Abstract
The varied rates of protection induced by Mycobacterium bovis BCG vaccine against tuberculosis has been attributed to many factors such as genetic variability among BCG strains, rapid clearance of BCG in some populations, and different levels of previous exposure of vaccinated populations to environmental mycobacteria. However, the methods and conditions employed to prepare this vaccine for human usage by various manufacturers have not been investigated as potential factors contributing to the variation in vaccine efficacy. A review of the literature indicates discrepancies between the approach for growing BCG vaccine in the laboratory to assess immune responses and protective ability in animal models, and that employed for production of the vaccine for administration to humans. One of the major differences is in the growth medium used for routine propagation in the laboratory and the one used for bulk vaccine production by manufacturers. Here we compared the immunogenicity of the BCG vaccine grown in Middlebrook 7H9 medium, the most commonly used medium in laboratory studies, against that grown in Sauton medium, which is used for growing BCG by most manufacturers. Our results showed clear differences in the behavior of BCG grown in these different culture media. Compared to BCG grown in Middlebrook 7H9 medium, BCG grown in Sauton media was more persistent inside macrophages, more effective at inhibiting apoptosis of infected cells, induced stronger inflammatory responses and stimulated less effective immunity against aerosol challenge with a virulent Mtb strain. These findings suggested that the growth medium used for producing BCG vaccine is an important factor that deserves increased scrutiny in ongoing efforts to produce more consistently effective vaccines against Mtb.
Keywords: Tuberculosis, BCG, Adaptive immunity, T cell
1. Introduction
Mycobacterium bovis BCG is the only currently approved vaccine for prevention of tuberculosis (TB), and it continues to be used routinely in many regions with high levels of endemic TB. The original BCG strain was developed by Calmette and Guerin between 1908 and 1919 by carrying out 231 passages of virulent M. bovis in glycerinated bile potato medium [1]. This long-term serial cultivation led to spontaneous attenuation, resulting in a strain of M. bovis that was no longer virulent in healthy animals or humans and therefore was suitable for use as a live attenuated vaccine. However, the level of protection against pulmonary TB provided by BCG vaccination has been highly variable in different controlled trials around the world, ranging from 0 to 80% [2–4]. Three main hypotheses have been proposed to explain this variable efficacy. First, BCG has acquired additional mutations through many years of passage in culture which reduce its ability to stimulate protective immunity against Mycobacterium tuberculosis. Second, exposure to environmental mycobacteria alters the response to BCG and may lead to tolerance that blunts the vaccine effect. Third, there is a rapid clearance of BCG by vaccine recipients in some populations that does not provide sufficient exposure to the vaccine for development of long-term immunological memory [5].
Dissemination of the BCG vaccine over many years and geographic regions has led to the derivation of multiple substrains from the original strain, with as many as 49 production substrains being used at various times and places including the four major BCG vaccines in current use (BCG-Pasteur, -Danish, -Glaxo, and -Japan) [6,7]. The multiplication of production sites has led to great variation in the culture conditions and the timing of the harvesting of BCG for vaccine production [8]. However, in most production laboratories that prepare BCG vaccines for administration to humans, the bacteria are grown as a pellicle on the surface of liquid Sauton medium [9]. In contrast, the liquid culture media employed to grow BCG for research purposes vary among different laboratories, with the most common medium used being Middlebrook 7H9. In fact, a review of the literature for the last 3 years led us to conclude that greater than 95% of published papers during this period evaluating immunogenicity and vaccine efficacy of BCG in vitro or in animal models have used organisms grown in Middlebrook 7H9 medium. Interestingly, it has been shown that BCG grown in Middlebrook 7H9 or Sauton media have different protein expression profiles, and different levels of sensitivity to reactive nitrogen intermediates [10]. Moreover, BCG vaccines grown in different culture media have been shown to induce distinct humoral immune responses in mice [11], and studies of other mycobacterial species have shown major effects of the growth medium composition on the secretion of virulence related proteins such as ESAT-6 and CFP-10 [12].
The above findings suggest that the growth medium used for preparation of BCG vaccines could have a significant impact on immunogenicity and vaccine efficacy, and that this may be an important factor contributing to variations in the properties of BCG strains both in laboratory studies and in clinical vaccine trials. In particular, it is striking that most BCG vaccines used for clinical trials or routine vaccination of humans have been grown in Sauton medium, whereas BCG used for laboratory based preclinical vaccine studies is usually grown in Middlebrook 7H9 medium. This discrepancy between methods used in the laboratory versus the clinical setting prompted us to investigate whether these two different growth conditions may have any influence on immunological properties and protective efficacy of the BCG vaccine in the mouse model. Our results show that this is indeed the case, and indicate that growth conditions prior to in vivo inoculation of BCG have a significant effect on the subsequent immune response in the vaccinated host.
2. Materials and methods
2.1. Mice
Six- to 8-week-old female wild type (C57BL/6) and severe combined immunodeficiency (SCID; also C57BL/6 background) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All mice were maintained in specific pathogen-free conditions, and were transferred to biosafety level 3 conditions for infection with M. tuberculosis. All procedures involving the use of animals were in compliance with protocols approved by the Albert Einstein College of Medicine Institutional Animal Use and Biosafety Committees.
2.2. Bacterial strains, media and culture
M. bovis BCG (Pasteur and Danish strains) were obtained from Statens Serum Institute (Copenhagen, Denmark), BCG-Tice was obtained from the American Type Culture Collection (ATCC; Manassas VA), and virulent M. tuberculosis strain H37Rv was obtained from the Trudeau Institute (Saranac Lake, NY). Middlebrook 7H9 medium (M7H9; Difco Laboratories/BD Diagnostic Systems, Sparks, MD) was supplemented with oleic acid-albumin-dextrose-catalase (OADC Enrichment; Difco Laboratories/BD Diagnostic Systems, Detroit, MI) and 0.05% tyloxapol (Sigma–Aldrich, St. Louis, MO). Sauton medium was prepared using a published recipe [13]. Bacterial CFU titers were determined by plating tissue homogenates or aliquots of bacterial suspension on Middlebrook 7H11 agar plates containing OADC (M7H11). For culturing M. tuberculosis from tissues of previously BCG immunized mice, 2 µg/ml of thiophene-2-carboxylic acid hydrazide (TCH, Sigma–Aldrich) was added to the plates to selectively inhibit the growth of residual BCG organisms.
2.3. Primary cell suspensions and cell lines
Bone marrow derived macrophages (BMM) from C57BL/6 mice were prepared as described in published protocols [14]. Briefly, bone marrow cells were flushed aseptically from the femurs and tibias and suspended in complete medium which consisted of DMEM supplemented with 10 mM HEPES, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 55 µM 2-mercaptoethanol, 100 units/ml penicillin and 100 µg/ml streptomycin (all from Invitrogen, Carlsbad, CA), plus 10% heat-inactivated fetal calf serum (FCS; Gemini Biological Products, Calabasas, CA). For BMM cultures, 10% conditioned medium from a culture of mouse L929 fibroblasts was added as a source of M-CSF, and the cells were plated in bacteriology grade non-tissue culture treated 10 cm diameter polystyrene dishes (ThermoFisher Scientific, Waltham, MA) at 2 × 106 cells per dish. After incubation in a 37 °C humidified 10% CO2 incubator for 7 days, the macrophage enriched adherent fraction was harvested. The human monocytic cell line THP-1 was obtained from ATCC and maintained in complete RPMI-1640.
2.4. In vitro infection of bone marrow derived macrophages
BMM were plated in 24-well plates and incubated overnight in complete DMEM. The cells were washed once with DMEM and then infected with BCG grown in M7H9 medium (BCG-M) or Sauton medium (BCG-S) at an MOI of 10:1 for 4 h at 37 °C. The infected wells were washed three times with complete medium to remove extracellular bacteria and incubated at 37 °C. To enumerate bacterial colony forming units (CFUs) at various time points, cell lysates were prepared by removing the medium and lysing with 0.05% SDS. Serial dilutions of the lysate were plated on M7H11 agar, and incubated at 37 °C for 21 days before counting the CFUs.
2.5. Microscopy for cellular localization of infecting mycobacteria
BCG organisms grown in either of the two media (BCG-M or BCG-S) were stained with CFSE (Invitrogen-Molecular Probes, Carlsbad, CA) at 10 µM in PBS with 0.05% Tyloxapol for 30 min at 37 °C and then washed four times with PBS-T. The CFSE labeled BCG were used to infect cultures of BMM using a multiplicity of infection (MOI) of 10:1 for 3 h at 37 °C. Subsequently, the infected cells were harvested at various time points as indicated and stained with the lysotracker dye (Invitrogen-Molecular Probes) at 100 nM at room temperature for 40 min. Images were then obtained with a fluorescence microscope (Axiovert 200 M, Karl Zeiss Inc., Thornwood, NY) and analyzed using appropriate filters for co-localization of BCG with the lysotracker dye.
