Abstract
Dibenzo[def,p]chrysene (DBC) is a transplacental carcinogen in mice (15 mg/kg; gestation day (GD) 17). To mimic residual exposure throughout pregnancy, dams received 4 smaller doses of DBC (3.75 mg/kg) on GD 5, 9, 13 and 17. This regimen alleviated the previously established carcinogenic responses in the thymus, lung, and liver. However, there was a marked increase in ovarian tumors (females) and hyperplastic testes (males). [14C]-DBC (GD 17) dosing revealed transplacental distribution to fetal tissues at 10-fold lower concentrations than in paired maternal tissue and residual [14C] 3 weeks post dose. This study highlights the importance of developmental stage in susceptibility to environmental carcinogens.
Keywords: transplacental cancer, PAHs, T-cell lymphoma
INTRODUCTION
The “Fetal Basis of Adult Disease” or Barker Hypothesis is relevant to a number of chronic diseases in humans including diabetes, asthma, cardiovascular disease, and cancer as well as neurological and behavior toxicities [1–4]. With respect to cancer, a number of epidemiology studies have documented that exposure of pregnant women to environmental carcinogens such as cigarette smoke enhances the risk for the offspring of developing a number of cancers [5–7]. Animal models have documented that a number of chemicals are transplacental carcinogens including the tobacco specific nitrosamine NNK, the cooked meat mutagen PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine), benzene, arsenic, phenytoin, drugs targeting HIV and polycyclic aromatic hydrocarbons (PAHs) [8–16]. PAHs have been documented to be bioavailable to the fetus and to generate DNA damage [17–23]. Dibenzo[def,p]chrysene (DBC) is a potent environmental PAH in animal models of carcinogenesis and has recently been reclassified by the International Agency for Research on Cancer (IARC) from possible carcinogenic (2B) to probably carcinogenic in humans (2A) [24]. Our laboratory has demonstrated that exposure of pregnant mice (C57B6129F1 dams bred to 129 males) to a single dose of DBC (15 mg/kg by gavage) during late pregnancy was carcinogenic to the offspring [16]. Over half the offspring had to be euthanized due to an aggressive T-cell lymphoblastic lymphoma that invaded almost every organ. The surviving mice at 10 months had a 100% incidence of pulmonary tumors (multiplicity of about 15) and approximately 70% of the males (and almost no females) had liver lesions (primarily precancerous lesions and adenomas) [16]. Subsequent studies with cyp1b1 knockout mice demonstrated that fetal Cyp1b1 was required for development of the T-cell lymphoma [25]. Cross-fostering studies further demonstrated that the 2–3 day in utero exposure to DBC resulted in much higher lymphoma-associated mortality than exposure to residual DBC in the breast milk over the 3 weeks of nursing [26].
Developmental expression of CYPs in the 1 family is important in the transplacental toxicities of PAHs [27]. Studies with expressed mouse and human CYPs have found that CYP1A1 and CYP1B1 are effective in the bioactivation of PAHs to mutagenic and carcinogenic metabolites, primarily diol-epoxides. For example, benzo[a]pyrene-7,8-trans-dihydrodiol-9,10-syn-epoxide (BPDE) is one of the more potent mutagenic products of Cyp metabolism of benzo[a]pyrene and forms DNA adducts, primarily at 2′-deoxyguanosine and 2′-deoxyadenosine [28]. Expressed Cyp1a2 exhibits lower activity toward PAHs than other members of the Cyp1 family [28,29] and is not expressed at appreciable levels in the mouse until after birth [30]. Surprisingly, Cyp1a1 (not expressed constitutively in liver but inducible by aryl hydrocarbon receptor (Ahr) ligands, including PAHs) activity appears to provide protection against PAH toxicity as Cyp1a1 null mice exhibited enhanced sensitivity to PAHs [31]. Cyp1b1, like Cyp1a1, is not expressed constitutively in liver but is inducible via the Ahr (although not to the same degree as Cyp1a1); rather it is found constitutively in a number of extrahepatic tissues including testis, ovary, thymus, breast and prostate [32] and is expressed at high levels in the third trimester of pregnancy [30].
As the major enzymes involved in PAH bioactivation are expressed in a tissue- and developmental-specific manner during embryogenesis, and to better model human exposures, we examined DBC transplacental carcinogenesis when maternal exposure occurred during all trimesters. For comparison with a single 15 mg/kg dose on GD 17, the dose was divided into 4 smaller doses administered (3.75 mg/Kg by gavage) on GDs 5, 9, 13 and 17. These periods cover all three trimesters including the first which is often the most sensitive to teratogenic effects. We report here that this multiple-dosing regimen of DBC to the pregnant mouse produced a marked alteration in the carcinogenic response in the offspring. Studies with [14C]-DBC were also performed to determine the time-dependent levels of radioisotope distribution in maternal and fetal target tissues, as well as in urine and feces, following a single oral dose (15 mg/kg GD 17).
MATERIALS AND METHODS
Chemicals
DBC (CAS No.: 191-30-0; formerly referred to as dibenzo[a,l]pyrene) was obtained from the National Cancer Institute (NCI) Chemical Reference Standard Repository at Midwest Research Institute (Kansas City, MO) and was greater than 98% purity as determined by HPLC with UV detection [16]. Uniformly labeled [14C]-DBC was obtained from the NCI Radiochemical Carcinogen Reference Standard Repository previously operated by Chemsyn Science Laboratories (Lenexa, KS) with a specific activity of 51.4 mCi/mmol. This material was purified by Tjaden Biosciences, LLC (Burlington, IA) to >99% radiochemical purity. Other reagents utilized in this study were as described previously [16,25,26].
