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- Pinsky DJ, Patton S, Mesaros S, Brovkovych V, Kubaszewski E, Grunfeld S, Malinski T. Mechanical transduction of nitric oxide synthesis in the beating heart. Circ Res. 1997;81(3):372–379. doi: 10.1161/01.res.81.3.372. [DOI] [PubMed] [Google Scholar]
- Popp O, Heidinger M, Ruiz-Heinrich L, Ries C, Jochum M, Gil-Parrado S. The calpastatin-derived calpain inhibitor CP1B reduces mRNA expression of matrix metalloproteinase-2 and -9 and invasion by leukemic THP-1 cells. Biol Chem. 2003;384(6):951–8. doi: 10.1515/BC.2003.107. [DOI] [PubMed] [Google Scholar]
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273(49):32833–41. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- Qureshi I, et al. Homocysteine-induced vascular dysregulation is mediated by the NMDA receptor. Vasc Med. 2005;10(3):215–223. doi: 10.1191/1358863x05vm626oa. [DOI] [PubMed] [Google Scholar]
- Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007;92(6):1952–65. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan AC, et al. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2–dependent ERK1/2 activation. J Biol Chem. 2001;276(17):14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- Rassow J, Maarse AC, Krainer E, Kubrich M, Muller H, Meijer M, Craig EA, Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994;127(6 Pt 1):1547–556. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267(5205):1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Robillon JF, Canivet B, Candito M, Sadoul JL, Jullien D, Morand P, Chambon P, Freychet P. Type 1 diabetes mellitus and homocyst(e)ine. Diabete Metab. 1994;20(5):494–6. [PubMed] [Google Scholar]
- Rodriguez WE, Tyagi N, Deng AY, Adeagbo A, Joshua IG, Tyagi SC. Congenic expression of tissue inhibitor of metalloproteinase in Dahl-salt sensitive hypertensive rats is associated with reduced LV hypertrophy. Arch Physiol Biochem. 2008;114(5):340–348. doi: 10.1080/13813450802535978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ruan L, Chen W, Srinivasan SR, Xu J, Sun M, Toprak A, Berenson GS. Relation of plasma homocysteine to arterial stiffness in black and white young adults (from the Bogalusa Heart Study) Am J Cardiol. 2009;103(7):985–8. doi: 10.1016/j.amjcard.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Rucklidge GJ, Milne G, McGaw BA, Milne E, Robins SP. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochim Biophys Acta. 1992;1156(1):57–61. doi: 10.1016/0304-4165(92)90095-c. [DOI] [PubMed] [Google Scholar]
- Saari H, Sorsa T, Lindy O, Suomalainen K, Halinen S, Konttinen YT. Reactive oxygen species as regulators of human neutrophil and fibroblast interstitial collagenases. Int J Tissue React. 1992;14(3):113–20. [PubMed] [Google Scholar]
- Schmalfeldt B, et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res. 2001;7(8):2396–404. [PubMed] [Google Scholar]
- Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- Schulz R, Heusch G. Connexin43 and ischemic preconditioning. Adv Cardiol. 2006;42:213–27. doi: 10.1159/000092571. [DOI] [PubMed] [Google Scholar]
- Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100(11 Suppl):S87–9. [PubMed] [Google Scholar]
- Si-Tayeb K, Monvoisin A, Mazzocco C, Lepreux S, Decossas M, Cubel G, Taras D, Blanc JF, Robinson DR, Rosenbaum J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am J Pathol. 2006;169(4):1390–1401. doi: 10.2353/ajpath.2006.060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Communal C, Sawyer DB, Colucci WS. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res. 2000;45(3):713–19. doi: 10.1016/s0008-6363(99)00370-3. [DOI] [PubMed] [Google Scholar]
- Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa T. Elevated plasma homocysteine level is an independent predictor of coronary heart disease events in patients with type 2 diabetes mellitus. Ann Intern Med. 2004;140(2):94–100. doi: 10.7326/0003-4819-140-2-200401200-00009. [DOI] [PubMed] [Google Scholar]
- Sood HS, Cox MJ, Tyagi SC. Generation of nitrotyrosine precedes activation of metalloproteinase in myocardium of hyperhomocysteinemic rats. Antioxid Redox Signal. 2002;4(5):799–804. doi: 10.1089/152308602760598954. [DOI] [PubMed] [Google Scholar]
- Spiess C, Beil A, Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97(3):339–47. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- Susin SA, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Takenouchi Y, Kobayashi T, Taguchi K, Matsumoto T, Kamata K. Relationship among superoxide-related enzyme, PPARs, and endothelium-dependent relaxation in murine aortas previously organ-cultured in high-glucose conditions. Can J Physiol Pharmacol. 2010;88(7):760–69. doi: 10.1139/y10-045. [DOI] [PubMed] [Google Scholar]
- Takeshita K, et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164(2):449–56. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaddon HS, Vaidya D, Simon AM, Paul DL, Jalife J, Morley GE. High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ Res. 2000;87(10):929–36. doi: 10.1161/01.res.87.10.929. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Ogura N, Abiko Y. Stimulation of plasminogen activator/plasmin system in gingival fibroblast cells by oxygen radicals. Arch Oral Biol. 1997;42(4):263–270. doi: 10.1016/s0003-9969(97)00010-1. [DOI] [PubMed] [Google Scholar]