2.6. Apoptosis assay
Apoptosis of THP-1 macrophages was quantitated by flow cytometric analysis following incubation with CaspGLOW™ Fluorescein Active Caspase-3 staining reagent (eBioscience, San Diego, CA), Annexin V-Alexa Fluor® 647 (Invitrogen) and 1 µg/ml propidium iodide (PI; Sigma–Aldrich, St. Louis, MO). Briefly, 3 × 105 THP-1 cells per well were plated in 96-well plates in complete RPMI-1640 with 50 nM phorbol 12-myristate 13-acetate (PMA; Sigma–Aldrich). After 24-h the cells were washed with PBS, and then infected with either BCG-S or BCG-M at 10:1 MOI followed by incubation at 37 °C for 72 h. 1 h prior to harvest, the cells were incubated with FITC-DEVD-FMK in complete media following the manufacturer’s instructions. The cells were then washed twice in Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) and then stained with Annexin V-Alexa Fluor® 647. After incubation for 15 min at 37 °C, the cells were washed twice with Annexin V binding buffer and transferred to FACS tubes on ice. Immediately prior to acquisition PI was added to a final concentration of 1 µg/ml, and the cells were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA)
2.7. Detection of cytokines in vivo by multiplex capture ELISA
C57BL/6 mice were injected intravenously with 1 × 106 CFU in the lateral tail vein and serum was collected at various time points by retro-orbital bleeding. Samples were analyzed for cytokine levels by capture ELISA using the Meso Scale Discovery multiplex assay system based on electrochemiluminescent detection (MSD, Gaithersburg, MD). The standard MSD 10-plex mouse TH1/TH2 cytokine assay was used as directed by the manufacturer, which allows detection of murine IFNγ, IL-1β, IL–2, IL-4, IL-5, KC/CXCL1, IL-10, IL-12 (total p40) and TNFα.
2.8. Multiparameter FACS analyses
For analysis of multifunctional CD4+ T cells (MFT cells), splenocyte suspensions were prepared from animals immunized with either BCG-S or BCG-M administered intraperitoneally (5 × 106 CFU) or subcutaneously (1 × 106 CFU) 2 or 8 weeks previously, as stated. Single cell suspensions in complete RPMI-1640 medium were plated at 1.5 × 106 cells per well in 0.2 ml in 96-well round-bottom plates. Cells were restimulated with 10 µg/ml of each of the following synthetic peptide antigens (Invitrogen): FQDAYNAAGGHNAVF (Ag85B-P25; residues 240–254 of MTb/BCG Ag85B, I-Ab restricted); QIMYNYPAM (residues 3–11 of MTb/BCG TB10.4/10.3, H-2Kb restricted); ESSAAFQAAHARFVAA (residues 46–61 of MTb/BCG TB9.8, I-Ab restricted) plus 1 µg/ml soluble anti-CD28 mAb (clone 37.51; eBiosciences). These epitopes have been previously described and are available in the Immune Epitope Database (http://www.immuneepitope.org), with the exception of the TB9.8 epitope which was mapped in the course of this study (Supplementary Fig. 1). After incubation (37 °C, 5% CO2) for 2 h, 10 µg/ml Brefeldin A (Sigma–Aldrich) was added for an additional 4 h. Cells were labeled with blue live/dead viability dye BluVID (Invitrogen), washed, preincubated with ~10 µg/ml mAb 2.4G2 to block Fc receptors and stained with the following fluorochrome conjugated mAbs (all from BD Biosciences, Franklin Lakes, NJ): anti-CD3e-FITC (clone 145-2C11), anti-CD4-APC-Cy7 (clone RM4–5), and anti-CD8α-Pacific Blue (clone 53-6.7). Cells were then fixed with 2% paraformaldehyde for 10 min and washed with permeabilization buffer (PBS with 1 mM Ca2+, 1 mM Mg2+, 1 mM HEPES, 2% FCS and 0.1% saponin), and then blocked for 30 min in permeabilization buffer + 10% normal mouse serum (Jackson Immunoresearch, West Grove, PA). The following fluorochrome conjugated anti-cytokine mAbs were then added: anti-IL-2-APC (clone JES6-5H4; BD), anti-IFN-γ-Alexa Fluor® 700 (clone XMG1.2; BD) anti-TNF-α-PE-Cy7 (clone MP6-XT22; BD) and anti-IL-17A PerCP-Cy5.5 (clone eBio17B7; eBioscience, San Diego, CA). Samples were acquired on a BD Biosciences LSR II flow cytometer with FACSDiva™ software. For analysis of multifunctional cytokine producing T cells a Boolean gating strategy was employed using FlowJo software (Treestar, Ashland, OR).
2.9. IFNγ ELISPOT assay
IFNγ secretion by individual CD4+ T and CD8+ T cells from the spleens of mice infected intraperitoneally 2 weeks earlier with BCG-M or BCG-S (1 × 106 or 5 × 106 CFU, as indicated) was quantitated by ELISPOT assay after stimulation in vitro with peptide epitopes of MTb antigens (Ag85B240–254, TB10.3/10.43–11 and TB9.846–61) and purified recombinant M. tuberculosis Hsp65 protein (BEI Resources, Manassas, VA). After treatment with RBC lysis buffer (Sigma–Aldrich, St. Louis, MO), splenic T cells were purified using the Dynal Mouse T Cell Negative Isolation Kit (Invitrogen). The separated T cells were cultured in 96-well ELISPOT plates (Millipore, Danvers, MA) that had been coated previously with IFNγ capture antibody (Clone R4.6A2; BD Biosciences) for 16 h at room temperature (RT), followed by blocking with 1% BSA for 2 h at RT. Bulk splenocytes from a naïve mouse (9 × 105/well) were added as antigen presenting cells (APCs) with and without antigens (5 µg/ml), and incubated for 24 h at 37 °C in a 5% humidified CO2 incubator. Cells were removed and the plates were washed with PBS and then with PBS containing 0.05% Tween 20. Biotinylated anti-IFNγ detection antibody (clone 4S.B3; BD Biosciences) was added for 2 h at 37 °C, followed by washing with PBS + 0.05% Tween 20. Streptavidin–alkaline phosphatase (Sigma–Aldrich) was added to the plates for 1 h (37 °C). BCIP/NBT substrate (Sigma–Aldrich) was then added and spots were allowed to develop. The reaction was stopped by washing the wells with water, and spots were counted using an automated ELISPOT reader (Autoimmun Diagnostika, Strassberg, Germany).
2.10. Vaccination, challenge and infection studies
For vaccination/challenge studies, wild type C57BL/6 mice were vaccinated subcutaneously with either BCG-M or BCG-S (1 × 106 CFU/mouse in 0.2 ml PBS + 0.05% tyloxapol). Aerogenic challenge was done 2 months later using a whole-body exposure aerosol chamber (University of Wisconsin Mechanical Engineering Workshop, Madison, WI) custom fitted to a class III biosafety cabinet (Baker, Sanford, ME) to deliver 50–100 CFU per animal of virulent strain M. tuberculosis H37Rv. Mice were sacrificed at 4 weeks and 12 weeks after challenge. Lungs and spleens of individual mice were aseptically removed and homogenized separately in 5 ml normal saline plus 0.05% Tween 80 using a Seward Stomacher 80 blender (Tekmar, Cincinnati, OH). The homogenates were diluted serially and plated on Middlebrook 7H11 agar. Lung tissues were processed for histopathology using standard paraffin fixation, sectioning and H&E staining. For systemic BCG infection to assess time to death in SCID mice (C57BL/6 background), animals received 1 × 106 CFU of either BCG-M or BCG-S intravenously via the lateral tail vein. Survival was determined by time to spontaneous death or to severe morbidity requiring sacrifice, as specified in our approved animal use protocol (e.g., loss of >25% of body weight).
2.11. Adoptive transfer of T cells from immunized to naïve C57BL/6 mice
C57BL/6 mice were injected intraperitoneally with 4 mg/mouse of cyclophosphamide to give partial lymphocyte depletion that promotes engraftment of transferred cells [15,16], and 2 days later received adoptive transfer of 6 × 106 isolated total T cells (Pan T cell isolation kit, Miltenyi Biotec, Germany) from mice immunized subcutaneously 3 months previously with either BCG-M or BCG-S (1 × 106 CFU). The next day the recipient mice were subjected to a low dose (50–100 CFU) aerosol challenge with virulent MTb H37Rv. After 4 weeks, the spleens and lungs were harvested for CFU counts.