Animals and Diets
Breeding pairs of mice (B6129SF1/J females and 129S1/SvImJ males, eight weeks of age) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under pathogen-free conditions (micro-isolator cages from Life Products, Inc., Seaford, DE with Care FRESH bedding) at 20° ± 1°C and 50% ± 10% humidity with a light/dark cycle of 12 hours. Following arrival, mice were acclimated for 1 week prior to the initiation of breeding. Offspring were housed separately by sex and litter and fed AIN93G until three months of age and then AIN93M (both from Research Diets, New Brunswick, NJ) diet until the study was terminated at 10 months. Mice were observed daily for any signs of pain or discomfort and if any were observed the mice were euthanized by CO2 asphyxiation and a cervical dislocation performed prior to necropsy. All procedures used for the handling and treatment of mice in this study were approved by the Oregon State University Institutional Animal Care and Use Committee.
Study Design
Females were paired with males and monitored daily for the appearance of a vaginal plug (denoted GD 0). The dams were dosed either with a single gavage of 15 mg/kg DBC in corn oil (5 ml/kg) on GD 17 or with four total gavages (3.75 mg/kg DBC each) given to the dam on GD 5, 9, 13 and 17. Offspring were housed and monitored as described above until 10 months of age. To determine the distribution of total [14C]-DBC equivalents to the fetus, a subset of dams were randomly chosen and administered 300 μCi [14C]-DBC/kg, diluted to a specific activity of 20 μCi/mg DBC (to yield a dose of 15 mg/kg on GD 17), again by gavage in corn oil. The first experiment consisted of a time course with four dams dosed on GD 17 and four respective litters collected at each time point (2, 4, 6 or 8 hours after dosing). Dams and fetuses were euthanized appropriately (CO2 asphyxiation followed by cervical dislocation for dams and decapitation for fetuses), blood was collected into heparinized vials and spun at 15,000 g for 15 minutes to obtain plasma. Tissues and plasma (as well as maternal urine and feces) were stored at −80°C until analysis. A second cross foster experiment was conducted to assess transplacental versus translactational exposure as previously described [26]. Briefly, dams were gavaged with [14C]-DBC (n=3) or corn oil (n=3) on GD 17 and monitored for the delivery of offspring. After birth, neonates from DBC-treated mothers were randomly assigned to a foster corn oil-treated mother and vice versa so as to receive exposure only in utero or through lactation. At post-natal day 21 (PND 21), when these mice are normally weaned, tissues and plasma from a total of 40 pups (6 litters) and the 3 dams administered [14C]-DBC were euthanized and tissues collected as described above, again pooling within a litter as the dam represents the experimental unit.
Histopathology
At 10 months of age surviving mice were euthanized by CO2 asphyxiation, followed by cervical dislocation, and a number of tissues (thymus, lung, liver, spleen, heart, kidney, testis, ovary, uterus, colon, skin, and any (abnormal) lymph nodes) examined first by gross necropsy and then fixed in 10% formalin. Fixed tissues were routinely processed to paraffin blocks, and hematoxylin and eosin-stained sections were analyzed by a board-certified histopathologist as previously described [16].
Sample preparation for liquid scintillation [14C]-DBC analysis
Fetal tissues including lung, liver, GI tract (stomach through colon with contents) were pooled by tissue type within a litter and solubilized directly as described previously [33]. Maternal plasma, spleen and lung or homogenized portions of liver, GI tract (with contents), placenta, and kidney, were solubilized accordingly. Feces required extended solubilization time and bleach to remove color. Samples were then clarified with 1:5 H2O2: 2-propanol, treated with glacial acetic acid to remove chemiluminescence and stored overnight in the dark before measuring radioactivity by liquid scintillation.
Statistical Analysis
Litter size and sex ratio were assessed with Fisher’s exact test comparison of vehicle control and DBC treatment groups and found to not be significantly different at p<0.05. Comparisons of tumor multiplicity between four low doses of DBC and a single dose of DBC evaluated the number of tumors per mouse for those with tumors. A mixed-effects linear model was used to determine if there was statistically significant evidence between dose groups in body weight and multiplicity. The random effects of gender and litter were included in the model. There was statistically significant evidence of differences in body weight between the control and DBC groups (p<0.001), as well as differences in multiplicity between the four low doses and single high dose groups (p<0.001). Multiplicity was analyzed as tumors per animal including those with zero (i.e., overall multiplicity). In addition, there was evidence of considerable variance across the random effects gender and litter in the measurement of body weight. Statistical analyses were performed using Matlab R2011a (Version 7.12.0.635). Maternal and pooled-litter (fetal) [14C]-DBC concentrations in both the time-dependent tissue distribution and cross-foster studies were roughly log normal and hence log transformed for analysis. Each tissue (or ratio of tissues) of interest was analyzed separately. [14C]-DBC concentrations were compared between the four time points by overall ANOVA (n=4 dams/litters sacrificed per time point) followed by trend and/or other contrasts. For the cross-fostered study there were n=3 pairs, so that the data are shown for each cross-foster litter pair and the by-tissue paired t-tests,comparing the exposure routes, had low power (2 denominator degrees of freedom and considerable residual variation).