- Tarkun I, Cetinarslan B, Canturk Z, Tarkun P, Agacdiken A, Komsuoglu B. Homocysteine concentrations in type 2 diabetic patients with silent myocardial ischemia: a predictive marker. J Diabetes Complications. 2004;18(3):165–8. doi: 10.1016/j.jdiacomp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Taskinen MR, Sullivan DR, Ehnholm C, Whiting M, Zannino D, Simes RJ, Keech AC, Barter PJ. Relationships of HDL cholesterol, ApoA-I, and ApoA-II with homocysteine and creatinine in patients with type 2 diabetes treated with fenofibrate. Arterioscler Thromb Vasc Biol. 2009;29(6):950–955. doi: 10.1161/ATVBAHA.108.178228. [DOI] [PubMed] [Google Scholar]
- Tikhomirov O, Carpenter G. Bax activation and translocation to mitochondria mediate EGF-induced programmed cell death. J Cell Sci. 2005;118(Pt 24):5681–90. doi: 10.1242/jcs.02676. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22(6):1141–9. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- Tummalapalli CM, Tyagi SC. Responses of vascular smooth muscle cell to extracellular matrix degradation. J Cell Biochem. 1999;75(3):515–27. [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi N, Vacek JC, Givvimani S, Sen U, Tyagi SC. Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J Recept Signal Transduct Res. 2010;30(2):78–87. doi: 10.3109/10799891003614808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi SC. Homocyst(e)ine and heart disease: pathophysiology of extracellular matrix. Clin Exp Hypertens. 1999;21(3):181–98. doi: 10.3109/10641969909068660. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109(8):1048–55. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, van Veen TA, van Kempen MJ, Wilms-Schopman FJ, Potse M, Krueger O, Willecke K, Opthof T, Jongsma HJ, de Bakker JM. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circulation. 2001;103(11):1591–8. doi: 10.1161/01.cir.103.11.1591. [DOI] [PubMed] [Google Scholar]
- Van LG, et al. A matrix-assisted laser desorption ionization post-source decay (MALDI-PSD) analysis of proteins released from isolated liver mitochondria treated with recombinant truncated Bid. Cell Death Differ. 2002a;9(3):301–8. doi: 10.1038/sj.cdd.4400966. [DOI] [PubMed] [Google Scholar]
- Van LG, et al. Endonuclease G: a mitochondrial protein released in apoptosis and involved in caspase-independent DNA degradation. Cell Death Differ. 2001;8(12):1136–42. doi: 10.1038/sj.cdd.4400944. [DOI] [PubMed] [Google Scholar]
- Van LG, Van GM, Depuydt B, Srinivasula SM, Rodriguez I, Alnemri ES, Gevaert K, Vandekerckhove J, Declercq W, Vandenabeele P. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002b;9(1):20–26. doi: 10.1038/sj.cdd.4400970. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102(1):43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001;52(1):40–50. doi: 10.1016/s0008-6363(01)00364-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106(12):1543–9. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1(2):75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- Weber KT, Pick R, Jalil JE, Janicki JS, Carroll EP. Patterns of myocardial fibrosis. J Mol Cell Cardiol. 1989;21(Suppl 5):121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35(Pt 5):1175–9. doi: 10.1042/BST0351175. [DOI] [PubMed] [Google Scholar]
- Wilcken DE, Wilcken B. The pathogenesis of coronary artery disease. A possible role for methionine metabolism. J Clin Invest. 1976;57(4):1079–82. doi: 10.1172/JCI108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E, Mai Q, Sudhir K, Weiss RH, Ives HE. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123(3):741–7. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SC, Schellenberger U, Wang H, Kinder DH, Talhouk JW, Larrick JW. Activation of CPP32-like proteases is not sufficient to trigger apoptosis: inhibition of apoptosis by agents that suppress activation of AP24, but not CPP32-like activity. J Exp Med. 1997;186(7):1107–17. doi: 10.1084/jem.186.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97(4):354–62. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274(13):9116–21. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Zhao W, Venkataraman S, Robbins ME, Buettner GR, Kregel KC, Oberley LW. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002;277(23):20919–26. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000;279(4):F671–8. doi: 10.1152/ajprenal.2000.279.4.F671. [DOI] [PubMed] [Google Scholar]
- Zhou HZ, Ma X, Gray MO, Zhu BQ, Nguyen AP, Baker AJ, Simonis U, Cecchini G, Lovett DH, Karliner JS. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem Biophys Res Commun. 2007;358(1):189–95. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. 2009;35(2):120–129. doi: 10.1002/biof.17. [DOI] [PubMed] [Google Scholar]
- Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(9):1470–76. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]