2.12. Western blot analyses
For the analysis of humoral responses, serum samples were obtained from C57BL/6 mice by retro-orbital bleeding 2 weeks after immunization with BCG-M or BCG-S (5 × 106 CFU inoculated intraperitoneally), and were analyzed by Western blotting for reactivity to MTb protein antigens. Preparations of MTb total lysate, culture filtrate proteins, cell wall associated proteins and cytosolic proteins prepared from M. tuberculosis strain H37Rv were provided by Colorado State University via the BEI Resources reagent procurement program (BEI Resources). Samples of these preparations were electrophoresed in 4–15% SDS-PAGE gels (20 µg of total protein per lane), which were then blotted onto PVDF membranes using a dry-blot apparatus (BioRad, Hercules, CA) according to the manufacturer’s instructions. Membranes were preincubated in buffer (PBS + 0.05%Tween 20 containing 5% dried milk to block nonspecific protein interections), and then washed and incubated with the serum samples diluted 1:1000 in lysis buffer (150 mM NaCl, 100 mM Tris-buffered saline (pH 8), 1% Tween 20, 1 mM EDTA) containing complete protease inhibitor cocktail (Roche, Basel, Switzerland). After 2 h of incubation at room temperature, the membranes were washed and incubated with anti-mouse IgG-HRP (Invitrogen) diluted 1:5000. After a further 1 h of incubation at room temperature, the membranes were washed and developed using the Super-Signal West Pico chemiluminescent substrate (Thermo Scientific, Rockford, IL), following instructions provided by the supplier. Images were obtained by exposing X-ray film to the membrane. The Western-blot for cyclooxygenase-2 (COX-2) was done using a polyclonal rabbit anti-COX-2 antiserum (Cayman, Ann Arbor, MI) at 1:120 dilution followed by a secondary polyclonal HRP-anti-rabbit IgG at 1:5000 dilution (Invitrogen). Anti-mouse β-actin-HRP was used at 1:5000 dilution to detect the housekeeping protein β-actin (Invitrogen).
2.13. Statistical analyses
GraphPad Prism 5.0 software was used for statistical analyses. Unless otherwise stated in the figure legends, two-way Analysis of Variance (ANOVA) with Bonferroni post-test was used to evaluate paired replicates. Log rank test was used to evaluate differences between survival curves. p values of less than 0.05 were considered significant.
3. Results
3.1. Increased resistance to killing in vitro and in vivo of BCG grown in Sauton medium
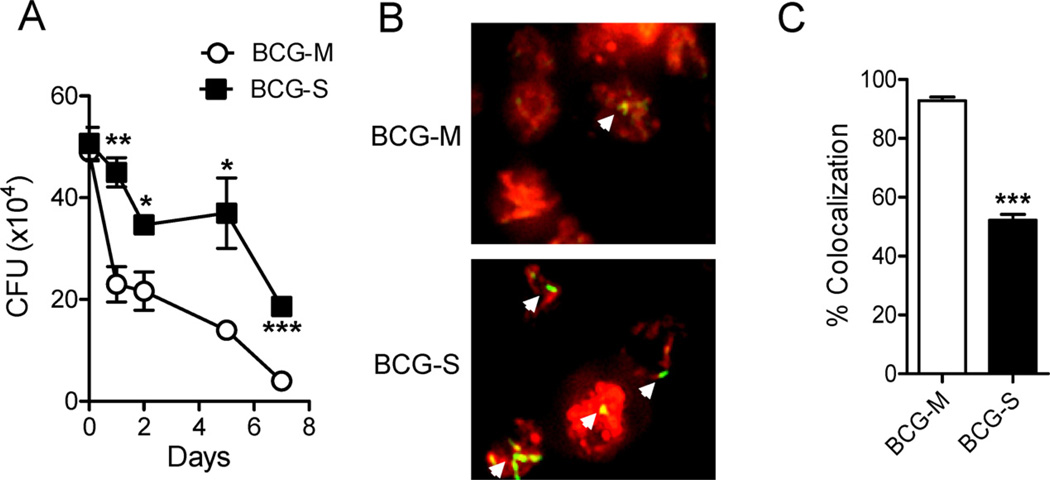
In order to assess the influence of growth conditions on the intracellular survival of BCG, we infected BMM with BCG organisms (Pasteur strain) grown in either Sauton (BCG-S) or M7H9 (BCG-M) media, and analyzed the numbers of viable bacilli (CFUs) at different time points. We observed a significantly higher survival of BCG-S compared to BCG-M, starting from 24 h through 7 days post infection (Fig. 1A). This effect was observed with three different substrains of BCG (Pasteur, Danish and Tice strains), indicating that it was broadly applicable and not restricted only to BCG-Pasteur (Supplementary Fig. 1). By infecting macrophages with CFSE-labeled bacilli, we noted that many of the intracellular BCG-M bacilli showed a dramatic loss of fluorescence 24 h after infection, suggesting that the organisms were killed and degraded (Fig. 1B). Nearly all of the relatively few bacteria that were still visualized by their green fluorescence showed colocalization with the Lysotracker dye (Fig. 1B and C), indicating their delivery to acidified compartments. In contrast, BMM infected with BCG-S showed many intracellular bacteria at 24 h post infection (Fig. 1B), and only about half of these showed detectable colocalization with Lysotracker (Fig. 1C).
Fig. 1.
In vitro survival of BCG-M and BCG-S in BMM. (A) Bone marrow derived macrophages were infected at an MOI of 10:1, and CFU counts were obtained at various time points after infection. Data shown are representative of 3 independent experiments (*p < 0.05, **p < 0.01, ***p < 0.005). (B) Bone marrow derived macrophages were infected with BCG-M or BCG-S labeled with CFSE (indicated by green or yellow color) and stained with Lysotracker dye (red color) at 24 h after infection, followed by analysis using fluorescence microscopy (magnification 20×). Representative images are shown from 2 independent experiments that yielded similar results. White arrows indicate CFSE-labeled bacteria. (C) Quantification of co-localization of Lysotracker with CFSE stained BCG (***p < 0.0001). Note that the Pasteur strain of BCG was used in the experiments shown in this figure, and in all subsequent figures except where otherwise state.
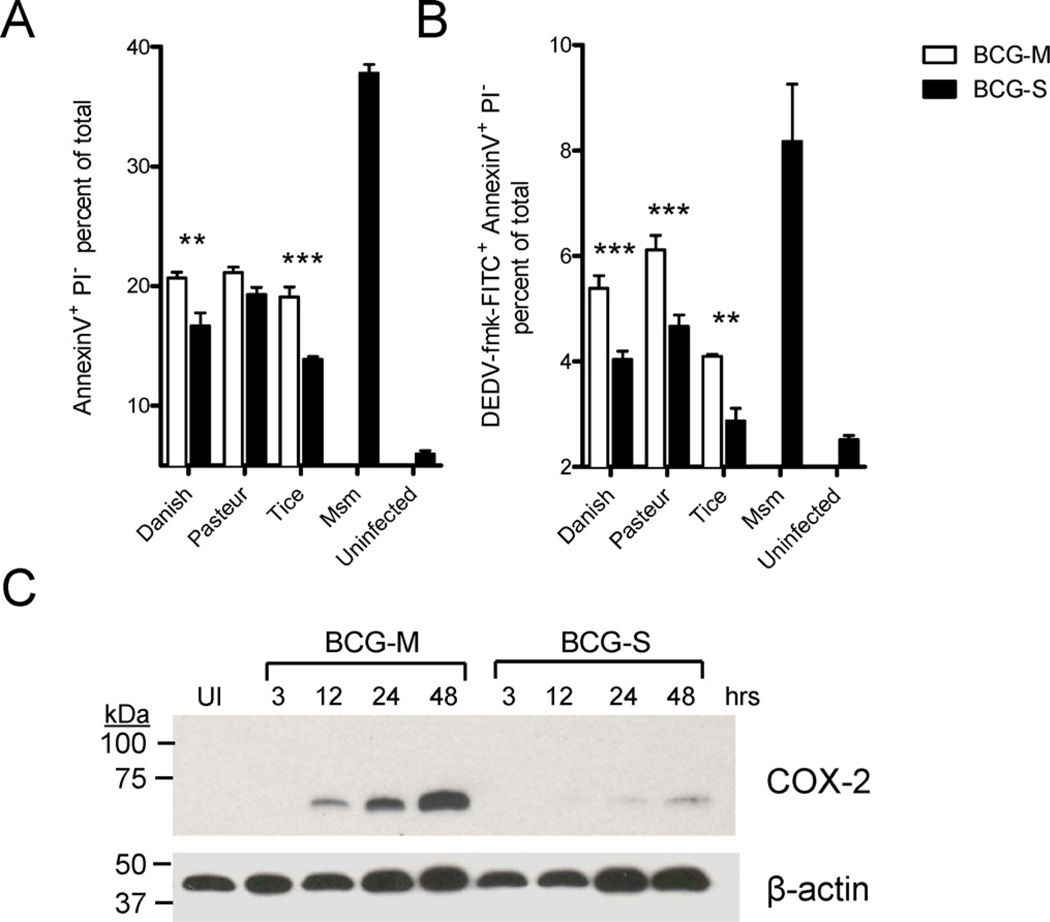
The induction of apoptosis by infected macrophages has been linked to bactericidal effects that promote the killing of intracellular mycobacteria [17]. Since we observed a significant difference in the survival in macrophages of BCG cultivated in the two growth conditions, we assessed whether these differences correlated with effects on inhibition of the ability of the host cell to initiate programmed cell death. For this purpose we used staining of BCG-infected THP-1 macrophages with Annexin V, propidium iodide and a fluorescent inhibitor of caspase activation (FLICA) reagent that specifically labels active caspase-3. Consistent with earlier reports, we found that BCG was in general a relatively weak inducer of apoptosis compared to some other mycobacterial species, e.g Mycobacterium smegmatis (Supplementary Fig. 2) [18]. However, our results showed that BCG-M promoted stronger staining of the cell surface with Annexin V and more pronounced intracellular caspase-3 activation compared to BCG-S (Fig. 2A and B, and Supplementary Fig. 2).