RESULTS and DISCUSSION
Maternal and Fetal Toxicity
Previous studies, utilizing this same cross of mouse strains and dosing with DBC on GD 17, did not result in any maternal or fetal toxicities as evidenced by the lack of an impact on the sex ratio (1.20 and 1.09, respectively), litter size (7.8 and 7.1) or birth weight [16]. In the present study there was no treatment-related effect on litter size or offspring gender (Table 1). Treatment of dams with multiple doses of DBC did not result in smaller pups at birth but there was a significant difference with respect to body weight of the pups upon weaning (11.8 g versus 9.6 g, p< 0.0001).
TABLE 1.
Sex, Birth Weights and Litter Size
| Group (# litters) | Males, Females (M/F) | Pups/Litter | B.W. (at weaning.) |
|---|---|---|---|
| Controls (5) | 17, 16 (1.06) | 6.6 ± 1.0 | 11.8 ± 0.9 |
| DBC (5) | 18, 13 (1.38) | 6.2 ± 2.1 | 9.6 ± 1.6* |
p< 0.0001
DBC Transplacental Carcinogenesis
The greatest difference in tumor response in the offspring from in utero exposure following treatment of dams with DBC at 4 doses of 3.75 mg/kg (maternal body weight) at GDs 5, 9, 13 and 17, compared to a single dose of 15 mg/kg at GD 17, was a total lack of mortality due to an aggressive T-cell lymphoblastic lymphoma. This lymphoma appears from ages 3–6 months much like what is observed in Tp53 (−/−) mice on the same genetic background [34].
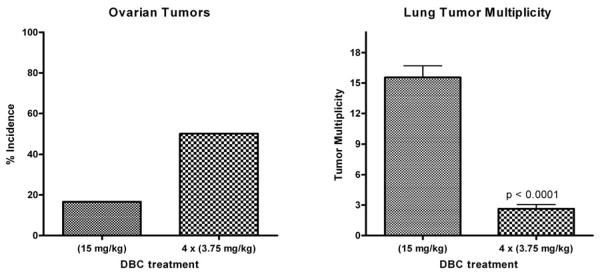
Following termination of the study, when offspring were 10 months of age, a marked difference in target organ response was observed between treatment regimes. The incidence of lung tumors in all our previous studies employing the single high dose was 100% and the average lung tumor multiplicity was 13–15 (tumors/tumor-bearing mouse) [16,25,26,35–37]. When a smaller DBC dose is administered over all trimesters, the lung tumor incidence was reduced to 80% (data not shown) and the multiplicity significantly reduced to 2.6 ± 0.4 (p< 0.0001) (Figure 1, right panel). Gestational age at exposure to ethylnitrosourea (ENU) markedly influences the multiplicity, size and morphology of lung tumors [38,39]. The greatest period of susceptibility to transplacental ENU lung tumorigenesis appears to be around GD 16, possibly due to variable rates of clonal expansion in the developing tissue [39], with lung morphogenesis beginning on GD 10 [40]. In addition to changes in cellular proliferation and differentiation throughout gestation, age-specific susceptibility to mutagenic transplacental carcinogens such as ENU or PAHs may be due to differences in expression of metabolic enzymes or in repair pathway activation. As treatments were administered in this study on GD 5, 9, 13, and 17, it is possible that this window of susceptibility and the lower individual doses were both factors in the reduced lung tumor multiplicity observed. The reduction in liver lesions was also striking. Previously, the single high dose resulted in a lesion (precancerous lesions and adenomas) incidence of 70% (almost exclusively in males) while the multiple low doses yielded only a 6.3% incidence (data not shown).
Figure 1.
Offspring born to a mother treated with multiple doses of DBC (3.75 mg/kg GDs 5, 9, 13, 17) versus a single dose at 15 mg/kg GD 17 have increased ovarian tumor incidence (left panel) and decreased lung tumor multiplicity (right panel).
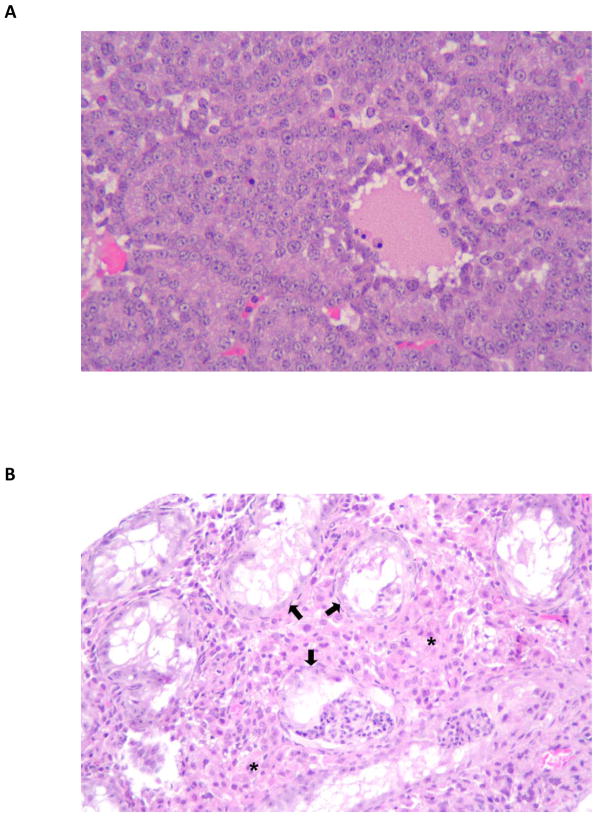
In contrast to the reduced response in lymphoma, lung and liver tumors, a marked increase in the incidence of ovarian tumors was observed (Figure 1, left panel and Figure 2A). Histologically, the ovarian tumors presented as sex-cord stromal tumors and included granulosa cell tumors, granulosa cell-theca cell tumor, and luteoma. Some of the tumors had extensive necrosis and hemorrhage. It is possible that exposure to DBC earlier in gestation (during organogenesis and gonad differentiation) is responsible for this pathology. Exposure of C57BL/6 mice to the PAHs benzo[a]pyrene (BaP) and 7,12-dimethylbenz[a]anthracene (DMBA) in utero during early development (maternal treatment with 6 mg/kg total just prior to conception) reduced the number of primordial follicles by over two thirds [41]. Interestingly, the ovarian toxicity also occurred following lactational exposure but the greatest impact was if the mothers of female offspring were exposed both pre-pregnancy and during lactation. The toxicity was shown by Jurisicova et al., to be aryl hydrocarbon receptor (Ahr)-dependent with the use of Ahr antagonists [41]. We did not determine the impact of Ahrb-1/d (responsive) versus Ahrd/d (non-responsive) genotype on the degree of ovarian toxicity nor did we examine lactational exposure as a separate factor (pups were exposed to residual DBC from distribution to maternal breast during gestation). The uteri of ovarian tumor-bearing mice were examined and all showed cystic endometrial hyperplasia, a fairly common finding in older mice but previously not seen to any great degree in 10 month-old offspring from the B6129F1 × 129 cross.
Figure 2.
(A) Photomicrograph of a granulosa cell tumor in the ovary of a 10-month-old offspring born to a mother treated with DBC (3.75 mg/kg GDs 5, 9, 13, 17). (B) This unusually small testis collected at necropsy from a 10-month-old offspring born to a mother treated with DBC (3.75 mg/kg GDs 5, 9, 13, 17) shows atrophic seminiferous tubules (arrows). Note the increased number of interstitial (Leydig) cells (asterisks).
In male offspring, abnormally small testes were seen, a pathology not previously observed with the late gestation single DBC dose (Figure 2B). Histologically, these testes showed diffuse atrophy of seminiferous tubules, marked Leydig cell hyperplasia, and scattered granulomas. Igsajim et al., [42] found that exposure of mice on GD 14 to the high potency Ahr ligand TCDD resulted in an Ahr-dependent negative impact on prostate development. TCCD has been known to be a reproductive toxicant in mice and other rodents, reducing ano-genital distance and sperm viability later in life [43,44]. Leydig cell hyperplasia has been documented in male mice following transplacental exposure to estradiol and diethylstilbestrol [45,46]. Evidence also exists in humans for maternal smoking similarly producing a negative impact on testicular development, possibly due in part to PAH exposure [47,48].
Our results suggest differential tissue susceptibility to DBC, depending on age of development at exposure. These findings are similar to studies with an environmental mixture of PAHs, cigarette smoke, that differentially affects cancer incidence and target organ depending on the developmental window of exposure in mice [49]. An alternative explanation, provided by Buters et al., is that lower individual doses of DBC exert less acute toxicity to immune cells thereby prolonging survival time and allowing alternative tissue malignancies to develop [50].
[14C]-DBC Distribution
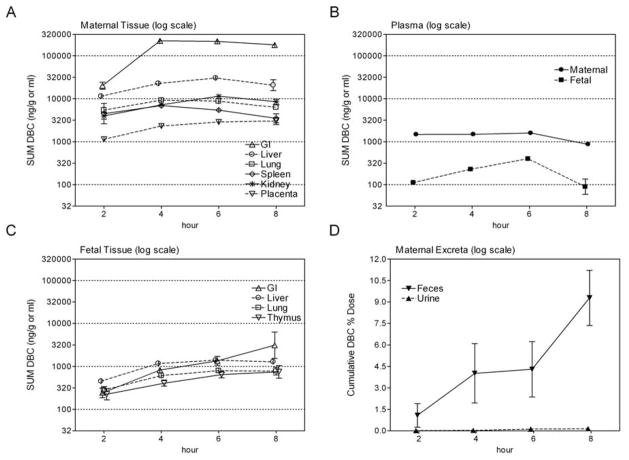
Tissue levels of [14C]-DBC equivalents (DBC plus metabolites) were measured in maternal and fetal tissues, as well as in urine, plasma, and feces in separate animals euthanized at 2, 4, 6 and 8 hours following a single (15 mg/kg, ~10 μCi) dose by gavage on GD 17. The geometric mean of maternal plasma from mice sacrificed at 2, 4, or 6 hours was fairly constant at about 1.5 μg/ml DBC equivalents (Figure 3B) whereas the animals euthanized at 8 hours had about 1.8-fold lower levels (Cmax of approximately 3 μM). Maternal GI tract with contents exhibited a significant increase between animals euthanized at 2 hours and animals euthanized at 4 hours and the majority of radioisotope (~75%) remained there throughout the 8 hour period (Figure 3A). Interestingly, the placenta was the only maternal tissue shown to increase in concentration of [14C]-DBC equivalent between 6 and 8 hours. The placental tissue in mice administered [14C]-benzo[a]pyrene (BaP) has similarly been shown to retain radioactivity much longer than other maternal organs [51]. When expressed as a ratio relative to plasma concentration, the following maternal tissues exhibited an approximately constant percent increase per hour (kidney, 23.6%; liver, 19.6%; lung, 9.9%, and placenta, 26.4%).