Fig. 2.
Increased apoptosis of host cells with infection by BCG-M. (A and B) THP-1 cells were infected with BCG-M or BCG-S and analyzed 72 h later by FACS for the induction of apoptosis by staining with Annexin V-Alexa Fluor® 647 and Propidium Iodide (A), and FITC-DEVD-fmk (B) (**p < 0.01, ***p < 0.001). (C) BMMs were infected and lysates obtained at various time points to measure COX-2 expression levels by Western Blot. Each lane was loaded with an equal amount of lysate comprising 3 × 106 cell equivalents and normalized according to the signal for the beta-actin control. UI indicates uninfected BMMs. Results shown are representative of two experiments.
It has been shown that induction of cyclooxygenase-2 (COX-2) is a hallmark of pro-apoptotic species or strains of mycobacteria [19]. Consistent with the increased induction of macrophage apoptosis by BCG-M, we found that BCG-S markedly decreased the induction of COX-2 in BMMs as compared to BCG-M at 12–48 h following infection (Fig. 2C). This further supported the conclusion that BCG-M had a decreased ability to block macrophage apoptosis compared to BCG-S.
3.2. Elevated inflammatory cytokine levels and increased bacterial persistence in mice infected with BCG-S
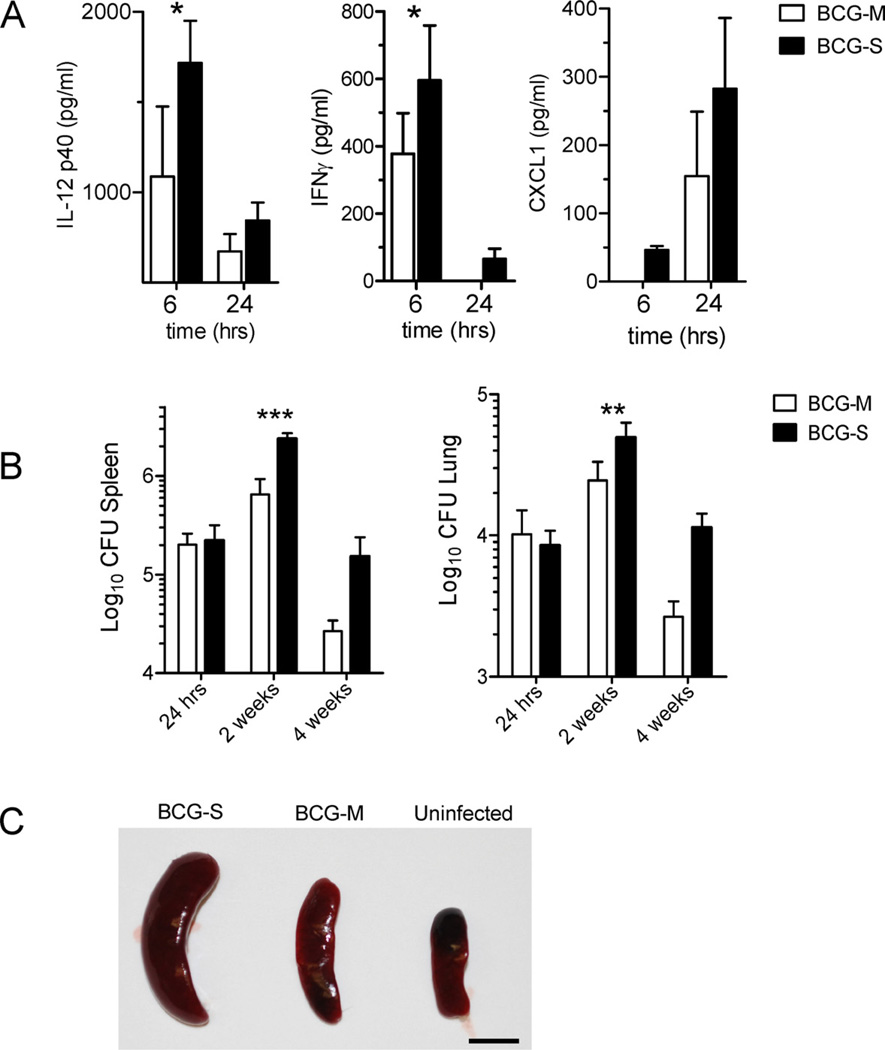
The predominant cytokine response to mycobacterial infection in mice and humans is of the Th1 type, characterized by IFN-γ, IL-12 and TNF [20,21]. These cytokines orchestrate the initial inflammatory responses in the infected tissue, which paves the way for an antigen specific adaptive T cell response. Comparing the cytokines induced in the sera of mice infected by BCG grown in different media using a multiplex cytokine detection method, we found that IFN-γ, IL-12p40 and the chemokine CXCL1 were expressed at higher levels in mice vaccinated with BCG-S as compared to those vaccinated with BCG-M (Fig. 3A). In addition to these cytokines, we also detected IL-10 but this was produced at similar levels in mice vaccinated with the two different BCG preparations (data not shown). Interferon-γ was detectable at relatively high levels as early as 6 h after vaccination, which may indicate that it was derived from innate immune effector cells such as natural killer (NK) and natural killer T (NKT) cells. A similar early response was seen with IL-12p40, which is normally released by APCs like dendritic cells (DCs). The chemokine CXCL1, which appeared to peak at later time points and also showed a trend toward increased production in BCG-S infected mice, has been implicated in the recruitment of myeloid and lymphoid cells to the site of infection.
Fig. 3.
Increased inflammatory cytokine response and enhanced bacterial survival in vivo with BCG-S. (A) Cytokine levels in sera of mice infected i.v. with either 1 × 106 BCG-M or BCG-S were analyzed by multiplex capture ELISA at 6 h and 24 h post infection. Results are shown for IL-12p40, IFNγ, and CXCL1, which were the only three cytokines in the 10-plex analysis system that showed significantly different levels betweens the two groups of mice. Bars represent means of cytokine levels from 3 mice in each group, and error bars show standard errors. (B) In vivo CFU loads of BCG in the spleens (left) and lungs (right) after intravenous infection of C57BL/6 mice with 1 × 106 CFU of BCG-M or BCG-S. Results are averages from 3 mice in each group. For (A) and (B): *p < 0.05, **p < 0.01, ***p < 0.001. (C) Representative spleens are shown from C57BL/6 mice that were infected intraperitoneally with either 5 × 106 CFU of BCG-S or BCG-M for 2 weeks, or from a mouse that received only saline injection (uninfected). Scale bar corresponds to 1 cm.
Based on the increased survival in vitro, inhibition of apoptosis and COX-2 induction by BCG-S, we hypothesized that BCG-S would be more persistent in vivo than BCG-M. Indeed, we found significantly higher levels of CFUs in the lungs and spleens of BCG-S infected mice, especially at 2 weeks post infection (Fig. 3B). Additionally, we observed markedly enlarged livers (data not shown) and spleens in mice that were infected by intravenous inoculation with BCG-S as compared with organs from mice similarly infected with BCG-M (Fig. 3C). Taken together, these results clearly suggested that the ex vivo growth of BCG in Sauton medium rendered the bacilli more resistant to killing after their uptake by phagocytes. This increased ability of BCG-S to persist in mice was part of a phenotype that more closely resembled that of fully virulent mycobacteria such as M. tuberculosis.
3.3. Elevated T cell responses to mycobacterial antigens in mice infected with BCG-S
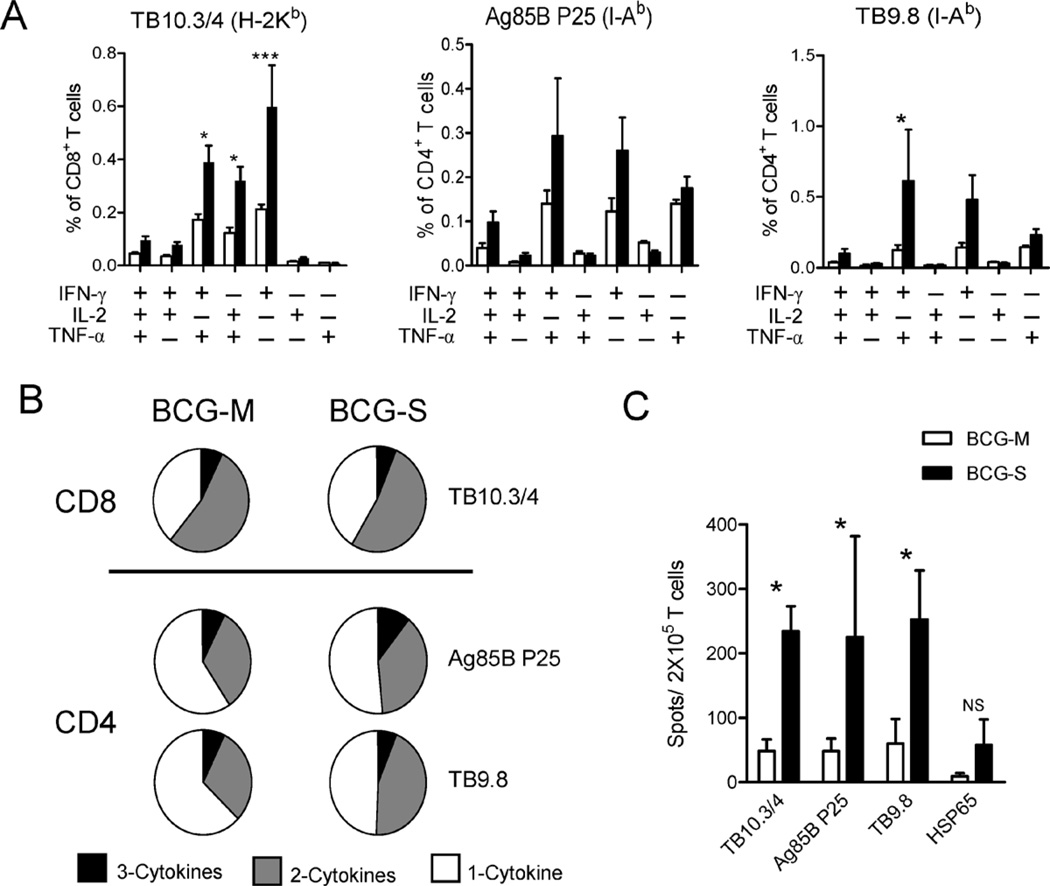
We reasoned that the significant differences in cytokine responses induced by the BCG grown in different conditions may have an impact on the magnitude or quality of T cell activation. Since multifunctional Th1 cells simultaneously secreting these three cytokines have been correlated in a published study with protection against M. tuberculosis challenge in BCG immunized mice [22], we focused particularly on the differences in the multifunctional T cell compartment in mice immunized with either BCG-M or BCG-S. We infected C57BL/6 mice by intraperitoneal injection of BCG-S or BCG-M, and analyzed the CD4+ and CD8+ T cell responses to specific mycobacterial antigens using intracellular cytokine staining for IFN-γ, TNF and IL-2 and multiparameter flow cytometry [22]. Splenocytes were stimulated with two MHC class II restricted (I-Ab) epitopes (P25 from Ag85B and Peptide 10 of TB9.8) and with one MHC class I restricted (H-2Kb) presented epitope of the protein TB10.3/4. T cells from BCG-S infected mice showed increased antigen specific responses to all of these epitopes, reflecting increases in the frequencies of most of the cytokine producing subsets assessed by this analysis (Fig. 4A). The overall proportions of antigen responsive T cells producing one, two or three of the cytokines measured were calculated. This showed marked similarity for CD8+ T cells from BCG-S and BCG-M infected mice. However, CD4+ T cell responses to both epitopes studied showed a moderate shift toward greater multifunctionality in BCG-S infected mice (Fig. 4B). Additionally, we found higher frequencies of IFNγ producing T cells from the BCG-S immunized mice in ELISPOT assays in response to the three epitopes used in the ICS analysis, and a trend in this direction was also seen for responses to recombinant Mtb Hsp65 protein which contains both MHC class I and II presented epitopes (Fig. 4C). This effect was not limited to a single BCG strain as we observed significantly enhanced T-cell responses to BCG-S across three distinct strains (Supplementary Fig. 3). Overall, mice immunized with BCG-S showed elevated levels of cytokine producing CD4+ and CD8+ T cells and a shift to greater multifunctional cytokine secretion in the CD4+ compartment compared to mice immunized with BCG-M.
Fig. 4.
Induction of more robust T cell responses to mycobacterial antigens by BCG-S. (A) Multiparameter FACS with intracellular staining for cytokines in splenocytes from animals immunized 2 weeks previously with 5 × 106 CFU of BCG-M (open bars) or BCG-S (filled bars) intraperitoneally, and restimulated in vitro with peptides corresponding to epitopes of the indicated mycobacterial antigens plus soluble anti-CD28 mAb. The graphs show the percentages of total CD8+ T cells (for TB10.3/10.4) or CD4+ T cells (for Ag85 P25 and TB9.8) producing IFNγ, IL-2 or TNFα and combinations of two or three of these cytokines; *p < 0.05, ***p < 0.001. (B) Pie charts summarizing frequencies of cells producing one, two or three cytokines in the experiment shown in A. (C) ELISPOT assay for IFNγ producing cells in splenocytes from mice immunized i.p. with 5 × 106 CFU of either BCG-M or BCG-S 2 weeks earlier. The cells were stimulated in vitro with peptides corresponding to epitopes for the indicated mycobacterial antigens recognized by CD4+ T cells (Ag85B, TB9.8) or CD8+ T cells (TB10.3/4) as indicated, and with recombinant Hsp65 protein that contains epitopes for both CD4+ and CD8+ T cell responses; *p < 0.05. All results shown are means and SD for groups of 5 mice immunized with each BCG preparation.