Figure 3.
Pregnant mice received a single oral dose of 15 mg [14C]-DBC/kg body weight on GD 17. Transplacental transfer to fetus and maternal distribution of [14C]-DBC, or metabolite equivalents (SUM DBC), was evaluated at 2, 4, 6, and 8 hours post-dose. Bars represent the mean and standard error, n=4 litters or 4 dams per time-point. Total [14C]-DBC for maternal and fetal tissues over time is depicted in panels A and C, respectively, whereas the maternal and fetal plasma levels are shown in panel B. Time-dependent cumulative excretion of [14C]-DBC equivalents in feces and urine is shown in panel D.
Compared to maternal plasma, fetal tissues exhibited 4- to 13-fold lower levels of [14C]-DBC over the 2 to 8 hours after gavage (Figure 3B). Levels of [14C] were still increasing at 8 hours in the fetal GI tract and thymus but appeared to have reached a plateau in lung and liver (Figure 3B). The concentrations at 8 hours were 773, 748 and 1274 ng [14C]-DBC equivalents/g tissue (wet wt.) in fetal thymus, lung and liver, respectively (Figure 3C). For animals euthanized at 8 hours, maternal lung and liver levels were 8- and 16-fold higher, than the respective fetal tissue (Figure 3A and 3C). The fetal tissue:plasma ratios did not increase over the first 6 hours nor did they differ between tissues, however at 8 hours this ratio increased dramatically as plasma levels dropped, with fetal GI tract:plasma significantly higher than other tissues.
The elimination of [14C]-DBC in maternal urine and feces was also followed over the 2–8 hour time-course demonstrating that the great majority of [14C]-DBC equivalents were eliminated in the feces as expected for an oral exposure (Figure 3D).
However, this study was not designed to characterize the pharmacokinetics of DBC or its metabolites directly nor was it of sufficient duration to fully define the clearance of [14C]-DBC as shown in Figure 4. Such a study is currently in progress where the pharmacokinetics of DBC and its diol and tetrol metabolites are being evaluated more extensively in cohorts of 36 non-pregnant and 36 pregnant (GD 17) mice (B6129SF1/J) administered DBC at the same dose level (15 mg/kg) used in the current studies. Preliminary results indicate that the half-lives for the clearance of DBC from blood, determined in subgroups of 3–4 mice sacrificed at various times over 48 hours increased from approximately 7.2 hours in non-pregnant mice to 14 hours in pregnant mice. This longer half-life corresponded to increased peak concentrations and areas under the curve for DBC in blood of pregnant mice (13.7 ± 10.4 μM and 2911 μmol × min/L, respectively) vs. non-pregnant mice (3.5 0.3 and 1136 μmol × min/L, respectively). Work is in progress to more completely evaluate the impact of pregnancy on the pharmacokinetics of DBC and its metabolites in blood, feces, urine, tissues, and fetuses (where applicable) from these mice. Such data will facilitate the continued development and refinement of a physiologically based pharmacokinetic model for DBC [52] that will ultimately be used to relate dose levels used in these mouse carcinogenicity studies to relevant human exposure scenarios.
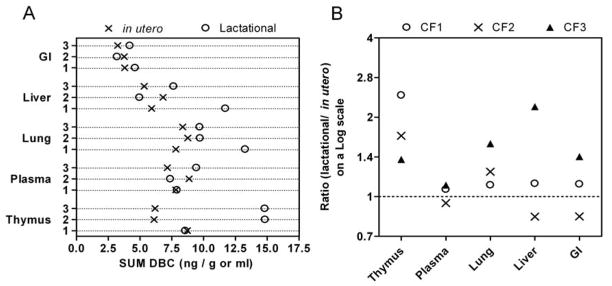
Figure 4.
Neonatal tissue and plasma concentrations at weaning (PND21) are similar following in utero or lactational exposure to [14C]-DBC. Three litters were exposed in utero following oral gavage of the mother with [14C]-DBC and at birth exchanged with a litter from a vehicle-treated dam (cross-fostered) such that the paired litter received DBC exposure via lactation. Raw data for three pairs of cross-fostered litters demonstrate variable tissue profiles according to the cross-fostered group (panel A). The log 2 ratio of SUM DBC (DBC or metabolite equivalents) from paired lactational/ in utero exposure litters (panel B) is generally greater than 1 (represented by the dotted horizontal line).
In our previous studies on the transplacental carcinogenesis properties of DBC, we performed a study with a cross-foster design [26]. We were somewhat surprised to see that the majority of the T-cell lymphoblastic lymphoma mortality was due to the short 2–3 day in utero exposure; pups exposed only through breast milk had little mortality. Lung tumor multiplicity at 10 months of age was also significantly higher in offspring only exposed in utero. We speculated that the difference may be that DBC, like other PAHs, had a relatively short half-life in the dam and by parturition, little DBC remained to partition into breast milk. It was outside the scope of this study to examine the residual DBC at the onset of lactational exposure or to measure partitioning of DBC into breast milk. When cross-fostered litters were matched according to exposed dam, we were unable to detect any significant difference in the concentration of [14C]-DBC equivalents in offspring exposed only in utero versus through continuous lactational exposure at the end of 3 weeks (Figure 4). This demonstrates that DBC reaches the previously characterized target organs (thymus, lung, and liver) following exposure to a single dose of DBC (15 mg/kg on GD 17) both transplacentally and translactationally. However, our results represent total [14C] with no further identification of the chemical identity. Thus, a more thorough study of the pharmacokinetics of DBC in this transplacental model requires that we characterize DBC metabolites and DBC-covalent adducts in the various maternal and fetal tissues throughout gestation and lactation, and is now ongoing as described above.