3.4. Better protection against virulent M. tuberculosis in BCG-M vaccinated mice
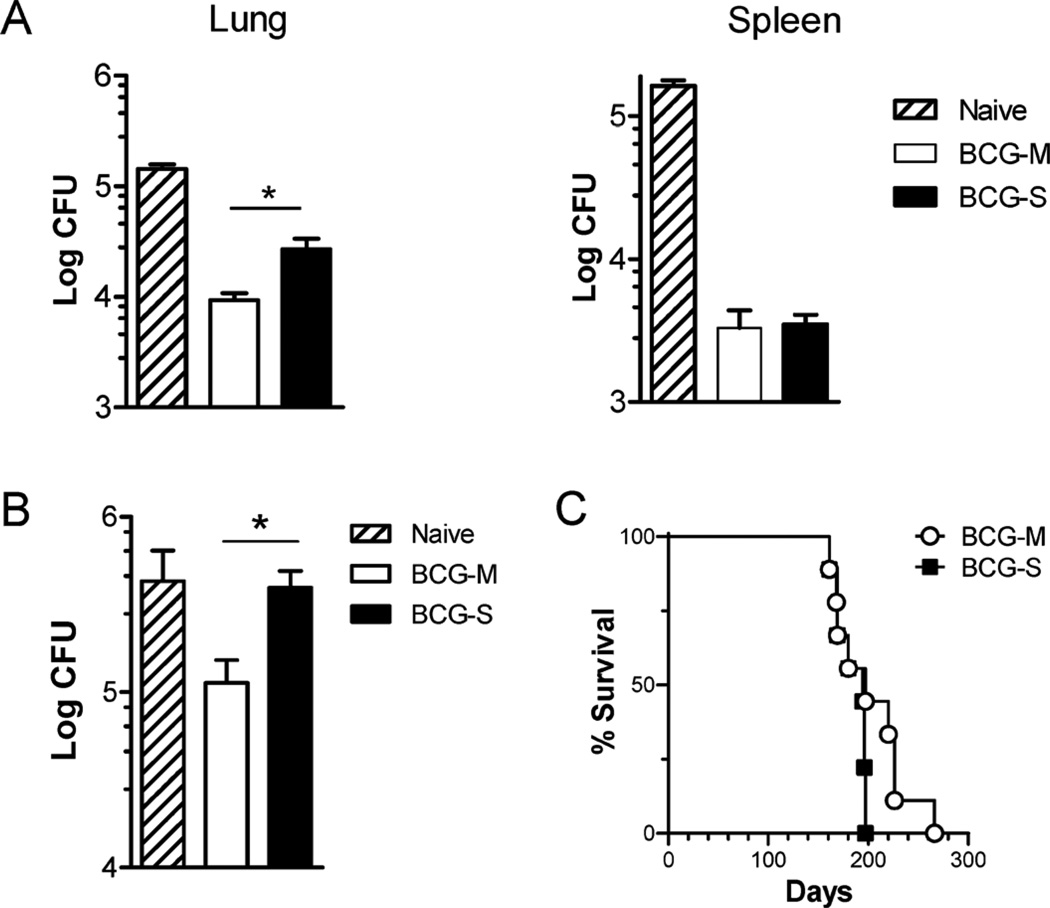
Having observed a pronounced difference in the T cell responses among mice immunized with the two BCG preparations, we tested if these differences could influence the level of protection against a virulent MTb challenge. Surprisingly, despite the finding that mice immunized with BCG-S showed more robust T cell responses, we observed that the mice immunized with BCG-M showed significantly better control of Mtb infection in their lungs and equivalent control of infection in their spleens compared to mice immunized with BCG-S (Fig. 5A). Moreover, using an adoptive transfer protocol in which T cells purified from immunized mice were transferred into unimmunized recipients, we also tested the ability of memory T cells from long-term immunized mice to protect naïve recipient mice against virulent MTb. This also supported the conclusion that BCG-M immunized mice developed superior protective immunity against Mtb, as naïve animals that received T cells from BCG-M immunized mice showed lower CFU counts of Mtb in their lungs than mice that received T cells from BCG-S immunized mice (Fig. 5B).
Fig. 5.
Improved vaccine efficacy against M. tuberculosis challenge with BCG-M. C57BL/6 mice were vaccinated subcutaneously with 1 × 106 CFU of BCG-M or BCG-S, or sham-vaccinated with saline. Two months later, animals were challenged by aerosol infection with 50–100 CFU of virulent M. tuberculosis (strain H37Rv). (A) Bars show means and SD for CFU of M. tuberculosis in lungs (left) and spleens (right) at 3 months after challenge for groups of 5 mice. Results shown are representative of two independent experiments. (B) CFU counts from lungs of mice challenged with M. tuberculosis after adoptive transfer of T cells from donor mice immunized with either BCG-M or BCG-S (1 × 106 CFU subcutaneously) or sham immunized with saline (naïve) mice as indicated. Results shown are representative of two independent experiments, each with 5 mice per group. In (A) and (B), *p < 0.05 (unpaired t test). (C) Survival curves for SCID mice (N = 9 mice per group) after i.v. infection with 1 × 106 BCG-M or BCG-S. Differences between the survival curves were not significant (p > 0.05, Log rank test).
In addition to protective efficacy, another important aspect to consider in the assessment of TB vaccines is safety in immuno-compromised hosts, particularly with live vaccines derived from pathogenic organisms as is the case for BCG. We thus evaluated the virulence of BCG-S versus BCG-M by inoculation of severely immunodeficient SCID mice, which lack T cells and B cells. This experiment showed no significant difference in the survival of SCID mice infected with BCG-M or BCG-S (Fig. 5C), indicating that the two BCG preparations were similar with respect to their capacity to cause disease in hosts lacking adaptive immune responses. This supported the view that the differences observed with BCG cultured in the different growth medium did not affect the safety of the BCG vaccine.
3.5. BCG-M primes stronger Th17 responses than BCG-S
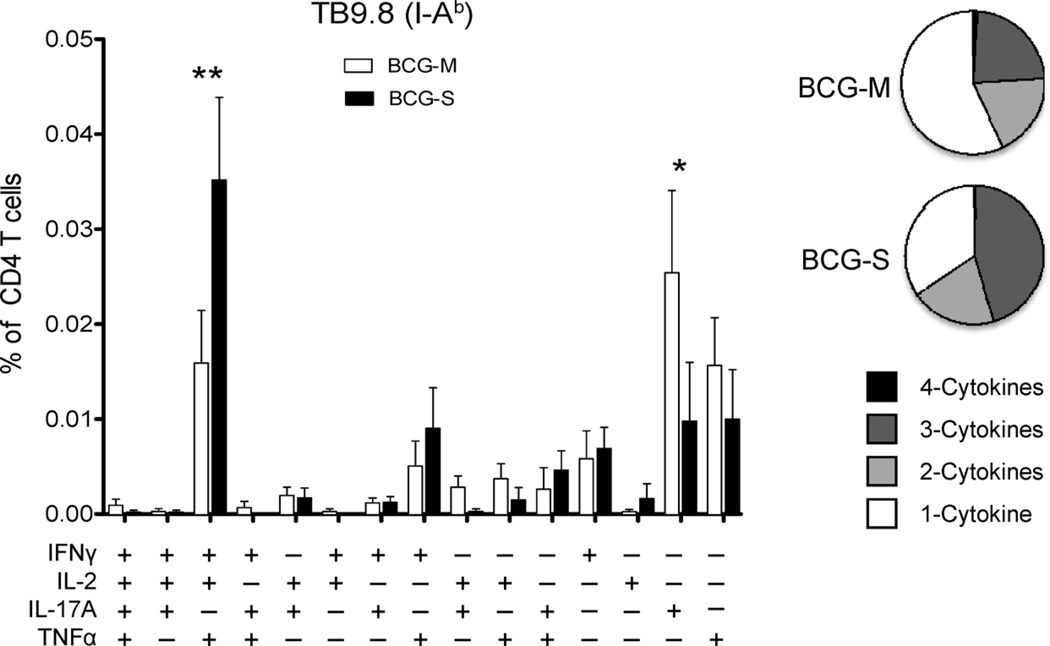
IL-17 producing CD4 (Th17) T cells have been suggested as a correlate of vaccine mediated protection, accelerating the emergence of protective Th1 cells in lungs of Mtb infected animals [23]. In order to evaluate vaccine-induced antigen-specific Th17 responses, we immunized mice subcutaneously with 1 × 106 BCG-M or BCG-S. After 8 weeks, we restimulated splenic T-cells with the I-Ab restricted TB9.8 epitope peptide as described earlier, and used multiparameter FACS analysis to quantitate cells producing IFNγ, IL-2, TNFα and IL-17A. As in our studies of mice infected by the i.p. route (Fig. 4), we again observed that there was a shift toward greater multifunctionality in mice receiving BCG-S compared to BCG-M (Fig. 6). However, BCG-M immunized mice showed significantly greater proportions of IL-17 producing CD4+ T cells (Fig. 6). In summary, the comparison between BCG-M and BCG-S vaccinated animals revealed no correlation between levels of multifunctional T cells in the spleens and the superior protection induced by BCG-M. However, enhanced Th17 responses in the spleen appeared to correlate well with enhanced vaccine induced protection in BCG-M vaccinated mice.
Fig. 6.
Induction of stronger Th17 responses by BCG-M. Multiparameter FACS with intracellular staining for cytokines in splenocytes from animals immunized 8 weeks previously with 1 × 106 CFU of BCG-M or BCG-S subcutaneously, and restimulated in vitro with I-Ab restricted TB9.8 peptide plus soluble anti-CD28 mAb. The bar graph shows the percentages of total CD4+ T cells producing IFNγ, IL-2, IL-17A or TNFα and combinations of two or three or four of these cytokines. Open bars correspond to BCG-M immunized mice, and filled bars are BCG-S vaccinated mice (N = 5 mice per group; *p < 0.05, **p < 0.005). Pie charts show proportions of CD4+ cells producing one, two, three or four of the cytokines analyzed following stimulation with TB9.8 peptide.