DBC-DNA adducts in fetal lung, reported in one of our previous studies [35], was similar to maternal lung. The major DNA adducts of DBC are derived from Cyp1-dependent epoxygenation, followed by hydrolysis to the trans-dihydrodiol (DBCD) which is epoxygenated a second time to 11,12-trans-dihydrodiol-13,14-epoxide (DBCDE, 4 possible enantiomers). These fjord region PAH diol-epoxides, in most animal models [53–55], are more carcinogenic than the bay region BPDEs. Cyp1b1 (and human CYP1B1) are very efficient at carrying out both epoxygenations, followed by Cyp1a1 with a smaller contribution from Cyp1a2 [28,29].
The absolute requirement for Cyp1b1 expression in fetal thymus [25] for induction of T-cell lymphoma suggests that one or more of the key steps in the bioactivation of DBC occurs in the fetal compartment. Given the very high chemical reactivity of the most potent of the diol-epoxide metabolites, DBCDE, it is unlikely that it crosses the placenta and distributes to fetal thymus tissues without hydrolysis or reaction with nucleophiles.
In conclusion, we have demonstrated in this manuscript that the target tissue response for PAH-dependent transplacental carcinogenesis in this mouse model is dependent, to a large degree, on the stage of development during exposure. The high sensitivity to developing T-cell lymphoma when the dam is dosed on GD 17 is consistent with the developmental expression of Cyp1b1 (thymus expresses the highest level of Cyp1b1 mRNA during late gestation in both mouse and human) [34,56]. Current studies are underway to determine if the ontogeny of expression of various members of the Cyp1 family are critical for prediction of target organs for transplacental carcinogenesis (or other developmental toxicities) and if these genes could be promising targets for chemoprevention [57,58] of transplacental cancer. Finally, studies of the potency of actual PAH environmental mixtures as transplacental carcinogens are underway to better assess potential human health risk.
Acknowledgments
The authors would like to thank Marilyn Henderson, Tammie McQuistan, and Abby Benninghoff for their technical expertise and Mandy Louderback for animal handling assistance. In addition, we would like to acknowledge Dr. Clifford Pereira, Department of Statistics, Oregon State University, for his advice on statistical modeling. Support from this study was provided by the following PHS grants from NIH, P42 ES016465, P01 CA90890, T32ES07060 and P30 ES03850 along with assistance from The Linus Pauling Institute at Oregon State University.
Footnotes
Conflict of interest statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morley R. Fetal origins of adult disease. Semin Fetal Neonatal Med. 2006;11:73–78. doi: 10.1016/j.siny.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 3.Dolinoy DC, Das R, Weidman JR, Jirtle RL. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res. 2007;61:30–37. doi: 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]
- 4.Burde GC, Lillycrop KA. Nutrition, epigenetics and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 5.Sasco SJ, Vainio H. From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol. 1999;18:192–201. doi: 10.1191/096032799678839905. [DOI] [PubMed] [Google Scholar]
- 6.John ES, Savitz DA, Sandler DP. Prenatal exposure to parents’ smoking and childhood cancer. Am J Epidemiol. 1991;133:123–132. doi: 10.1093/oxfordjournals.aje.a115851. [DOI] [PubMed] [Google Scholar]
- 7.Sandler DP, Everson RB, Wilcox AJ, Browder JP. Cancer risk in adulthood from early life exposure to parents’ smoking. Am J Public Hlth. 1985;75:487–492. doi: 10.2105/ajph.75.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson LM. Introduction and overview. Perinatal carcinogenesis: growing a node for epidemiology, risk management, and animal studies. Toxicol Appl Pharmacol. 2004;199:85–90. doi: 10.1016/j.taap.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LM, Hecht SS, Dixon DE, Dove LF, Kovatch RM, Amin S, Hoffmann D, Rice JM. Evaluation of the transplacental tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. Cancer Res. 1989;49:3770–3775. [PubMed] [Google Scholar]
- 10.Fujii K, Nomoto KI, Nakamura K. Tumor induction in mice administered neonatally with 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole or 3-amino-1-methyl-5H-pyrido[4,3-b]indole. Carcinogenesis. 1987;8:1721–1723. doi: 10.1093/carcin/8.11.1721. [DOI] [PubMed] [Google Scholar]
- 11.Badham HJ, LeBrun DP, Rutter A, Winn LM. Transplacental benzene exposure increases tumor incidence in mouse offspring: possible role of fetal benzene metabolism. Carcinogenesis. 2010;31:1142–1148. doi: 10.1093/carcin/bgq074. [DOI] [PubMed] [Google Scholar]
- 12.Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxiocl Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Murracy JC, Hill RM, Hegemier S, Hurwitz FI. Lymphoblastic lymphoma following prenatal exposure to phenytoin. J Pediatr Hematol Oncol. 1996;18:241–243. doi: 10.1097/00043426-199605000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Miller MS, Jones AB, Park SS, Anderson LM. The formation of 3-methylcholanthrene-initiated lung tumors correlates with induction of cytochrome P450IA1 by the carcinogen in fetal but not adult mice. Toxicol Appl Pharmacol. 1990;104:235–245. doi: 10.1016/0041-008x(90)90298-9. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z, Loehr CV, Fischer KA, Louderback MA, Krueger SK, Dashwood RH, Kerkvliet NI, Pereira CB, Jennings-Gee JE, Dance ST, Miller MS, Bailey GS, Williams DE. In utero exposure of mice to dibenzo[a,l]pyrene produces lymphoma in the offspring: role of the aryl hydrocarbon receptor. Cancer Res. 2006;66:755–762. doi: 10.1158/0008-5472.CAN-05-3390. [DOI] [PubMed] [Google Scholar]
- 17.Perera FP, Tang D, Tu YH, Cruz LA, Borjas M, Bernert T, Whyatt RM. Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environm Hlth Perspect. 2004;112:1133–1136. doi: 10.1289/ehp.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, The World Trade Center area, Poland and China. Cancer Epidemiol Biomarkers Prev. 2005;14:709–714. doi: 10.1158/1055-9965.EPI-04-0457. [DOI] [PubMed] [Google Scholar]
- 19.Perera FP, Rauh V, Tsai W-Y, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu Y-H, Diza D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environm Hlth Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedrychowski W, Galas A, Pac A, Flak E, Caman D, Rauh V, Perera F. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20:775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 21.Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environm Hlth Perspect. 1999;107(suppl3):451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen C, Sørensen LD, Asmussen I, Autrup H. Transplacental exposure to tobacco smoke in human-adduct formation in placenta and umbilical cord blood vessels. Teratog Carcinog Mutagen. 1992;12:51–60. doi: 10.1002/tcm.1770120202. [DOI] [PubMed] [Google Scholar]
- 23.Whyatt RM, Jedrychowski W, Hemminki K, Santella RM, Tsai W-Y, Yang K, Perera FP. Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol Biomarkers Prev. 2001;16:581–588. [PubMed] [Google Scholar]
- 24.World Health Organization, International Agency for Research on Cancer. Some non-heterocyclic polcyclic aromatic hydrocarbons and some related exposures. Vol. 92. Lyon: France; 2010. IARC monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 25.Castro DJ, Baird WM, Pereira C, Giovanini J, Löhr C, Fischer K, Yu Z, Gonzalez FJ, Krueger SK, Williams DE. Fetal Cyp1b1 in mice and transplacental carcinogenesis from maternal exposure to dibenzo[a,l]pyrene. Cancer Prev Res. 2008;1:128–134. doi: 10.1158/1940-6207.CAPR-07-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castro DJ, Löhr CV, Fischer KA, Pereira C, Williams DE. Lymphoma and lung cancer in offspring born to pregnant mice dosed with dibenzo[a,l]pyrene: the importance of in utero versus lactational exposure. Toxicol Appl Pharmacol. 2008;233:454–458. doi: 10.1016/j.taap.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M, Nelson GB, Moore JE, McCoy TP, Dai J, Manderville RA, Ross JA, Miller MS. Induction of Cyp1a1 and Cyp1b1 and formation of DNA adducts in C57BL/6, Balb/c, and F1 mice following in utero exposure to 3-methylcholanthrene. Toxicol Appl Pharmacol. 2005;209:28–38. doi: 10.1016/j.taap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM 2009. Drug Metabol Dispos. 2001;29:1176–1182. [PubMed] [Google Scholar]
- 29.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochrome P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 31.Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Molec Pharmacol. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 32.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 33.Yu Z. PhD Thesis. Oregon State University; 2005. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by dibenzo[a,l]pyrene in the B6129 mouse model: role of the aryl hydrocarbon receptor. [Google Scholar]
- 34.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Mahadevan B, Löhr CV, Fischer KA, Louderback MA, Krueger SK, Pereira CB, Albershardt DJ, Baird WM, Bailey GS, Williams DE. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon, dibenzo[a,l]pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 36.Castro DJ, Löhr CV, Fischer KA, Waters CK, Webb-Robertson B-J, Dashwood DH, Bailey GS, Williams DE. Identifying efficacious approaches to chemoprevention with chlorophyllin, purified chlorophylls and freeze-dried spinach in a mouse model of transplacental carcinogenesis. Carcinogenesis. 2009;30:315–320. doi: 10.1093/carcin/bgn280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro DJ, Yu Z, Löhr CV, Pereira CB, Giovanini J, Fischer KA, Orner GA, Dashwood RH, Williams DE. Chemoprevention of dibenzo[a,l]pyrene transplacental carcinogenesis in mice born to mothers administered green tea: primary role of caffeine. Carcinogenesis. 2008;29:1581–1586. doi: 10.1093/carcin/bgm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehm S, Ward JM, Anderson LM, Riggs CW, Rice JM. Transplacental induction of mouse lung tumors: stage of fetal organogenesis in relation to frequency, morphology, size, and neoplastic progression of N-nitrosoethylurea-induced tumors. Toxicol Pathol. 1991;19:35–46. doi: 10.1177/019262339101900105. [DOI] [PubMed] [Google Scholar]