3.6. Distinct humoral responses to BCG-S versus BCG-M
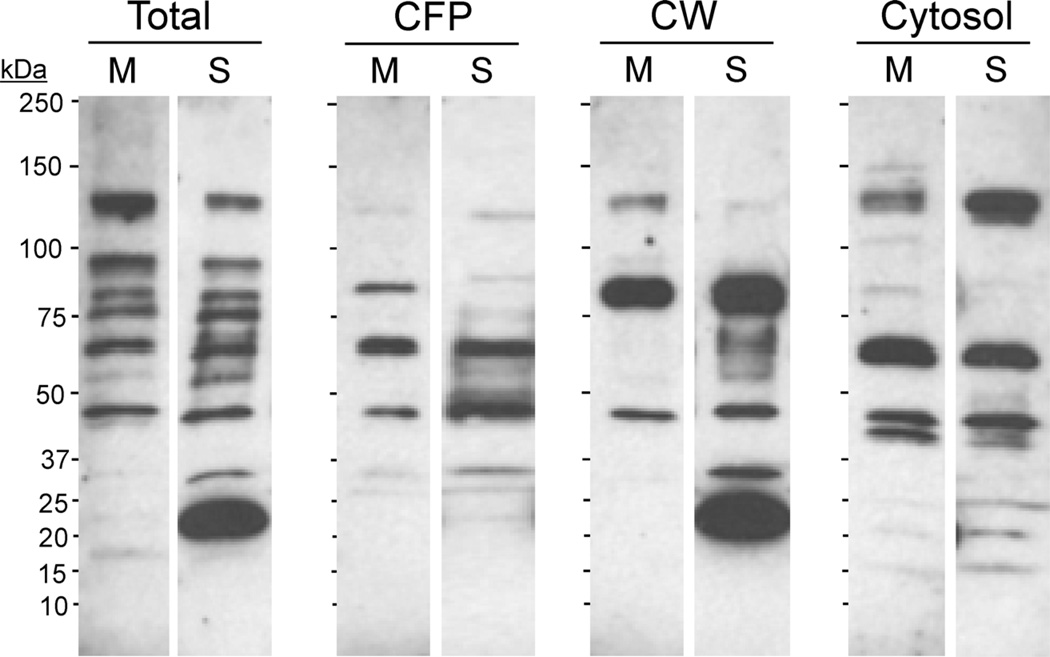
Similar to previous reports on the differences in antibody responses induced by BCG vaccine when grown in different culture media [11], we observed that the sera from mice immunized with either BCG-M or BCG-S showed distinct patterns of antibody reactivities when used to probe various fractions from M. tuberculosis in Western blotting. The most remarkable difference that was observed was in the serum antibody reactivity with a MTb cell wall associated protein with apparent molecular mass of approximately 20 kDa. This was detected strongly by sera from BCG-S immunized mice, but minimally or not at all by sera from BCG-M immunized animals (Fig. 7). The difference in the level of antibody production against this target may reflect markedly increased expression of this protein antigen when BCG is grown in Sauton medium. Alternatively, it could be related to differences in protein secretion by the mycobacteria which have been previously found to be influenced by the composition of the growth media [12]. Although the target protein has not been definitively identified, its size, immunogenicity and cell wall localization would be consistent with the 19 kDa lipoprotein, which is an extensively studied antigen and immunomodulatory component of Mtb and BCG.
Fig. 7.
Distinct humoral responses induced by BCG-S and BCG-M. Pooled sera from five mice infected 2 weeks earlier with 5 × 106 CFU i.p. of either BCG-M (indicated by M above relevant lanes) or BCG-S (indicated by S) were analyzed for antibody reactivity by Western blotting of M. tuberculosis proteins. Blots show reactivity with total Mtb lysate (Total), culture filtrate proteins (CFP), cell wall fraction (CW) or cytosolic fraction (Cytosol) of M. tuberculosis.
4. Discussion
Despite the fact that the BCG vaccine has been administered to people for nearly a century, the factors influencing its protective efficacy against Mtb infection remain poorly understood. In the current study, we have examined the potential impact of the growth medium used to propagate BCG on its subsequent behavior in infected cells and animals. We were motivated to carry out this study by the observation that many vaccine producers have used and continue to use Sauton medium for growth of production lots of BCG for administration to humans, whereas the majority of laboratory investigators propagate BCG in Middlebrook 7H9 medium. Our results presented in this study demonstrated that these two media have a significant impact on the ability of the BCG strain to survive in infected macrophages and mice. Thus, growth in Sauton medium conferred a relative resistance to BCG against killing by primary macrophages, which correlated with an ability to grow and persist longer in mice when compared to the same BCG strain grown in Middlebrook 7H9 medium. These differences were associated with alterations in both the cellular and humoral immune responses to BCG, and with different levels of protective vaccine efficacy in the mouse M. tuberculosis vaccination/challenge model.
Previous reports have established that attenuated strains of mycobacteria induce higher COX-2 expression resulting in increased apoptosis of macrophages, while virulent strains show lower levels of COX-2 induction and reduced apoptosis [19]. The combination of enhanced replication in macrophages, persistence in vivo and lower levels of COX-2 induction with reduced apoptosis by BCG-S resembles the characteristics of virulent mycobacteria [24]. The higher bacterial load in combination with a strong pro-inflammatory cytokine response is likely to result in higher T cell activation in BCG-S immunized mice, which was reflected by our studies using ICS and ELISPOT to quantitate and characterize T cell responses against several defined mycobacterial epitopes. In addition, it has been shown that IL-12 and IFNγ play an important role in the protective immunity induced by BCG in humans, along with an IFNγ independent role of CD4+ T cells that has been reported in the mouse model [25–27]. Accordingly the augmented pro-inflammatory cytokine profiles and higher T cell activation that we observed might have been predicted to translate in better protection by the BCG-S. However, this did not prove to be the case, as the higher frequencies of multifunctional T cells induced by BCG-S did not appear to translate into better protection against a challenge with MTb. Surprisingly, mice immunized with BCG-M actually showed better control of subsequent Mtb challenge, despite the fact that we consistently detected weaker vaccine-induced Th1 responses in these animals. Similarly, the T cells induced by BCG-S did not protect better than those by BCG-M in a T cell adoptive transfer experiment in naive mice.
Although the presence of multifunctional T cells following BCG vaccination has been associated with protection against Mtb in mice [22], this remains an area of controversy that requires additional investigation. One study in mice reported that the presence of multifunctional T cells in the lungs but not in the spleen correlated with protection against Mtb challenge [28,29], although this was in the setting of an intranasal boost with viral vectors expressing a single mycobacterial antigen. Subsequent work has confirmed that systemic multifunctional T cell responses are not a clear correlate of protective immunity against TB in vaccinated mice [29]. Moreover, it has been demonstrated that the multifunctional CD4+ T cell frequencies were elevated in the blood of patients with TB compared with household contacts who were exposed to infection but did not develop disease, suggesting that these enhanced responses may not be highly protective against TB [30]. Our study found that T cells producing one, two or three Th1-type cytokines were all more abundant with a shift toward a greater proportion of multifunctional CD4+ T cells in BCG-S immunized mice. Since BCG-S vaccination protected less well against Mtb challenge, our T cell analysis was consistent with the earlier reports showing a lack of correlation between systemic multifunctional T cell responses and control of Mtb infection. Nevertheless, it was surprising to us that the BCG-S vaccinated animals, which had such robust T cell responses to mycobacterial antigens, showed inferior protection against Mtb challenge when compared to BCG-M vaccinated mice.
It has been reported that Th1 responses against dominant mycobacterial antigens can act as decoys that divert the immune response from other potential antigens that may be more capable of stimulating protective immunity [31–33]. In support of this idea, a recent study has put forth data favoring a hypothesis for how hyper-conservation of human T cell epitopes among different strains of MTb can be interpreted as evidence of a distinct evolutionary strategy for immune subversion [34]. Additionally, multifunctional T cells reactive to immunodominant antigens such as ESAT6 or CFP10 have been associated with progression of disease [35]. Consistent with reports from Cooper and colleagues, we observed that mice with higher frequencies of IL-17A producing CD4 T cells following vaccination were better protected from an aerogenic challenge with Mtb [36]. It has also been shown that BCG-specific Th17 cells can offer immuno-deficient animals protection from Mtb challenge in the absence of IFNγ [37], and that mice lacking IL-17A are unable to develop a granulomatous response capable of controlling the spread of infection [38]. These models suggest that an expanded population of IL-17 producing CD4+ T cells respond quickly to aerogenic Mtb infection and traffic to the lung where they accelerate the recruitment of effector cells into involved tissues. These recruited effectors include neutrophils, macrophages, IFNγ-producing Th1 cells and potentially other cell types that contribute to the development of a granulomatous inflammatory lesion capable of restraining bacterial growth [36,38]. Our observation that BCG-M vaccinated mice have a significantly larger pool of antigen-specific IL-17A producing CD4 T cells is consistent with this model, and may explain the enhanced protection versus BCG-S immunized mice.