- 39.Branstetter DG, Stoner GD, Budd C, Conran PB, Goldblatt PJ. Effect of gestational development on lung tumor size and morphology in the mouse. Cancer Res. 1988;48:379–386. [PubMed] [Google Scholar]
- 40.Warburton D, Gauldie J, Bellusci S, Shi W. Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Amer Thorac Soc. 2006;3:668–672. doi: 10.1513/pats.200605-122SF. [DOI] [PubMed] [Google Scholar]
- 41.Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Hernández AB, Nunez G, Casper RF. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117:3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igsajim S, Fukuzawa B, Ishimura R, Kawakami T, Wu Q, Nagano R, Zaha H, Sone H, Yonemoto J, Tohyama C. Comparative contribution of the aryl hydrocarbon receptor gene to perinatal state development and dioxin-induced toxicity between the urogenital complex and testis in the mouse. Biol Reprod. 2010;82:636–643. doi: 10.1095/biolreprod.109.080812. [DOI] [PubMed] [Google Scholar]
- 43.Bably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of males rats to 2,3,7,8-tetrachlorodibezo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicol Appl Pharmacol. 1992;114:118–126. doi: 10.1016/0041-008x(92)90103-y. [DOI] [PubMed] [Google Scholar]
- 44.Gray LE, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda Y, Konishi H, Tanimura T. Leydig cell hyperplasia in fetal mice treated transplacentally with ethinyl estradiol. Teratol. 1986;33:281–288. doi: 10.1002/tera.1420330305. [DOI] [PubMed] [Google Scholar]
- 46.Pérez-Martinez C, Garcia-Iglesias MJ, Ferreras-Estrada MC, Bravo-Moral AM, Espinosa-Alvarez J, Escudero-Diez A. Effects of in-utero exposure to zeranol or diethylstilboestrol on morphological development of the fetal testis in mice. J Comp Pathol. 1996;114:407–418. doi: 10.1016/s0021-9975(96)80016-8. [DOI] [PubMed] [Google Scholar]
- 47.Coutts SM, Fulton N, Anderson RA. Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Human Reprod. 2007;22:2912–2918. doi: 10.1093/humrep/dem300. [DOI] [PubMed] [Google Scholar]
- 48.Fowler PA, Cassie S, Rhind SM, Brewer MJ, Collinson JM, Lea RG, Baker PJ, Bhattacharya S, O’Sharghnessy PJ. Maternal smoking during pregnancy specifically reduces human fetal Desert Hedgehog gene expression during testis development. J Clin Endocrinol Metabol. 2008;93:619–626. doi: 10.1210/jc.2007-1860. [DOI] [PubMed] [Google Scholar]
- 49.Balansky R, Ganchev G, Iltcheva M, Nikolov M, Steele VE, De Flora S. Differential carcinogenicity of cigarette smoke in mice exposed either transplacentally, early in life or in adulthood. Internatl J Cancer. 2011 doi: 10.1002/ijc.26103. in press. [DOI] [PubMed] [Google Scholar]
- 50.Buters J, Quintanilla-Martinez L, Schober W, Soballa VJ, Hintermair J, Wolff T, Gonzalez FJ, Greim H. CYP1B1 determines susceptibility to low doses of 7,12-dimethylbenz[a]anthracene-induced ovarian cancers in mice: correlation of CYP1B1-mediated DNA adducts with carcinogenicity. Carcinogenesis. 2003;24:327–334. doi: 10.1093/carcin/24.2.327. [DOI] [PubMed] [Google Scholar]
- 51.Neubert D, Tapken S. Transfer of benzo(a)pyrene into mouse embryos and fetuses. Arch Toxicol. 1988;62:236–239. doi: 10.1007/BF00570149. [DOI] [PubMed] [Google Scholar]
- 52.Crowell SR, Amin SG, Anderson KA, Krishnegowda G, Sharma AK, Soelberg JJ, Williams DE, Corley RA. Preliminary physiologically based pharmacokinetic models for benzo[a]pyrene and dibenzo[def,p]chrysene in rodents. Toxicol Appl Pharmacol. 2011 doi: 10.1016/j.taap.2011.09.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavalieri EL, Higginbotham S, Ramakrishna NVS, Devanesan PD, Todorovic R, Rogan EG, Salmasi S. Comparative dose-response tumorigenicity studies of dibenzo[a,l]pyrene versus 7-12-dimethylbenz[a]anthracene and benzo[a]pyrene and two dibenzo[a,l]pyrene dihydrodiols in mouse skin and rat mammary gland. Carcinogenesis. 1991;12:1939–1944. doi: 10.1093/carcin/12.10.1939. [DOI] [PubMed] [Google Scholar]
- 54.Higgingbotham S, Ramakrishna NVS, Johansson SL, Rogan EG, Cavalieri EL. Tumor-initiating activity of dibenzo[a,l]pyrene versus 7–12-dimethybenz[a]anthracene and benzo[a]pyrene at low doses in mouse skin. Carcinogenesis. 1993;14:875–878. doi: 10.1093/carcin/14.5.875. [DOI] [PubMed] [Google Scholar]
- 55.Prahalad AK, Ross JA, Nelson GB, Roop BC, King LC, Nesnow S, Mass MJ. Dibenzo[a,l]pyrene-induced DNA adduction, tumorigenicity, and Ki-ras oncogene mutation in strain A/J mouse lung. Carcinogenesis. 1997;18:1955–1963. doi: 10.1093/carcin/18.10.1955. [DOI] [PubMed] [Google Scholar]
- 56.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys. 2005;61:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Guengerich PF, Chun YJ, Kim D, Gilliam EM, Shimada TT. Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res. 2003;523–524:173–182. doi: 10.1016/s0027-5107(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 58.Chun YJ, Kim S. Discovery of cytochrome P450 1B1 inhibitors as new promising anti-cancer agents. Medicinal Res Rev. 2003;23:657–668. doi: 10.1002/med.10050. [DOI] [PubMed] [Google Scholar]