In light of these findings, we hypothesize that in spite of the high levels of Mtb reactive and cytokine producing T cells induced by BCG-S, a vaccine grown in the Middlebrook 7H9 medium may actually induce a more favorable immune response that could increase the efficacy of BCG against Mtb infection in animal models and potentially in humans. Although we confined this study to two types of growth media, the results suggest the need for a broader assessment of the impact of other media compositions and growth conditions on the performance of BCG vaccines. In addition, it is possible to envision potential effects on safety and toxicity of BCG vaccines that may be associated with the media or other growth conditions. For example, many studies have reported local adverse effects following BCG vaccination in humans, such as injection site abscess, lymphadenitis and adenopathy [39–41]. Interestingly, we found that BCG-S induced exaggerated inflammation of the organs in mice as compared with the BCG-M, which could potentially be associated some of these adverse effects.
Overall, the findings presented in the current study support the idea that Sauton medium, which is frequently used in the production of clinical grade BCG vaccines, induces BCG to acquire properties more similar to virulent mycobacteria in terms of its ability to withstand unfavorable conditions in the host cell and modulate immune responses. Thus, consideration should be given to examining the effects of growth media on the efficacy of BCG in clinical studies, and to the possibility that BCG grown in Middle-brook 7H9 or other defined media may yield better vaccine efficacy. It has been predicted that a 1% improvement in protective efficacy would prevent approximately 83,000 cases of tuberculosis each year while saving 18,000 lives [42]. Taking this into account, we suggest that by appropriately modifying the culture medium for the current BCG vaccine, an improvement in its protective efficacy that would lead to meaningful clinical impact might be attained. Moreover, in recent years many genetically modified live-attenuated vaccines for TB have been developed, and these may show similar effects of growth conditions on subsequent immunogenicity and protective efficacy in vivo. The results of the current study emphasize the importance of identifying the most favorable culture medium for preparing laboratory and clinical grade production lots to be used in initial testing and subsequent administration of such novel vaccines.
Supplementary Material
Acknowledgements
This work was supported by NIH/NIAID grants PO1AI063537 (SAP, WRJ and JC) and 1RO1AI093649 (SAP). MG was supported by NIH Training Grant 5T32CA009173. Flow cytometry studies were carried out using core facilities supported by the Einstein Cancer Center (NIH/NCI CA013330) and the Einstein Center for AIDS Research (NIH AI-51519).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2011.12.044.
References
- 1.Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuberc Lung Dis. 1999;79(4):243–250. doi: 10.1054/tuld.1999.0206. [DOI] [PubMed] [Google Scholar]
- 2.Ritz N, Curtis N. Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb) 2009 July;89(4):248–251. doi: 10.1016/j.tube.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995 November;346(8986):1339–1345. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. J Am Med Assoc. 1994 March;271(9):698–702. [PubMed] [Google Scholar]
- 5.Russell DG, Barry CE, III, Flynn JL. Tuberculosis: what we don’t know, can, and does, hurt us. Science. 2010 May;328(5980):852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung AS, Tran V, Wu Z, Yu X, Alexander DC, Gao GF, et al. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics. 2008;9:413. doi: 10.1186/1471-2164-9-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA. 2007 March;104(13):5596–5601. doi: 10.1073/pnas.0700869104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonah C. Packaging BCG: standardizing an anti-tuberculosis vaccine in interwar Europe. Sci Context. 2008 June;21(2):279–310. doi: 10.1017/s0269889708001725. [DOI] [PubMed] [Google Scholar]
- 9.Eickhoff TC. The current status of BCG immunization against tuberculosis. Annu Rev Med. 1977;28:411–423. doi: 10.1146/annurev.me.28.020177.002211. [DOI] [PubMed] [Google Scholar]
- 10.Florio W, Batoni G, Esin S, Bottai D, Maisetta G, Favilli F, et al. Influence of culture medium on the resistance and response of Mycobacterium bovis BCG to reactive nitrogen intermediates. Microbes Infect. 2006 February;8(2):434–441. doi: 10.1016/j.micinf.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Petricevich VL, Ueda C, Alves RC, da Silva MA, Moreno C, Melo AR, et al. A single strain of Mycobacterium bovis bacillus Calmette-Guerin (BCG) grown in two different media evokes distinct humoral immune responses in mice. Braz J Med Biol Res. 2001 January;34(1):81–92. doi: 10.1590/s0100-879x2001000100010. [DOI] [PubMed] [Google Scholar]
- 12.Converse SE, Cox JS. A protein secretion pathway critical for Mycobacterium tuberculosis virulence is conserved and functional in Mycobacterium smegmatis. J Bacteriol. 2005 February;187(4):1238–1245. doi: 10.1128/JB.187.4.1238-1245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatfull G. Molecular genetics of Mycobacteria. ASM Press; 2000. [Google Scholar]
- 14.Skinner PS, Furney SK, Jacobs MR, Klopman G, Ellner JJ, Orme IM. A bone marrow-derived murine macrophage model for evaluating efficacy of antimycobacterial drugs under relevant physiological conditions. Antimicrob Agents Chemother. 1994 November;38(11):2557–2563. doi: 10.1128/aac.38.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salem ML, Al-Khami AA, El-Naggar SA, Diaz-Montero CM, Chen Y, Cole DJ. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J Immunol. 2010 February;184(4):1737–1747. doi: 10.4049/jimmunol.0902309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding ZC, Blazar BR, Mellor AL, Munn DH, Zhou G. Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 2010 March;115(12):2397–2406. doi: 10.1182/blood-2009-11-253336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan H, Lee J, Ren F, Chen M, Kornfeld H, Remold HG. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol. 2008 October;9(10):1189–1197. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007 August;117(8):2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008 November;205(12):2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008 December;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995 June;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 22.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 July;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 23.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010 December;21(6):455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behr M, Schurr E, Gros P. TB: screening for responses to a vile visitor. Cell. 2010 March;140(5):615–618. doi: 10.1016/j.cell.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998 May;280(5368):1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 26.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, et al. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996 December;335(26):1941–1749. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 27.Cowley SC, Elkins KL. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol. 2003 November;171(9):4689–4699. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 28.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008 October;181(7):4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchilian EZ, Desel C, Forbes EK, Bandermann S, Sander CR, Hill AV, et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant (delta)ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect Immun. 2009 February;77(2):622–631. doi: 10.1128/IAI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol. 2009 March;39(3):723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 31.Hovav AH, Mullerad J, Davidovitch L, Fishman Y, Bigi F, Cataldi A, et al. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect Immun. 2003 June;71(6):3146–3154. doi: 10.1128/IAI.71.6.3146-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delogu G, Brennan MJ. Comparative immune response to PE and PE PGRS antigens of Mycobacterium tuberculosis. Infect Immun. 2001 September;69(9):5606–5611. doi: 10.1128/IAI.69.9.5606-5611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen P. Vaccine strategies against latent tuberculosis infection. Trends Microbiol. 2007 January;15(1):7–13. doi: 10.1016/j.tim.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyper-conserved. Nat Genet. 2010 June;42(6):498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young JM, Adetifa IM, Ota MO, Sutherland JS. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS ONE. 2010;5(6):e11237. doi: 10.1371/journal.pone.0011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007 April;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 37.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun. 2010 October;78(10):4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010 April;184(8):4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 39.Lotte A, Wasz-Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res. 1984;21:107–193. [PubMed] [Google Scholar]
- 40.Guld J, Magnus K, Tolderlund K, Biering-Sorensen K, Edwards PQ. Suppurative lymphadenitis following intradermal B.C.G. vaccination of the newborn; a preliminary report. Br Med J. 1955 October;2(4947):1048–1054. doi: 10.1136/bmj.2.4947.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lotte A, Wasz-Hockert O, Poisson N, Engbaek H, Landmann H, Quast U, et al. Second IUATLD study on complications induced by intradermal BCG-vaccination. Bull Int Union Tuberc Lung Dis. 1988 June;63(2):47–59. [PubMed] [Google Scholar]
- 42.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003 May;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.